Alternate Causes for Pathogenesis of Exfoliation Glaucoma, a Multifactorial Elastotic Disorder: A Literature Review
Abstract
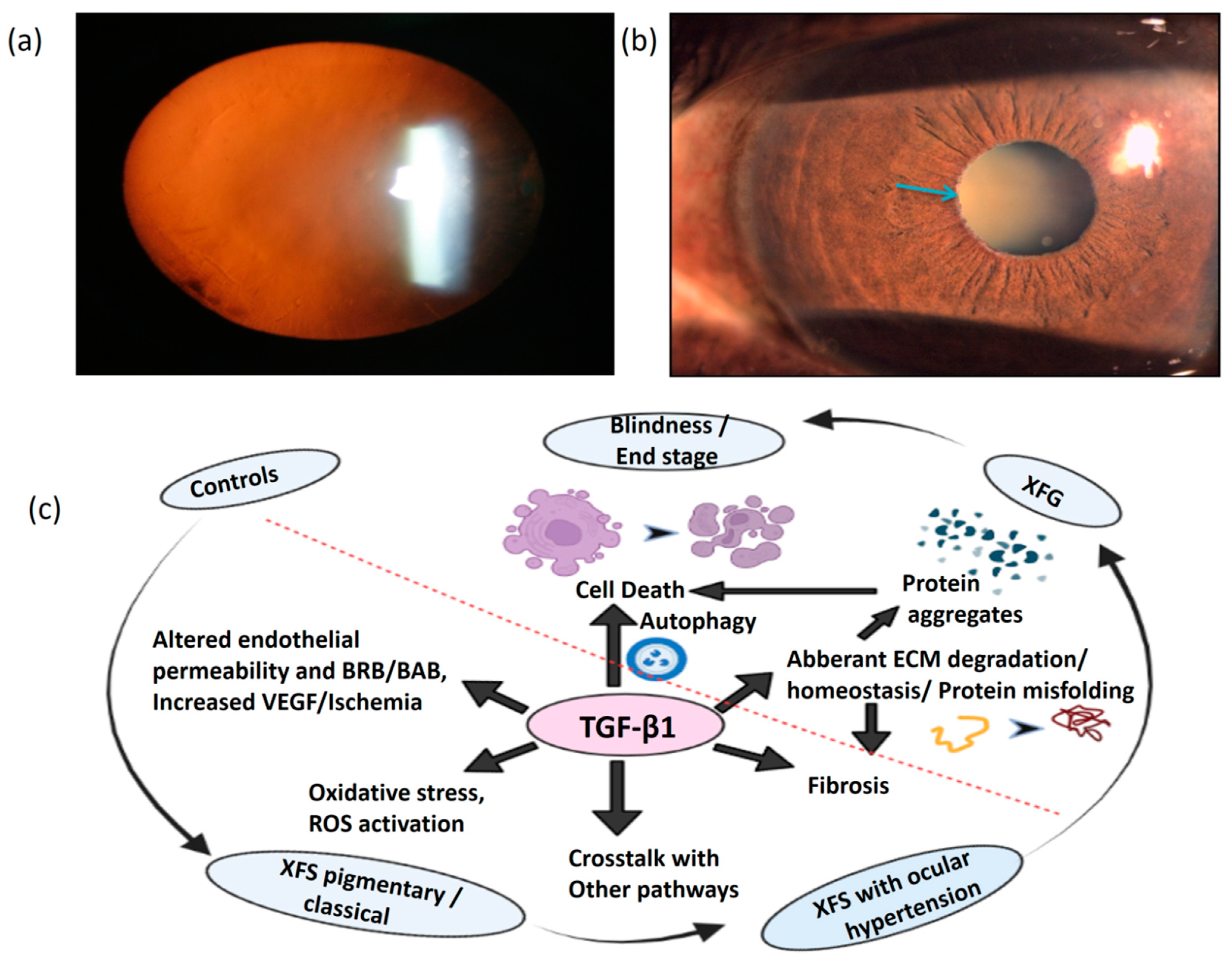
1. Introduction
2. Methods
3. Potential Role of miRNAs in XFG
4. Autophagy and Mitochondrial Dysfunction and Protein Aggregate Clearance
5. The Blood–Aqueous Barrier in Eyes with Exfoliation Syndrome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritch, R. The management of exfoliative glaucoma. Prog. Brain Res. 2008, 173, 211–224. [Google Scholar]
- Ritch, R.; Schlotzer-Schrehardt, U.; Konstas, A.G. Why is glaucoma associated with exfoliation syndrome? Progr. Retin. Eye Res. 2003, 22, 253–275. [Google Scholar] [CrossRef]
- Lee, R.K. The molecular pathophysiology of exfoliation glaucoma. Curr. Opin. Ophthalmol. 2008, 19, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.M.; Karger, R.A.; Hodge, D.O.; Burke, J.P.; Johnson, D.H.; Good, M.S. The risk of glaucoma in exfoliation syndrome. J. Glaucoma 2007, 16, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Thorleifsson, G.; Magnusson, K.P.; Sulem, P.; Walters, G.; Gudbjartsson, D.F.; Stefansson, H.; Jonsson, T.; Jonasdottir, A.; Jonasdottir, A.; Stefansdottir, G.; et al. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science 2007, 317, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Ozaki, M.; Lee, M.C.; Schlötzer-Schrehardt, U.; Thorleifsson, G.; Mizoguchi, T.; Igo, R.P.; Haripriya, A., Jr.; Williams, S.E.; Astakhov, Y.S.; et al. Genetic association study of exfoliation syndrome identifies a protective rare variant at LOXL1 and five new susceptibility loci. Nat. Genet. 2017, 49, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.; Ozaki, M.; Mizoguchi, T.; Allingham, R.R.; Li, Z.; Haripriya, A.; Nakano, S.; Uebe, S.; Harder, J.M.; Chan, A.S.; et al. A common variant mapping to CACNA1A is associated with susceptibility to exfoliation syndrome. Nat. Genet. 2015, 47, 387–392. [Google Scholar] [CrossRef]
- Dewundara, S.; Pasquale, L.R. Exfoliation syndrome: A disease with an environmental component. Curr. Opin. Ophthalmol. 2015, 26, 78–81. [Google Scholar] [CrossRef]
- Ghaffari, S.M.; Damji, K.F.; Unsworth, L.D. Recent advances in risk factors associated with ocular exfoliation syndrome. Acta Ophthalmol. 2020, 98, 113–120. [Google Scholar] [CrossRef]
- Rao, A.; Chakraborty, M.; Roy, A.; Sahay, P.; Pradhan, A.; Raj, N. Differential miRNA Expression: Signature for Glaucoma in Exfoliation. Clin. Ophthalmol. 2020, 14, 3025–3038. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat. Rev. Genet. 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Chen, K.; Rajewsky, N. The evolution of gene regulation by transcription factors and microRNAs. Nat. Rev. Genet. 2007, 8, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Bentwich, I.; Avniel, A.; Karov, Y.; Aharonov, R.; Gilad, S.; Barad, O.; Barzilai, A.; Einat, P.; Einav, U.; Meiri, E.; et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005, 37, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, L.; Tewari, A.; Coffin, S.L.; Xhako, E.; Pang, K.; Gennarino, V.A.; Johnson, J.L.; Blanco, F.A.; Liu, Z.; Zoghbi, H.Y. miR760 regulates ATXN1 levels via interaction with its 5′ untranslated region. Genes Dev. 2020, 34, 1147–1160. [Google Scholar] [CrossRef]
- Guo, R.; Shen, W.; Su, C.; Jiang, S.; Wang, J. Relationship between the pathogenesis of glaucoma and miRNA. Ophthalmic Res. 2017, 57, 194–199. [Google Scholar] [CrossRef]
- Undi, R.B.; Kandi, R.; Gutti, R.K. MicroRNAs as Haematopoiesis Regulators. Adv. Hematol. 2013, 2013, 695754. [Google Scholar] [CrossRef]
- Lu, B.; Christensen, I.T.; Ma, L.W.; Wang, X.L.; Jiang, L.F.; Wang, C.X.; Feng, L.; Zhang, J.S.; Yan, Q.C. miR-24-p53 pathway evoked by oxidative stress promotes lens epithelial cell apoptosis in age-related cataracts. Mol. Med. Rep. 2018, 17, 5021–5028. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Wang, S. MicroRNA-93 is overexpressed and induces apoptosis in glaucoma trabecular meshwork cells. Mol. Med. Rep. 2016, 14, 5746–5750. [Google Scholar] [CrossRef]
- Jayaram, H.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C. MicroRNA Expression in the Glaucomatous Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7971–7982. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen, L.M.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuda, S.; Kunikata, H.; Sato, J.; Kokubun, T.; Yasuda, M.; Nishiguchi, K.M.; Inada, T.; Nakazawa, T. Profiles of extracellular miRNAs in the aqueous humor of glaucoma patients assessed with a microarray system. Sci. Rep. 2014, 4, 5089. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, S.Y.; Bae, Y.S. Upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells. Mol. Cells 2014, 37, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Wang, Y.; Zhang, X.; Gao, K.; Chen, S.; Zhang, X. microRNA profiling in glaucoma eyes with varying degrees of optic neuropathy by using next-generation sequencing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2955–2966. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Liu, J.H.; Woung, L.C.; Lin, T.J.; Chiou, S.H.; Tseng, P.C.; Du, W.Y.; Cheng, C.K.; Hu, C.C.; Chien, K.H.; et al. MicroRNAs and cataracts: Cor-relation among let-7 expression, age and severity of lens opacity. Br. J. Ophthalmol. 2012, 96, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Sand, M.; Gambichler, T.; Sand, D.; Skrygan, M.; Altmeyer, P.; Bechara, F.G. MicroRNAs and the skin: Tiny players in the body’s largest organ. J. Dermatol. Sci. 2009, 53, 169–175. [Google Scholar] [CrossRef]
- Khee, S.G.; Yusof, Y.A.; Makpol, S. Expression of senescence-associated microRNAs and target genes in cellular aging and modulation by tocotrienol-rich fraction. Oxidative Med. Cell. Longev. 2014, 2014, 725929. [Google Scholar]
- Kim, J.; Yoon, H.; Chung, D.E.; Brown, J.L.; Belmonte, K.C.; Kim, J. miR-186 is decreased in aged brain and suppresses BACE 1 expression. J. Neurochem. 2016, 137, 436–445. [Google Scholar] [CrossRef]
- Lou, G.; Xu, W.; Dong, F.; Chen, G.; Liu, Y. Plasma miR-17, miR-20a, miR-20b and miR-122 as potential biomarkers for diagnosis of NAFLD in type 2 diabetes mellitus patients. Life Sci. 2018, 208, 201–207. [Google Scholar]
- Xiu, C.; Jiang, J.; Song, R. Expression of miR-34a in cataract rats and its related mechanism. Exp. Ther. Med. 2020, 19, 1051–1057. [Google Scholar] [CrossRef]
- Pan, Y.J.; Wei, L.L.; Wu, X.J.; Huo, F.C.; Mou, J.; Pei, D.S. MiR-106a-5p inhibits the cell migration and invasion of renal cell carcinoma through targeting PAK5. Cell Death Dis. 2017, 8, e3155. [Google Scholar] [CrossRef]
- Que, T.; Song, Y.; Liu, Z.; Zheng, S.; Long, H.; Li, Z.; Liu, Y.; Wang, G.; Liu, Y.; Zhou, J.; et al. Decreased miRNA-637 is an unfavorable prognosis marker and promotes glioma cell growth; migration and invasion via direct targeting Akt1. Oncogene 2015, 34, 4952–4963. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.B.; Xue, L.; Yang, J.; Ma, A.H.; Zhao, J.; Xu, M.; Tepper, C.G.; Evans, C.P.; Kung, H.J.; deVere White, R.W. An androgen-regulated miRNA suppresses Bak1 expression and induces BACE1 expression. J. Neurochem. 2016, 137, 436–445. [Google Scholar]
- Ye, D.; Zhang, T. Androgen-independent growth of pros-tate cancer cells. Proc. Natl. Acad. Sci. USA 2007, 104, 19983–19988. [Google Scholar]
- Thomson, J.M.; Newman, M.; Parker, J.S.; Morin-Kensicki, E.M.; Wright, T.; Hammond, S.M. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 2006, 20, 2202–2207. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Zheng, T.H.; Bai, L.; Liu, Z.J. MicroRNAs in the occurrence and development of primary hepatocellular carcinoma. Adv. Clin. Exp. Med. 2016, 25, 971–975. [Google Scholar] [CrossRef]
- Zhang, J.F.; He, M.L.; Fu, W.M.; Wang, H.; Chen, L.Z.; Zhu, X.; Chen, Y.; Xie, D.; Lai, P.; Chen, G.; et al. Primate-specific microRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by disrupting signal transducer and activator of transcription 3 signaling. Hepatology 2011, 54, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Fu, W.M.; He, M.L.; Wang, H.; Wang, W.M.; Yu, S.C.; Bian, X.W.; Zhou, J.; Lin, M.C.; Lu, G.; et al. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol. Biol. Cell 2011, 22, 3955–3961. [Google Scholar] [CrossRef]
- Youngblood, H.; Cai, J.; Drewry, M.D.; Helwa, I.; Hu, E.; Liu, S.; Yu, H.; Mu, H.; Hu, Y.; Perkumas, K.; et al. Expression of mRNAs; miRNAs; and lncRNAs in Human Trabecular Meshwork Cells Upon Mechanical Stretch. Investig. Ophthalmol. Vis. Sci. 2020, 61, 2. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Phillips, J.I.; Lozano, D.C.; Choe, T.E.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Gattey, D.M.; Saugstad, J.A.; Keller, K.E. Comparison of MicroRNA expression in aqueous humor of normal and primary open-angle glaucoma patients using PCR arrays: A pilot study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2884–2890. [Google Scholar] [CrossRef]
- Li, X.; Zhao, F.; Xin, M.; Li, G.; Luna, C.; Li, G.; Zhou, Q.; He, Y.; Yu, B.; Olson, E.; et al. Regulation of intraocular pressure by microRNA cluster miR-143/145. Sci. Rep. 2017, 7, 915. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Kosior-Jarecka, E.; Czop, M.; Gasińska, K.; Wróbel-Dudzińska, D.; Zalewski, D.P.; Bogucka-Kocka, A.; Kocki, J.; Żarnowski, T. MicroRNAs in the aqueous humor of patients with different types of glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2337–2349. [Google Scholar] [CrossRef] [PubMed]
- Hindle, A.G.; Thoonen, R.; Jasien, J.V.; Grange, R.M.H.; Amin, K.; Wise, J.; Ozaki, M.; Ritch, R.; Malhotra, R.; Buys, E.S. Identification of Candidate miRNA Biomarkers for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk-Socha, M.; Baczyńska, D.; Przeździecka-Dołyk, J.; Turno-Kręcicka, A. MicroRNA-125b overexpression in exfoliation syndrome. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2020, 29, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Dettoraki, M.; Karekla, A.; Lamprinakis, I.; Damaskos, C.; Gouliopoulos, N.; Tibilis, M.; Gazouli, M. Polymorphism analysis of miR182 and CDKN2B genes in Greek patients with primary open angle glaucoma. PLoS ONE 2020, 15, e0233692. [Google Scholar] [CrossRef]
- He, J.; Zhao, J.; Zhu, W.; Qi, D.; Wang, L.; Sun, J.; Wang, B.; Ma, X.; Dai, Q.; Yu, X. MicroRNA biogenesis pathway genes polymorphisms and cancer risk: A systematic review and meta-analysis. PeerJ 2016, 4, e2706. [Google Scholar] [CrossRef]
- Chatzikyriakidou, A.; Founti, P.; Melidou, A.; Minti, F.; Bouras, E.; Anastasopoulos, E.; Pappas, T.; Haidich, A.B.; Lambropoulos, A.; Topouzis, F. MicroRNA-related polymorphisms in exfoliation syndrome; pseudoexfoliative glaucoma; and primary open-angle glaucoma. Ophthalmic Genet. 2018, 39, 603–609. [Google Scholar] [CrossRef]
- Saeki, M.; Watanabe, M.; Inoue, N.; Tokiyoshi, E.; Takuse, Y.; Arakawa, Y.; Hidaka, Y.; Iwatani, Y. DICER and DROSHA gene expression and polymorphisms in autoimmune thyroid diseases. Autoimmunity 2016, 49, 514–522. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 2009, 15, 2488–2497. [Google Scholar]
- Chakraborty, M.; Sahay, P.; Rao, A. Primary Human Trabecular Meshwork Model for Pseudoexfoliation. Cells 2021, 10, 3448. [Google Scholar] [CrossRef]
- Sharma, S.; Chataway, T.; Klebe, S.; Griggs, K.; Martin, S.; Chegeni, N.; Dave, A.; Zhou, T.; Ronci, M.; Voelcker, N.H.; et al. Novel protein constituents of pathological ocular exfoliation syndrome deposits identified with mass spectrometry. Mol. Vis. 2018, 24, 801–817. [Google Scholar] [PubMed]
- Wolosin, J.M.; Ritch, R.; Bernstein, A.M. Is Autophagy Dysfunction a Key to Exfoliation Glaucoma? J. Glaucoma 2018, 27, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Sahay, P.; Chakraborty, M.; Prusty, B.K.; Srinivasan, S.; Jhingan, G.D.; Mishra, P.; Modak, R.; Suar, M. Switch to Autophagy the Key Mechanism for Trabecular Meshwork Death in Severe Glaucoma. Clin. Ophthalmol. 2021, 15, 3027–3039. [Google Scholar] [CrossRef]
- De Juan-Marcos, L.; Escudero-Domínguez, F.A.; Hernández-Galilea, E.; Cruz-González, F.; Follana-Neira, I.; González-Sarmiento, R. Investigation of Association between Autophagy-Related Gene Polymorphisms and Exfoliation Syndrome and Exfoliation Glaucoma in a Spanish Population. Semin. Ophthalmol. 2018, 33, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Want, A.; Gillespie, S.R.; Wang, Z.; Gordon, R.; Iomini, C.; Ritch, R.; Wolosin, J.M.; Bernstein, A.M. Autophagy and Mitochondrial Dysfunction in Tenon Fibroblasts from Exfoliation Glaucoma Patients. PLoS ONE 2016, 11, e0157404. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial aging and age-related dysfunction of mitochondria. BioMed Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef] [PubMed]
- Manickam, A.H.; Michael, M.J.; Ramasamy, S. Mitochondrial genetics and therapeutic overview of Leber’s hereditary optic neuropathy. Indian J. Ophthalmol. 2017, 65, 1087–1092. [Google Scholar]
- Tanwar, M.; Dada, T.; Sihota, R.; Dada, R. Mitochondrial DNA analysis in primary congenital glaucoma. Mol. Vis. 2010, 16, 518–533. [Google Scholar]
- Izzotti, A.; Longobardi, M.; Cartiglia, C.; Sacca, S.C. Mitochondrial damage in the trabecular meshwork occurs only in primary open-angle glaucoma and in pseudoexfoliative glaucoma. PLoS ONE 2011, 6, e14567. [Google Scholar] [CrossRef]
- Porter, K.; Nallathambi, J.; Lin, Y.; Liton, P.B. Lysosomal basification and decreased autophagic flux in oxidatively stressed trabecular meshwork cells: Implications for glaucoma pathogenesis. Autophagy 2013, 9, 581–594. [Google Scholar] [CrossRef]
- Liton, P.B.; Lin, Y.; Gonzalez, P.; Epstein, D.L. Potential role of lysosomal dysfunction in the pathogenesis of primary open angle glaucoma. Autophagy 2009, 5, 122–124. [Google Scholar] [CrossRef][Green Version]
- Abu-Amero, K.K.; Morales, J.; Bosley, T.M. Mitochondrial abnormalities in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, P.; Simpson, D.A.; Sambare, C.; Duffy, S.; Lechner, J.; Dastane, A.; Dervan, E.W.; Vallabh, N.; Chelerkar, V.; Deshpande, M.; et al. Whole-mitochondrial genome sequencing in primary open-angle glaucoma using massively parallel sequencing identifies novel and known pathogenic variants. Genet. Med. Off. J. Am. Coll. Med. Genet. 2015, 17, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amero, K.K.; Bosley, T.M.; Morales, J. Analysis of nuclear and mitochondrial genes in patients with exfoliation glaucoma. Mol. Vis. 2008, 14, 29–36. [Google Scholar]
- Sirohi, K.; Swarup, G. Defects in autophagy caused by glaucoma-associated mutations in optineurin. Exp. Eye Res. 2016, 144, 54–63. [Google Scholar] [CrossRef]
- Bosley, T.M.; Hellani, A.; Spaeth, G.L.; Myers, J.; Katz, L.J.; Moster, M.R.; Milcarek, B.; Abu-Amero, K.K. Down-regulation of OPA1 in patients with primary open angle glaucoma. Mol. Vis. 2011, 17, 1074–1079. [Google Scholar]
- Davies, V.; Votruba, M. Focus on molecules: The OPA1 protein. Exp. Eye Res. 2006, 83, 1003–1004. [Google Scholar] [CrossRef]
- Lenaers, G.; Reynier, P.; Elachouri, G.; Soukkarieh, C.; Olichon, A.; Belenguer, P.; Baricault, L.; Ducommun, B.; Hamel, C.; Delettre, C. OPA1 functions in mitochondria and dysfunctions in optic nerve. Int. J. Biochem. Cell Biol. 2009, 41, 1866–1874. [Google Scholar] [CrossRef]
- Singh, L.N.; Crowston, J.G.; Lopez Sanchez, M.I.G.; Van Bergen, N.J.; Kearns, L.S.; Hewitt, A.W.; Yazar, S.; Mackey, D.A.; Wallace, D.C.; Trounce, I.A. Mitochondrial DNA Variation and Disease Susceptibility in Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4598–4602. [Google Scholar] [CrossRef]
- Coca-Prados, M. The blood-aqueous barrier in health and disease. J. Glaucoma 2014, 23, S36–S38. [Google Scholar] [CrossRef]
- Bill, A. The blood-aqueous barrier. Trans. Ophthalmol. Soc. UK 1986, 105, 149–155. [Google Scholar] [PubMed]
- Küchle, M.; Vinores, S.A.; Mahlow, J.; Green, W.R. Blood-aqueous barrier in exfoliation syndrome: Evaluation by immunohistochemical staining of endogenous albumin. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pawlyk, B.; Connolly, E.; Adamian, M.; Miller, J.W.; Pasquale, L.R.; Haddadin, R.I.; Grosskreutz, C.L.; Rhee, D.J.; Li, T. Disruption of the blood-aqueous barrier and lens abnormalities in mice lacking lysyl oxidase-like 1 (LOXL1). Investig. Ophthalmol. Vis. Sci. 2014, 55, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Küchle, M.; Nguyen, N.X.; Hannappel, E.; Naumann, G.O. The blood-aqueous barrier in eyes with exfoliation syndrome. Ophthalmic Res. 1995, 27, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Yavrum, F.; Elgin, U.; Kocer, Z.A.; Fidanci, V.; Sen, E. Evaluation of aqueous humor and serum clusterin levels in patients with glaucoma. BMC Ophthalmol. 2021, 21, 25. [Google Scholar] [CrossRef]
- Doudevski, I.; Rostagno, A.; Cowman, M.; Liebmann, J.; Ritch, R.; Ghiso, J. Clusterin and complement activation in exfoliation glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2491–2499. [Google Scholar] [CrossRef]
- Zenkel, M.; Kruse, F.E.; Jünemann, A.G.; Naumann, G.O.; Schlötzer-Schrehardt, U. Clusterin deficiency in eyes with exfoliation syndrome may be implicated in the aggregation and deposition of pseudoexfoliative material. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1982–1990. [Google Scholar] [CrossRef]
- Yildirim, Z.; Yildirim, F.; Uçgun, N.I.; Sepici-Dinçel, A. The role of the cytokines in the pathogenesis of exfoliation syndrome. Int. J. Ophthalmol. 2013, 6, 50–53. [Google Scholar]
- Kondkar, A.; Azad, T.A.; Almobarak, F.; Kalantan, H.; Al-Obeidan, S.A.; Abu-Amero, K.K. Elevated levels of plasma tumor necrosis factor alpha in patients with exfoliation glaucoma. Clin. Ophthalmol. 2018, 12, 153–159. [Google Scholar] [CrossRef]
- Eraslan, N.; Elgin, U.; Şen, E.; Kilic, A.; Yilmazbas, P. Comparison of total/active ghrelin levels in primary open angle glaucoma; exfoliation glaucoma and exfoliation syndrome. Int. J. Ophthalmol. 2018, 11, 823–827. [Google Scholar]
- Bleich, S.; Roedl, J.; Von Ahsen, N.; Schlötzer-Schrehardt, U.; Reulbach, U.; Beck, G.; Kruse, F.E.; Naumann, G.O.; Kornhuber, J.; Jünemann, A.G. Elevated homocysteine levels in aqueous humor of patients with exfoliation glaucoma. Am. J. Ophthalmol. 2004, 138, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, M.; Voest, E.E.; Amano, S.; Beerepoot, L.V.; Takashima, S.; Tolentino, M.; Kim, R.Y.; Rohan, R.M.; Colby, K.A.; Yeo, K.T.; et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J. Clin. Investig. 1996, 98, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Borazan, M.; Karalezli, A.; Kucukerdonmez, C.; Bayraktar, N.; Kulaksizoglu, S.; Akman, A.; Akova, Y.A. Aqueous humor and plasma levels of vascular endothelial growth factor and nitric oxide in patients with exfoliation syndrome and exfoliation glaucoma. J. Glaucoma 2010, 19, 207–211. [Google Scholar] [CrossRef]
- Aiello, L.P.; Northrup, J.M.; Keyt, B.A.; Takagi, H.; Iwamoto, M.A. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch. Ophthalmol. 1995, 113, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
| miRNA | Accession Number | Function | Reference |
|---|---|---|---|
| hsa-miR-125b | MIMAT0000423 | Directly targets P53. TP53 gene is closely associated with lens epithelial cell apoptosis. (validated) | Drewry et al., 2018 |
| hsa-miR-6722-3p | MIMAT0025854 | Involved in mitogen-activated protein kinase (MAPK) signaling pathway; forkhead box, class O (FOXO) signaling pathway; and regulation of actin cytoskeleton. (predicted) | Kosior-Jarecka et al., 2021 |
| hsa-miR-184 | MIMAT0000454 | Involved mainly in response to hypoxia, cardiovascular system development, and apoptosis. Mutations in hsa-miR-184, which were linked with lens/corneal dystrophy and blindness. (predicted) | Kosior-Jarecka et al., 2021 |
| hsa-miR-4634 | MIMAT0019691 | Is a validated regulator of VAV3, whose deficiency in mice was associated with an ocular phenotype similar to glaucoma, including elevated IOP, selective loss of retinal ganglion cells, and optic nerve head cupping. (validated) | Kosior-Jarecka et al., 2021 |
| hsa-miR-1260b | MIMAT0015041 | May play a protective role in the course of glaucomatous neuropathy. Is also an essential regulator of vascular smooth muscle cell proliferation in response to hypoxia. (predicted) | Kosior-Jarecka et al., 2021 |
| miR-122-5p | MIMAT0000421 | Controls TGF-β1, protein binding, and ECM-related processes. Has been shown to regulate opteneuin pathway. (validated) | Rao et al., 2020; Drewryet al., 2018 |
| hsa-miR-124-3p | MIMAT0000422 | Controls TGF-β1, protein binding, and ECM-related processes. (predicted) | Rao et al., 2020 |
| hsa-miR-424-5p | MIMAAT0001341 | Is a tumor-suppressive miRNA. It regulates proliferation and invasion. It can also inhibit cell migration and epithelial–mesenchymal transition. (predicted) | Rao et al., 2020 |
| hsa-miR-30c-5p | MIMAT0000244 | Directly targets MAPK1 to regulate proliferation and migration. Negatively regulates protein secretion. (validated) | Rao et al., 2020 |
| hsa-miR-96-5p | MIMAT0000095 | Inhibits apoptosis by targeting caspase-9 gene. (validated) | Rao et al., 2020 |
| hsa-miR-142-5p | MIMAT0000433 | Acts as a negative regulator in TGF-β pathway by targeting SMAD3 and suppresses TGF-β-induced growth inhibition. (validated) | Rao et al., 2020 |
| hsa-miR-9-5p | MIMAT0000441 | Plays a role in growth, invasion, migration, and epithelial–mesenchymal transition. Negatively regulates cell adhesion and janus kinase–signal transducer and activator of transcription(JAK/STAT) pathway. (validated) | Rao et al., 2020 |
| hsa-miR-143-3p | MIMAT0000435 | It regulates proliferation, migration, and invasion. Negativelyregulates angiogenesis, actin cytoskeleton organization, regulation of blood vessel and endothelial cell proliferation. (validated) | Rao et al., 2020 |
| hsa-miR-302a-3p | MIMAT0000684 | Post-transcriptional gene silencing. Overexpression can inhibit proliferation and promote apoptosis. May modulate epithelial–mesenchymal transition. (validated) | Rao et al., 2020 |
| hsa-miR-223-3p | MIMAT00003280 | Plays a protective role against endothelial injury, inhibits cell proliferation and migration, and negatively regulates inflammatory response and necrotic cell death. (validated) | Rao et al., 2020 |
| hsa-miR-630 | MIMAT0003299 | Involved in the regulation of apoptosis. (predicted) | Drewryet al., 2018 |
| hsa-miR-451a | MIMAT0001631 | Associated with cell proliferation, migration, and apoptosis through targeting activating transcription factor 2 (ATF2) signaling pathway. It can inhibit hepatic gluconeogenesis and alleviate hyperglycemia. (predicted) | Drewryet al., 2018 |
| has-miR-637 | MIMAT0003307 | Involved in tryosine metabolism and endocytosis. (predicted) | Hindle, 2019 |
| hsa-miR-4725-3p | MIMAT0019844 | Involved in MAPK signaling pathway, p53 signaling pathway, and regulation of actin cytoskeleton. (predicted) | Hindle, 2019 |
| hsa-miR-433-3p | MIMAT0001627 | Involved in MAPK signaling pathway, p53 signaling pathway, FOXO signaling pathway, and regulation of actin cytoskeleton. (predicted) | Hindle, 2019 |
| hsa-miR-302d-3p | MIMAT0000718 | Involved in tryosine metabolism and endocytosis. (predicted) | Drewry et al., 2018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, M.; Rao, A. Alternate Causes for Pathogenesis of Exfoliation Glaucoma, a Multifactorial Elastotic Disorder: A Literature Review. Curr. Issues Mol. Biol. 2022, 44, 1191-1202. https://doi.org/10.3390/cimb44030078
Chakraborty M, Rao A. Alternate Causes for Pathogenesis of Exfoliation Glaucoma, a Multifactorial Elastotic Disorder: A Literature Review. Current Issues in Molecular Biology. 2022; 44(3):1191-1202. https://doi.org/10.3390/cimb44030078
Chicago/Turabian StyleChakraborty, Munmun, and Aparna Rao. 2022. "Alternate Causes for Pathogenesis of Exfoliation Glaucoma, a Multifactorial Elastotic Disorder: A Literature Review" Current Issues in Molecular Biology 44, no. 3: 1191-1202. https://doi.org/10.3390/cimb44030078
APA StyleChakraborty, M., & Rao, A. (2022). Alternate Causes for Pathogenesis of Exfoliation Glaucoma, a Multifactorial Elastotic Disorder: A Literature Review. Current Issues in Molecular Biology, 44(3), 1191-1202. https://doi.org/10.3390/cimb44030078






