Evidence from a Systematic Review and Meta-Analysis Pointing to the Antidiabetic Effect of Polyphenol-Rich Plant Extracts from Gymnema montanum, Momordica charantia and Moringa oleifera
Abstract
:1. Introduction
1.1. Gymnema montanum Effect on Diabetes
1.2. Momordica charantia Effect on Diabetes
1.3. Moringa oleifera Effects on Diabetes
2. Meta-Analysis
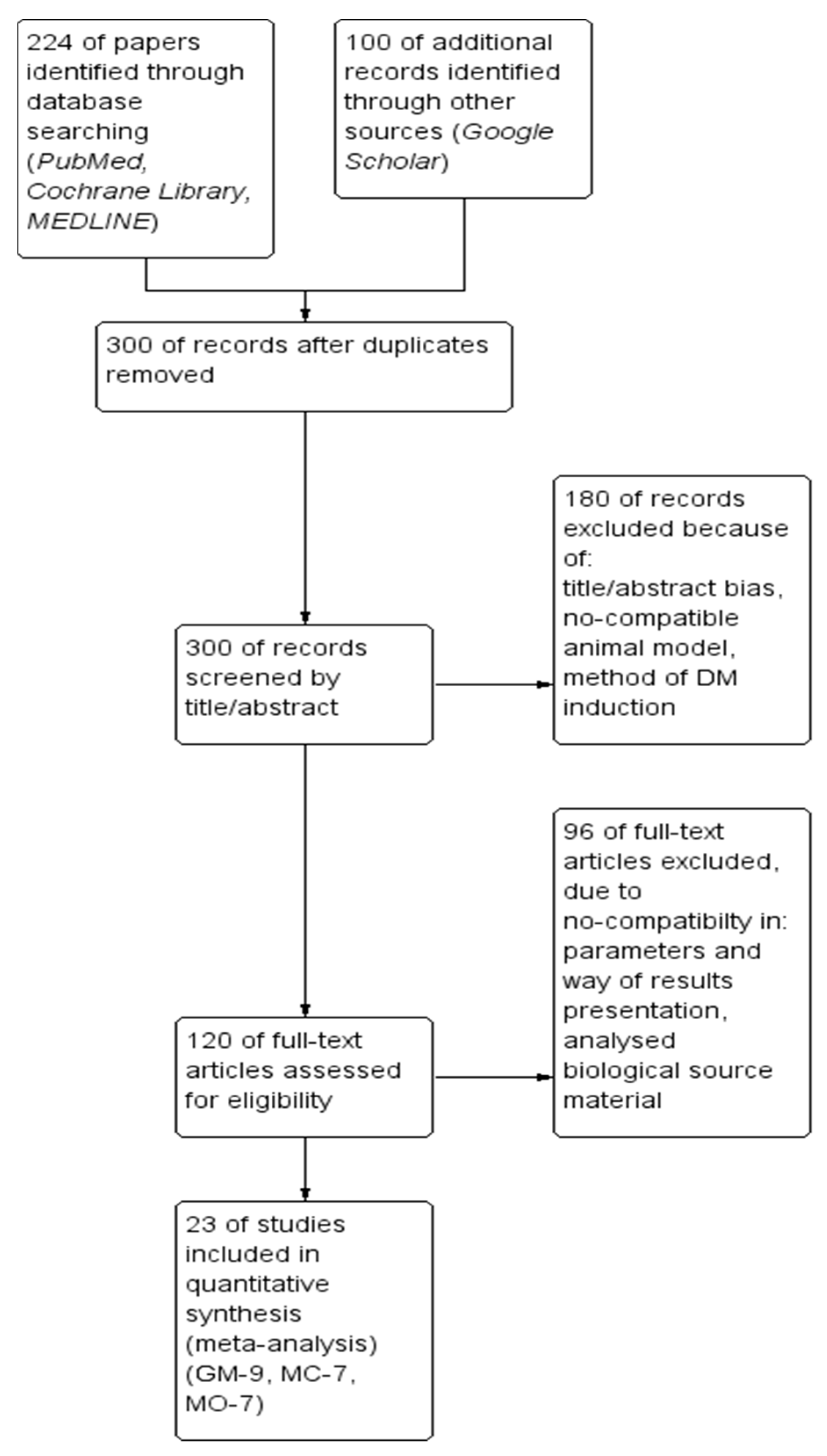
2.1. Literature Search, Data Screening and Extraction Protocol
- (1)
- original paper from an interventional experiment conducted on the suitable animal model (induced diabetic rats);
- (2)
- experiment design involving the intervention group and control group either with or without a standard antidiabetic drug intervention;
- (3)
- compatibility in the range of analyzed parameters and the manner of results presentation.
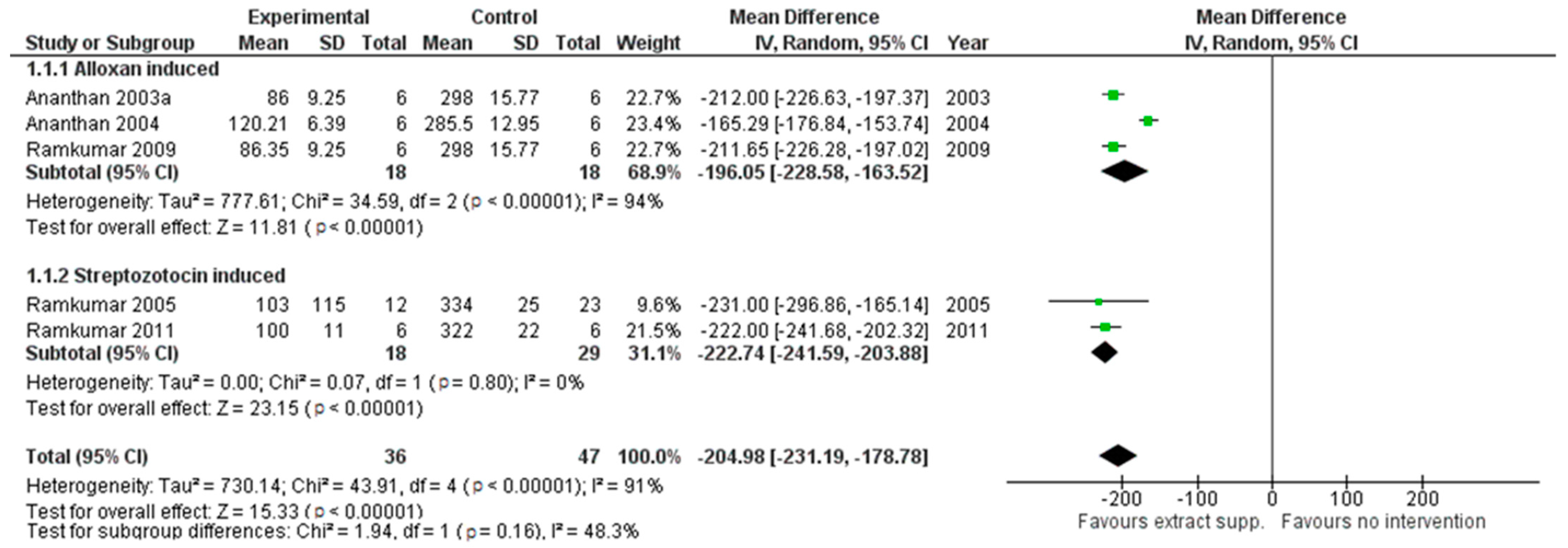
2.2. The Results for Physiological Parameters Analysis
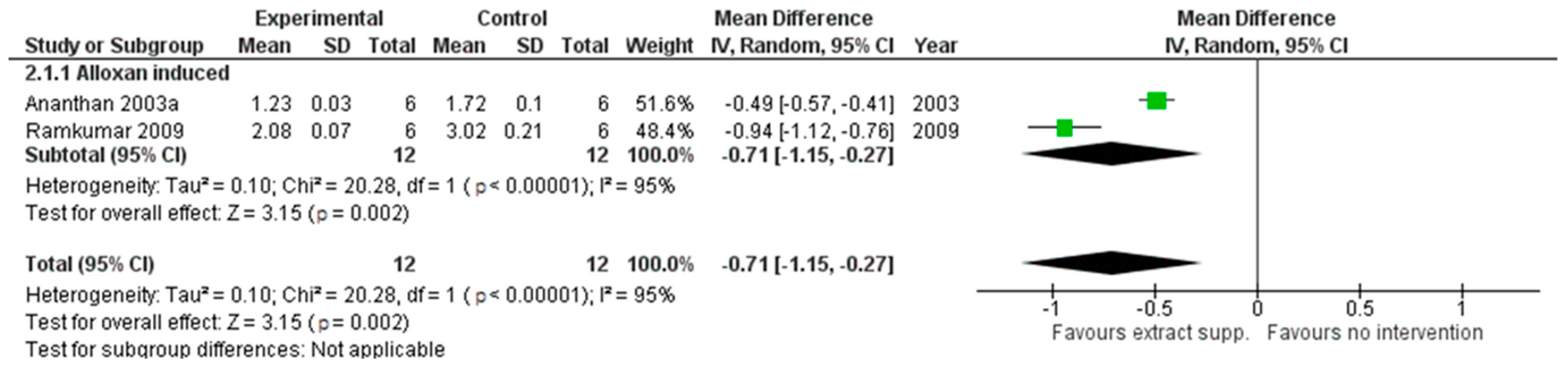
2.3. The Results of Parameters Related to Oxidative Status Analysis
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO Global Reports on Diabetes. 2016. Available online: https://www.who.int/diabetes/publications/grd-2016/en/ (accessed on 11 January 2022).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from The International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnefont-Rousselot, D.; Bastard, J.P.; Jaudon, M.C.; Delattre, J. Consequences of The Diabetic Status on the Oxidant/Antioxidant Balance. Diabetes Metab. 2000, 26, 163–176. [Google Scholar] [PubMed]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular Mechanisms Underlying Curcumin-Mediated Therapeutic Effects in Type 2 Diabetes and Cancer. Oxidative Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragan, S.; Andrica, F.; Serban, M.C.; Timar, R. Polyphenols-Rich Natural Products for the Treatment of Diabetes. Curr. Med. Chem. 2015, 22, 14–22. [Google Scholar] [CrossRef]
- Vajravelu, E.B.P. Rare and Endemic Species Collected after Fifty Years or More from South India. In An Assessment of Threatened Plants of India. In Proceedings of The Seminar Onrare and Endemic Species Re-Collected after Fifty Years or More from Southindia; Jain, S.K., Raw, R.R., Eds.; Botanical Survey Of India: Howrah, India, 1983; Volume 14. [Google Scholar]
- Ananthan, R.; Baskar, C.; Narmathabai, V.; Pari, L.; Latha, M.; Ramkumar, K.M. Antidiabetic Effect of Gymnema Montanum Leaves: Effect on Lipid Peroxidation Induced Oxidative Stress in Experimental Diabetes. Pharmacol. Res. 2003, 48, 551–556. [Google Scholar] [CrossRef]
- Venkateswaran, S.; Pari, L.; Saravanan, G. Effect of Phaseolus Vulgaris on Circulatory Antioxidants and Lipids in Rats with Streptozotocin-Induced Diabetes. J. Med. Food 2002, 5, 97–103. [Google Scholar] [CrossRef]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant Effects of Vitamins in Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Diabetol. Metab. Syndr. 2018, 10, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Christie-David, D.J.; Girgis, C.M.; Gunton, J.E. Effects of Vitamins C and D in Type 2 Diabetes Mellitus. Nutr. Diet. Suppl. 2015, 7, 21–28. [Google Scholar]
- Garg, M.C.; Bansal, D.D. Protective Antioxidant Effect of Vitamin C and Vitamin E in Streptozotocin-Induced Diabetic Rats. Indian J. Exp. Biol. 2000, 38, 101–104. [Google Scholar]
- Frei, B. Ascorbic Acid Protects Lipids in Human Plasma and Low-Density Lipoprotein Against Oxidative Damage. Am. J. Clin. Nutr. 1991, 54, 1113S–1118S. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Shulman, G.I. Free Fatty Acids in Obesity and Type 2 Diabetes: Defining Their Role in The Development of Insulin Resistance and Beta-Cell Dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. 3), 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jaiprakash, R.; Rani, M.A.; Venkataraman, B.V.; Andrade, C. Effect of Felodipine on Serum Lipid Profile in Short Term Streptozotocin Diabetes in Rats. Indian J. Exp. Biol. 1993, 31, 283–284. [Google Scholar] [PubMed]
- Wang, L.; Folsom, A.R.; Zheng, Z.J.; Pankow, J.S.; Eckfeldt, J.H. Investigators, AS Plasma Fatty Acid Composition and Incidence of Diabetes in Middle-Aged Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.A.; Pari, L. Combined N-Benzoyl-D-Phenylalanine and Metformin Treatment Reverse Changes in the Fatty Acid Composition of Streptozotocin Diabetic Rats. J. Basic Clin. Physiol. Pharmacol. 2006, 17, 17–28. [Google Scholar] [CrossRef]
- Sochor, M.; Zaher Baquer, N.; Mclean, P. Glucose Over and Under Utilization in Diabetes: Comparative Studies on the Changes in Activities of Enzymes of Glucose Metabolism in Rat Kidney and Liver. Mol. Phys. 1985, 7, 51–68. [Google Scholar]
- Ramkumar, K.M.; Vijayakumar, R.S.; Ponmanickam, P.; Velayuthaprabhu, S.; Archunan, G.; Rajaguru, P. Antihyperlipidaemic Effect of Gymnema Montanum: A Study on Lipid Profile and Fatty Acid Composition in Experimental Diabetes. Basic Clin. Pharmacol. Toxicol. 2008, 103, 538–545. [Google Scholar] [CrossRef]
- Cameron, N.E.; Cotter, M.A. Effect of Antioxidants on Nerve And vascular Dysfunctions in Experimental Diabetes. Diabetes Res. Clin. Pract. 1999, 45, 137–146. [Google Scholar] [CrossRef]
- Demaison, L.S.J.; Moreau, D.; Grynberg, A. Influence of the Phospholipid N-6/N-3 PUFA Ratio on the Mitochondrial Oxidative Metabolism Before and after Myocardial Ischemia. Biochem. Biophys. Acta 1994, 1227, 53–59. [Google Scholar]
- López-Vélez, M.; Martínez-Martínez, F.; Del Valle-Ribes, C. The Study of Phenolic Compounds as Natural Antioxidants in Wine. Crit. Rev. Food Sci. Nutr. 2003, 43, 233–244. [Google Scholar] [CrossRef]
- Grover, J.K.; Yadav, S.P. Pharmacological Actions and Potential Uses of Momordica Charantia: A Review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica Charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [Green Version]
- Raman, A.; Lau, C. Anti-Diabetic Properties and Phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine 1996, 2, 349–362. [Google Scholar] [CrossRef]
- Patel, S.; Patel, T.; Parmar, K.; Bhatt, Y.; Patel, Y.; Patel, N.M. Isolation, Characterization and Antimicrobial Activity of Charantin from Momordica Charantialinn. Fruit. Int. J. Drug Dev. Res. 2010, 2, 629–634. [Google Scholar]
- Kenny, O.; Smyth, T.J.; Hewage, C.M.; Brunton, N.P. Antioxidant Properties and Quantitative UPLC-MS Analysis of Phenolic Compounds from Extracts of Fenugreek (Trigonella Foenum-Graecum) Seeds and Bitter Melon (Momordica charantia) Fruit. Food Chem. 2013, 141, 4295–4302. [Google Scholar] [CrossRef] [PubMed]
- Horax, R.; Hettiarachchy, N.; Islam, S. Total Phenolic Contents And Phenolic Acid Constituents in 4 Varieties Of Bitter Melons (Momordica Charantia) and Antioxidant Activities of Their Extracts. J. Food Sci. 2005, 70, C275–C280. [Google Scholar] [CrossRef]
- Arafat, S.Y.; Nayeem, M.; Jahan, S.; Karim, Z.; Reza, H.M.; Md Hemayet, H.; Shohel, M.; Md Ashraful, A. Ellagic Acid-Rich momordica Charantia Fruit Pulp Supplementation Prevented Oxidative Stress, Fibrosis and Inflammation in Liver Ofalloxan-Induced Diabetic Rats. Orient Pharm. Exp. Med. 2016, 16, 267–278. [Google Scholar] [CrossRef]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic Acid Attenuates High-Carbohydrate, High-Fat Diet-Induced Metabolic Syndrome in Rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Kandasamy, N.; Ashokkumar, N. Protective Effect of Bioflavonoid Myricetin Enhances Carbohydrate Metabolic Enzymes and Insulin Signaling Molecules in Streptozotocin-Cadmium induced Diabetic Nephrotoxic Rats. Toxicol. Appl. Pharmacol. 2014, 279, 173–185. [Google Scholar] [CrossRef]
- Ahmed, I.; Adeghate, E.; Cummings, E.; Sharma, A.K.; Singh, J. Beneficial Effects and Mechanism of Action of Momordica Charantia Juice in The Treatment of Streptozotocin-induced Diabetes Mellitus In Rat. Mol. Cell Biochem. 2004, 261, 63–70. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, M. Regeneration Of Beta Cells in islets of langerhans of the pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (Bitter Gourd) Fruits. Indian J. Exp. Biol. 2007, 45, 1055–1062. [Google Scholar] [PubMed]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Effect of exogenous atp on Momordica charantia Linn. (Cucurbitaceae) induced inhibition of D-Glucose, L-Tyrosine and Fluid Transport Across Rat Everted Intestinal Sacs In Vitro. J. Ethnopharmacol. 2007, 110, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Wlazlo, N.; Beijers, H.J.; Schoon, E.J.; Sauerwein, H.P.; Stehouwer, C.D.; Bravenboer, B. High Prevalence Of Diabetes Mellitus in Patientswith Liver Cirrhosi. Diabet. Med. 2010, 27, 1308–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, Inflammation, and Insulin Resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Farah, N.; Bukhari, S.A.; Ali, M.; Naqvi, S.A.; Mahmood, S. Phenolic acid profiling and antiglycation studies of leaf and fruit extracts of tyrosine primed Momordica charantia seeds for possible treatment of diabetes mellitus. Pak. J. Pharm. Sci. 2018, 31, 2667–2672. [Google Scholar]
- Vargas-Sanchez, K.; Garay-Jaramillo, E.; Gonzalez-Reyes, R.E. Effects of Moringa oleiferaon Glycaemia and Insulin Levels: A Review of Animal and Human Studies. Nutrients 2019, 11, 2907. [Google Scholar] [CrossRef] [Green Version]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Stohs, S.J.; Hartman, M.J. Review of the Safety and Efficacy of Moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Ikram, M.; Sharma, S.; Khan, S.; Pandey, V.V.; Singh, A. Biological, the nutritional, and therapeutic significance of Moringa oleifera Lam. Phytother. Res. 2019, 33, 2870–2903. [Google Scholar] [CrossRef]
- Glover-Amengor, M.; Aryeetey, R.; Afari, E.; Nyarko, A. Micronutrient composition and acceptability of Moringa oleifera leaf-fortified dishes by children in Ada-East district, Ghana. Food Sci. Nutr. 2017, 5, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manguro, L.O.; Lemmen, P. Phenolics of Moringa oleifera leaves. Nat. Prod. Res. 2007, 21, 56–68. [Google Scholar] [CrossRef]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Lorenzo, J.M.; Afolayan, A.J.; Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food Res. Int. 2018, 106, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, N.A.; Gaytán-Martínez, M.; de la Luz Reyes-Vega, M.; Loarca-Piña, G. Glucosinolates and Isothiocyanates from Moringa oleifera: Chemical and Biological Approaches. Plant Foods Hum. Nutr. 2020, 75, 447–457. [Google Scholar] [CrossRef]
- Fahey, J.W.; Olson, M.E.; Stephenson, K.K.; Wade, K.L.; Chodur, G.M.; Odee, D.; Nouman, W.; Massiah, M.; Alt, J.; Egner, P.A.; et al. The Diversity of Chemoprotective Glucosinolates in Moringaceae (Moringa spp.). Sci. Rep. 2018, 8, 7994. [Google Scholar] [CrossRef] [Green Version]
- Abdul lRazis, A.F.; Konsue, N.; Ioannides, C. Isothiocyanates and Xenobiotic Detoxification. Mol. Nutr. Food Res. 2018, 62, e1700916. [Google Scholar] [CrossRef]
- Leone, A.; Fiorillo, G.; Criscuoli, F.; Ravasenghi, S.; Santagostini, L.; Fico, G.; Spadafranca, A.; Battezzati, A.; Schiraldi, A.; Pozzi, F.; et al. Nutritional Characterization and Phenolic Profiling of Moringa oleifera Leaves Grown in Chad, Sahrawi Refugee Camps, and Haiti. Int. J. Mol. Sci. 2015, 16, 18923–18937. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Torres-Castillo, J.A.; Castro-Lopez, C.; Rojas, R.; Sanchez-Alejo, E.J.; Ngangyo-Heya, M.; Martinez-Avila, G.C.G. Moringa plants: Bioactive compounds and promising applications in food products. Food Res. Int. 2018, 111, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Forster, N.; Ulrichs, C.; Schreiner, M.; Arndt, N.; Schmidt, R.; Mewis, I. Ecotype variability in growth and secondary metabolite profile in Moringa oleifera: Impact of sulfur and water availability. J. Agric. Food Chem. 2015, 63, 2852–2861. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, G.; De Petrocellis, L.; Schiano Moriello, A.; Bertoli, S.; Leone, A.; Battezzati, A.; Mazzini, S.; Bassoli, A.; Moringin, A. Stable Isothiocyanate from Moringa oleifera, Activates the Somatosensory and Pain Receptor TRPA1 Channel In Vitro. Molecules 2020, 25, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, J.; Khan, I.; Blundell, R. Moringa oleifera and glycemic control: A review of current evidence and possible mechanisms. Phytother. Res. 2019, 33, 2841–2848. [Google Scholar] [CrossRef]
- Irfan, H.M.; Asmawi, A.Z.; Khan, N.A.K.; Sadikun, A.; Mordi, M.N. Anti-diabetic activity-guided screening of aqueous-ethanol Moringaoleifera extracts and fractions: Identification of marker compounds. Trop. J. Pharm. Res. 2017, 16, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Bamagous, G.A.; Al Ghanidi, S.S.; Ibrahim, I.A.A.; Mahfoz, A.M.; Afify, M.A.; Alsugoor, M.H.M.; Shammah, A.A.; Arulselvan, P.; Rengarajan, T. Antidiabetic and antioxidant activity of ethyl acetate extract fraction of Moringa oleifera leaves in streptozotocin-induced diabetes rats via inhibition of inflammatory mediators. Asian Pac. J. Trop. Biomed. 2018, 8, 320–327. [Google Scholar] [CrossRef]
- Villarruel-Lopez, A.; de la Mora, D.A.L.; Vazquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuno, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127. [Google Scholar] [CrossRef] [Green Version]
- Arun Giridhari, V.; Malathi, D.; Geetha, K. Anti-diabetic property of drumstick (Moringa oleifera) leaf tablets. Int. J. Health Nutr. 2011, 2, 1–5. [Google Scholar]
- Fombang, E.N.; Saa, R.W. Antihyperglycemic activity of Moringa oleifera Lam leaf functional tea in rat models and human subjects. Food Nutr. Sci. 2016, 7, 1021–1032. [Google Scholar]
- Taweerutchana, R.; Lumlerdkij, N.; Vannasaeng, S.; Akarasereenont, P.; Sriwijitkamol, A. Effect of Moringa oleifera Leaf Capsules on Glycemic Control in Therapy-Naive Type 2 Diabetes Patients: A Randomized Placebo-Controlled Study. Evid. Based Complement. Altern. Med. 2017, 2017, 6581390. [Google Scholar] [CrossRef] [Green Version]
- Hafizur, R.M.; Maryam, K.; Hameed, A.; Zaheer, L.; Bano, S.; Sumbul, S.; Sana, A.; Saleem, R.; Naz, S.; Waraich, R.S.; et al. Insulin-releasing effect of some pure compounds from Moringa oleifera on mice islets. Med. Chem. Res. 2018, 27, 1408–1418. [Google Scholar] [CrossRef]
- Attakpa, E.; Sangaré, M.; Béhanzin, G.; Ategbo, J.; Seri, B.; Khan, N.A. Moringa oleifera Lam. stimulates activation of the insulin-dependent Akt pathway. Antidiabetic effect in diet-induced obesity (DIO) mouse model. Folia Biol. 2017, 63, 42–51. [Google Scholar]
- Olayaki, L.A.; Irekpita, J.E.; Yakubu, M.T.; Ojo, O.O. Methanolic extract of Moringa oleifera leaves improves glucose tolerance, glycogen synthesis and lipid metabolism in alloxan-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Kwon, O.; Chen, S.; Daruwala, R.; Eck, P.; Park, J.B.; Levine, M. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J. Biol. Chem. 2002, 277, 15252–15260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [PubMed] [Green Version]
- Waterman, C.; Rojas-Silva, P.; Tumer, T.B.; Kuhn, P.; Richard, A.J.; Wicks, S.; Stephens, J.M.; Wang, Z.; Mynatt, R.; Cefalu, W.; et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance, and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015, 59, 1013–1024. [Google Scholar] [CrossRef] [Green Version]
- Ndong, M.; Uehara, M.; Katsumata, S.; Suzuki, K. Effects of Oral Administration of Moringa oleifera Lam on Glucose Tolerance in Goto-Kakizaki and Wistar Rats. J. Clin. Biochem. Nutr. 2007, 40, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Leone, A.; Bertoli, S.; Di Lello, S.; Bassoli, A.; Ravasenghi, S.; Borgonovo, G.; Forlani, F.; Battezzati, A. Effect of Moringaoleifera Leaf Powder on Postprandial Blood Glucose Response: In Vivo Study on Saharawi People Living in Refugee Camps. Nutrients 2018, 10, 1494. [Google Scholar] [CrossRef] [Green Version]
- Azad, S.B.; Ansari, P.; Azam, S.; Hossain, S.M.; Shahid, M.I.; Hasan, M.; Hannan, J.M.A. Anti-hyperglycaemic activity of Moringa oleifera is partly mediated by carbohydrase inhibition and glucose fibre binding. Biosci. Rep. 2017, 37, BSR20170059. [Google Scholar] [CrossRef] [Green Version]
- Abd El Latif, A.; El Bialy Bel, S.; Mahboub, H.D.; AbdEldaim, M.A. Moringa oleifera leaf extract ameliorates alloxan-induced diabetes in rats by regeneration of beta cells and reduction of pyruvate carboxylase expression. Biochem. Cell Biol. 2014, 92, 413–419. [Google Scholar] [CrossRef]
- Nunthanawanich, P.; Sompong, W.; Sirikwanpong, S.; Makynen, K.; Adisakwattana, S.; Dahlan, W.; Ngamukote, S. Moringa Oleifera Aqueous Leaf Extract Inhibits Reducing Monosaccharide-Induced Protein Glycation And Oxidation Of Bovine Serum Albumin. Springerplus 2016, 5, 1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Kinetics Of Glycoxidation Of Bovine Serum Albumin By Methylglyoxal And Glyoxal And Its Prevention By Various Compounds. Molecules 2014, 19, 4880–4896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chumark, P.; Khunawat, P.; Sanvarinda, Y.; Phornchirasilp, S.; Morales, N.P.; Phivthong-Ngam, L.; Ratanachamnong, P.; Srisawat, S.; Pongrapeeporn, K.-U.S. The In Vitro And Ex Vivo Antioxidant Properties, Hypolipidaemic And Antiatherosclerotic Activities Of Water Extract Of Moringa Oleifera Lam. Leaves. J. Ethnopharmacol. 2008, 116, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Campos, E.; Valdez-Solana, M.; Avitia-Dominguez, C.; Campos-Almazan, M.; Flores-Molina, I.; Garcia-Arenas, G.; Tellez-Valencia, A. Effects of Moringaoleifera Leaf Extract on Diabetes-Induced Alterations in Paraoxonase 1 and Catalase in Rats Analyzed through Progress Kinetic and Blind Docking. Antioxidants 2020, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.; Aboua, G.; Omodanisi, E. Effects of Moringaoleifera on oxidative stress, apoptotic and inflammatory biomarkers in the streptozotocin-induced diabetic animal model. S. Afr. J. Bot. 2020, 129, 354–365. [Google Scholar] [CrossRef]
- Das, P.K.; Asha, S.Y.; Siddika, A.; Siddika, A.; Tareq, A.R.M.; Islam, F.; Khanam, J.A.; Rakib, A. Methanolic extract of Moringa oleifera leaves mediates anticancer activities through inhibiting NF-𝜅B and enhancing ROS in Ehrlich ascites carcinoma cells in mice. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 161–170. [Google Scholar] [CrossRef]
- Islam, A.; Manda, C.; Habib, A. Antibacterial potential of synthesized silver nanoparticles from leaf extract of Moringa oleifera. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 67–73. [Google Scholar] [CrossRef]
- Akter, T.; Rahman, M.A.; Moni, A.; Apu, M.A.I.; Fariha, A.; Hannan, M.A.; Uddin, M.J. Prospects for Protective Potential of Moringa oleifera against Kidney Diseases. Plants 2021, 10, 2818. [Google Scholar] [CrossRef]
- Ma, L.; Yu, A.H.; Sun, L.L.; Gao, W.; Zhang, M.M.; Su, Y.L.; Liu, H.; Ji, T. Two new bi desmosine triterpenoid saponins from the seeds of Momordica charantia L. Molecules 2014, 19, 2238–2246. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, Z.; Zamhuri, K.F.; Yaacob, A.; Siong, C.H.; Selvarajah, M.; Ismail, A.; Nazrul Hakim, M. In vitro antidiabetic activities and chemical analysis of polypeptide-k and oil isolated from seeds of Momordica charantia (bitter gourd). Molecules 2012, 17, 9631–9640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klomann, S.D.; Mueller, A.S.; Pallauf, J.; Krawinkel, M.B. Antidiabetic effects of bitter gourd extracts in insulin-resistant db/db mice. Br. J. Nutr. 2010, 104, 1613–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garau, C.; Cummings, E.; Phoenix, D.; Singh, J. Beneficial effect and mechanism of action of Momordica charantia in the treatment of diabetes mellitus: A mini-review. Int. J. Diabetes Metab. 2003, 11, 46–55. [Google Scholar]
- Ananthan, R.; Latha, M.; Pari, L.; Ramkumar, K.M.; Baskar, C.G.; Bai, V.N. Effect of Gymnemamontanumon blood glucose, plasma insulin, and carbohydrate metabolic enzymes in alloxan-induced diabetic rats. J. Med. Food 2003, 6, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kubola, J.; Siriamornpun, S. Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food Chem. 2008, 110, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.Y.; Hsu, C.; Chao, C.Y.; Wein, Y.S.; Kuo, Y.H.; Huang, C.J. Fractionation and identification of 9c, 11t, 13t-conjugated linolenic acid as an activator of PPARalpha in bitter gourd (Momordica charantia L.). J. Biomed. Sci. 2006, 13, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhang, M.; Liu, J.; Zhang, Y.; Zhang, R.-F.; Wei, Z.-C.; Ti, H.-H.; Liu, L.; Qiu, M.-H. Comparison of the content, antioxidant activity, andα-glucosidase inhibitory effect of polysaccharides from Momordica charantia L. species. Mod. Food Sci. 2014, 30, 102–108. [Google Scholar]
- Ramkumar, K.M.; Latha, M.; Venkateswaran, S.; Pari, L.; Ananthan, R.; Bai, V.N. Modulatory effect of Gymnema montanum leaf extract on brain antioxidant status and lipid peroxidation in diabetic rats. J. Med. Food 2004, 7, 366–371. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Rajaguru, P.; Latha, M.; Ananthan, R. Effect of Gymnema montanum leaves on red blood cell resistance to oxidative stress in experimental diabetes. Cell Biol. Toxicol. 2008, 24, 233–241. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Lee, A.S.; Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T.; Kang, K.P.; Lee, S.; Kim, W.; Park, S.K.; Lee, N.H.; et al. Gymnema montanum H. protects against alloxan-induced oxidative stress and apoptosis in pancreatic beta-cells. Cell. Physiol. Biochem. 2009, 24, 429–440. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Manjula, C.; Sankar, L.; Suriyanarayanan, S.; Rajaguru, P. Potential in vitro antioxidant and protective effects of Gymnema montanum H. on alloxan-induced oxidative damage in pancreatic beta-cells, HIT-T15. Food Chem. Toxicol. 2009, 47, 2246–2256. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.M.; Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Sujatha, J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 3390–3394. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, B.; Baijnath, H. An overview of the medicinal importance of Moringaceae. J. Med. Plants Res. 2012, 6, 5831–5839. [Google Scholar]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive Components in Moringa Oleifera Leaves Protect against Chronic Disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Sivanesan, I.; Keum, Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203. [Google Scholar] [CrossRef] [Green Version]
- Brilhante, R.S.N.; Sales, J.A.; Pereira, V.S.; Castelo-Branco, D.; Cordeiro, R.A.; de Souza Sampaio, C.M.; de Araújo Neto Paiva, M.; Santos, J.; Sidrim, J.J.C.; Rocha, M.F.G. Research advances on the multiple uses of Moringaoleifera: A sustainable alternative for socially neglected population. Asian Pac. J. Trop. Med. 2017, 10, 621–630. [Google Scholar] [CrossRef]
- Teixeira, E.M.; Carvalho, M.R.; Neves, V.A.; Silva, M.A.; Arantes-Pereira, L. Chemical characteristics and fractionation of proteins from Moringaoleifera Lam. leaves. Food Chem. 2014, 147, 51–54. [Google Scholar] [CrossRef]
- Adepoju-Bello, A.; Jolayemi, O.; Ehianeta, T.; Ayoola, G. Preliminary phytochemical screening, antioxidant and antihyperglycaemic activity of Moringa oleifera leaf extracts. Pak. J. Pharm. Sci. 2017, 30, 2217–2222. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Burzynska-Pedziwiatr, I.; Bukowiecka-Matusiak, M.; Wojcik, M.; Machala, W.; Bienkiewicz, M.; Spolnik, G.; Danikiewicz, W.; Wozniak, L.A. Dual stimulus-dependent effect of Oenothera paradoxa extract on the respiratory burst in human leukocytes: Suppressing for Escherichia coli and phorbol myristate acetate (PMA) and stimulating for formyl-methionyl-leucyl-phenylalanine (fMLP). Oxidative Med. Cell. Longev. 2014, 2014, 764367. [Google Scholar] [CrossRef] [Green Version]
- Bukowiecka-Matusiak, M.; Turek, I.A.; Woźniak, L.A. Natural phenolic antioxidants and their synthetic derivatives. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 4047–4061. [Google Scholar]
- Wozniak, L.A.; Cypryk, K.; Wojcik, M. Molecular mechanisms of diabetes prevention by structurally diverse antioxidants. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Bagchi, D., Sreejayan, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 315–330. [Google Scholar]
- Wojcik, M.; Krawczyk, M.; Wozniak, L.A. Antidiabetic Activity of Curcumin: Insight into Its Mechanisms of Action; Bagchi, D., Nair, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 385–401. [Google Scholar]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [Green Version]
- Patil, R.; Patil, R.; Ahirwar, B.; Ahirwar, D. Current status of Indian medicinal plants with antidiabetic potential: A review. Asian Pac. J. Trop. Biomed. 2011, 1, S291–S298. [Google Scholar] [CrossRef]
- Cortez-Navarrete, M.; Martınez-Abundis, E.; Pérez-Rubio, K.G.; Gonzalez-Ortiz, M.; Mendez-del Villar, M. Momordica charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. J. Med. Food 2018, 21, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.H.; Tuan, N.Q.; Park, M.H.; Quan, K.T.; Oh, J.; Heo, K.S.; Na, M.; Myung, C.S. Cucurbitane Triterpenoids fromthe Fruits of Momordica Charantia Improve Insulin Sensitivity and Glucose Homeostasis in Streptozotocin-Induced Diabetic Mice. Mol. Nutr. Food Res. 2018, 62, e1700769. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Eliza, J.; Mohamed, F.K.A. A novel dihydroxygymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J. Ethnopharmacol. 2009, 126, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
| Plant | Family | Occurrence Area | Identified Phytochemicals | Biological Activity of Extracts |
|---|---|---|---|---|
| Momordica charantia | Cucurbitaceae family | India, China, East Africa, Central and South America | triterpenoids and saponins (even up to 0.0432%) [82,83], | increase pancreatic insulin decrease insulin resistance [84], inhibit glucosidase, suppress the activity of disaccharides, reduce adiposity reduce xenobiotic metabolism [85] reduce oxidative stress [86] increase cytochrome P45 |
| polypeptides [87], | ||||
| flavonoids and phenolics (1.77 ± 0.72%) [83], | ||||
| alkaloids and sterols [24], | ||||
| unsaturated fatty acids (20.1%–64.3%), | ||||
| alkaloids, amino acids (up to 11.99%) | ||||
| Vitamins, | ||||
| polypeptide charantin [88], | ||||
| polysaccharides (5.91% to 10.62%) [89] | ||||
| Gymnema montanum | Apocynaceae family | India—Western Ghats | 11.57% w/w of carvacrol, | modulatory effect on glycolysis and gluconeogenesis [8] anti-hyperlipidemic properties [90,91] antiperoxidative properties [92,93] protect ß-cells against ROS [94,95] |
| 6.77% of erythritol, | ||||
| 4.58% of gallic acid, | ||||
| and 3.09% of quercetin [19] | ||||
| Moringa oleifera | Moringaceae family | Africa, India | vitamins, carotenoids, polyphenols, phenolic acids, flavonoids, alkaloids, glycosylates, isothiocyanates, tannins and saponins [96,97] lipids (stearic acid, palmitic acid and oleic acid), calcium, potassium, sodium, iron [98] | reduce insulin resistance [59], reduce hepatic gluconeogenesis [99], influence β-cell mass and function, increase insulin sensitivity in peripheral tissues [100] |
| Plant | Physiological Efficacy Parameters | Oxidative Stress Parameters | ||
|---|---|---|---|---|
| Momordica charantia | vs. control | no data analyzed Ø | ||
| Glycemia ↓ Insulinemia ↑ body weight ↔ glucose uptake by diaphragm↑ | ||||
| Gymnema montanum | vs. control | vs. drug | vs. control | vs. drug |
| Glycemia ↓ Insulinemia ↑ body weight ↑ food intake ↓ | Glycemia ↓ Insulinemia ↓ body weight ↔ food intake ↓ | TBARS ↓ Hydroperoxides ↓ | TBARS ↓ Hydroperoxides ↓ | |
| Moringa oleifera | vs. control | vs. control | ||
| Glycemia ↓ Insulinemia ↔ body weight ↑ | SOD ↓ CAT ↑ | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczyk, M.; Burzynska-Pedziwiatr, I.; Wozniak, L.A.; Bukowiecka-Matusiak, M. Evidence from a Systematic Review and Meta-Analysis Pointing to the Antidiabetic Effect of Polyphenol-Rich Plant Extracts from Gymnema montanum, Momordica charantia and Moringa oleifera. Curr. Issues Mol. Biol. 2022, 44, 699-717. https://doi.org/10.3390/cimb44020049
Krawczyk M, Burzynska-Pedziwiatr I, Wozniak LA, Bukowiecka-Matusiak M. Evidence from a Systematic Review and Meta-Analysis Pointing to the Antidiabetic Effect of Polyphenol-Rich Plant Extracts from Gymnema montanum, Momordica charantia and Moringa oleifera. Current Issues in Molecular Biology. 2022; 44(2):699-717. https://doi.org/10.3390/cimb44020049
Chicago/Turabian StyleKrawczyk, Michal, Izabela Burzynska-Pedziwiatr, Lucyna Alicja Wozniak, and Malgorzata Bukowiecka-Matusiak. 2022. "Evidence from a Systematic Review and Meta-Analysis Pointing to the Antidiabetic Effect of Polyphenol-Rich Plant Extracts from Gymnema montanum, Momordica charantia and Moringa oleifera" Current Issues in Molecular Biology 44, no. 2: 699-717. https://doi.org/10.3390/cimb44020049
APA StyleKrawczyk, M., Burzynska-Pedziwiatr, I., Wozniak, L. A., & Bukowiecka-Matusiak, M. (2022). Evidence from a Systematic Review and Meta-Analysis Pointing to the Antidiabetic Effect of Polyphenol-Rich Plant Extracts from Gymnema montanum, Momordica charantia and Moringa oleifera. Current Issues in Molecular Biology, 44(2), 699-717. https://doi.org/10.3390/cimb44020049







