Therapeutic Potential of 6-Gingerol in Prevention of Colon Cancer Induced by Azoxymethane through the Modulation of Antioxidant Potential and Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Animals
2.3. Animals Groups
2.4. Tissue Collection and Measurement of Antioxidant and Anti-Inflammatory Markers
2.5. Histopathological Analysis
2.6. Expressional Evaluation of PTEN Protein through Immunohistochemical Analysis
2.7. Statistical Analysis
3. Results
3.1. Effect of 6-Gingerol Treatment on Azoxymethane-Induced Colon Cancer through the Investigation of Antioxidant Enzyme Levels
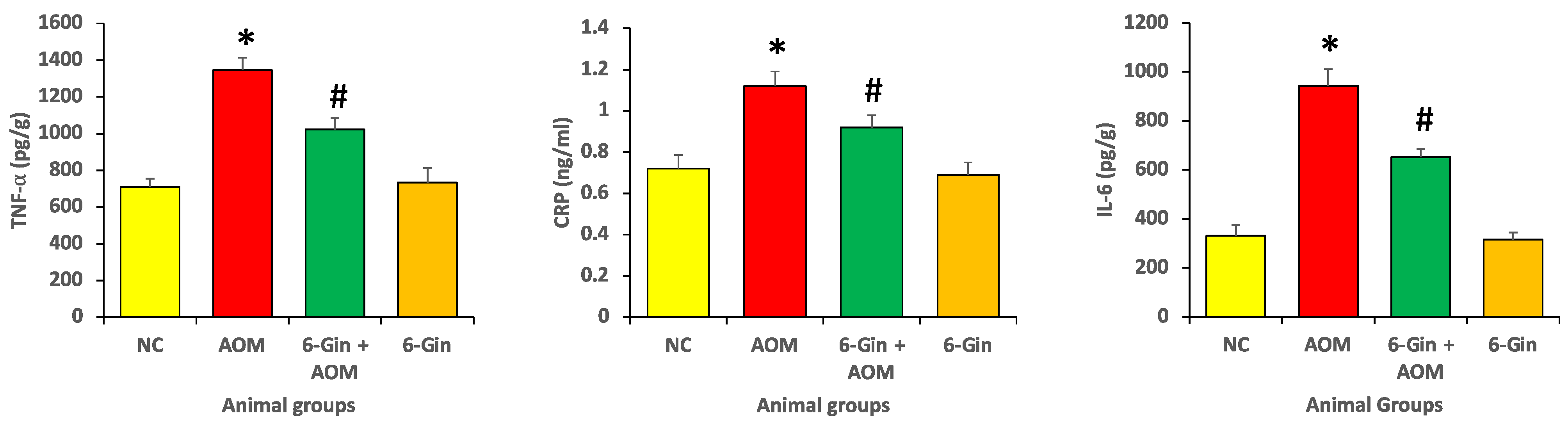
3.2. Effect of 6-Gingerol Treatment on Azoxymethane-Induced Colon Cancer through the Investigation of Inflammatory Protein Marker Levels
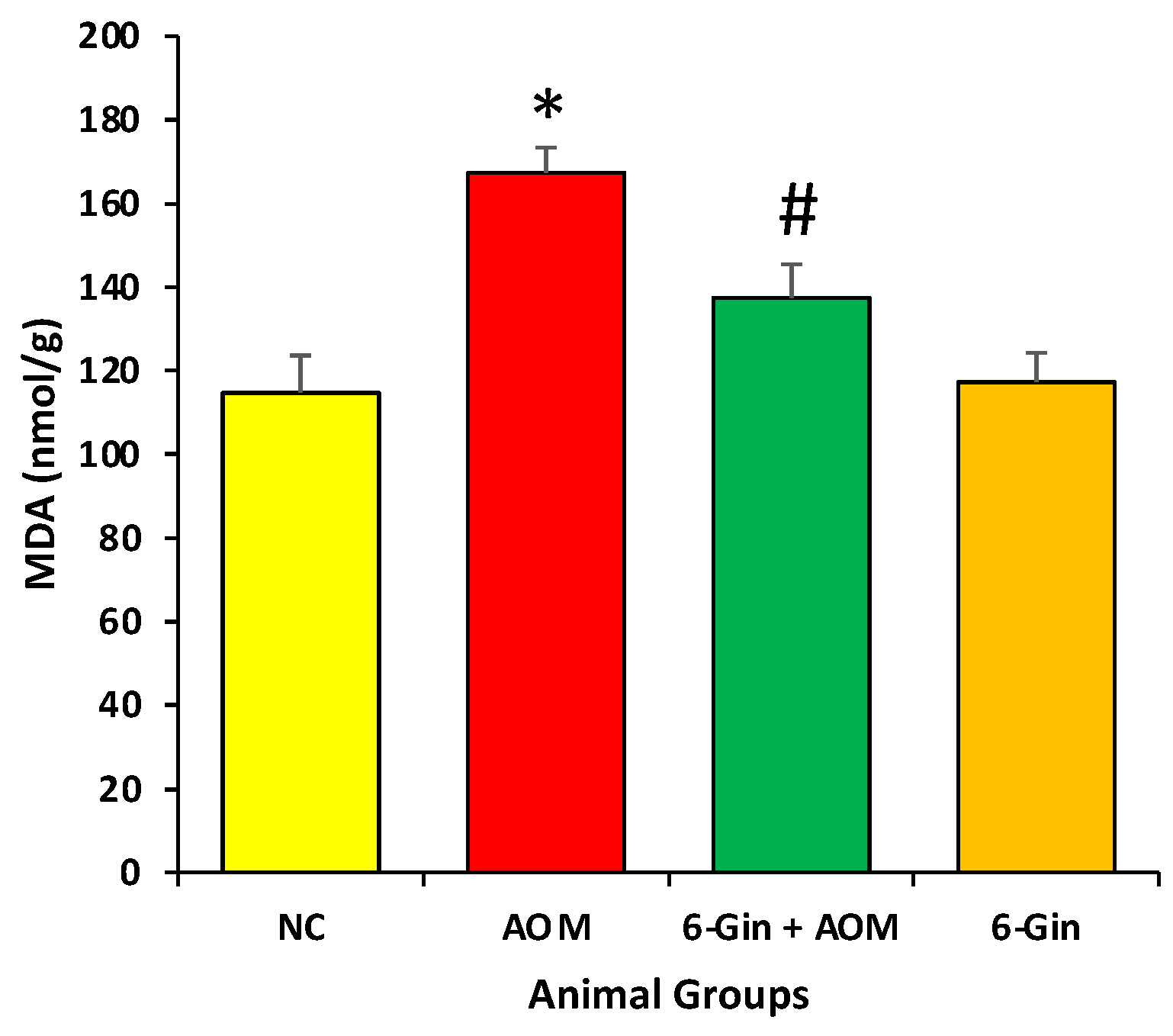
3.3. Effect of 6-Gingerol Treatment on Azoxymethane-Induced Colon Cancer through the Investigation of Lipid Peroxidation
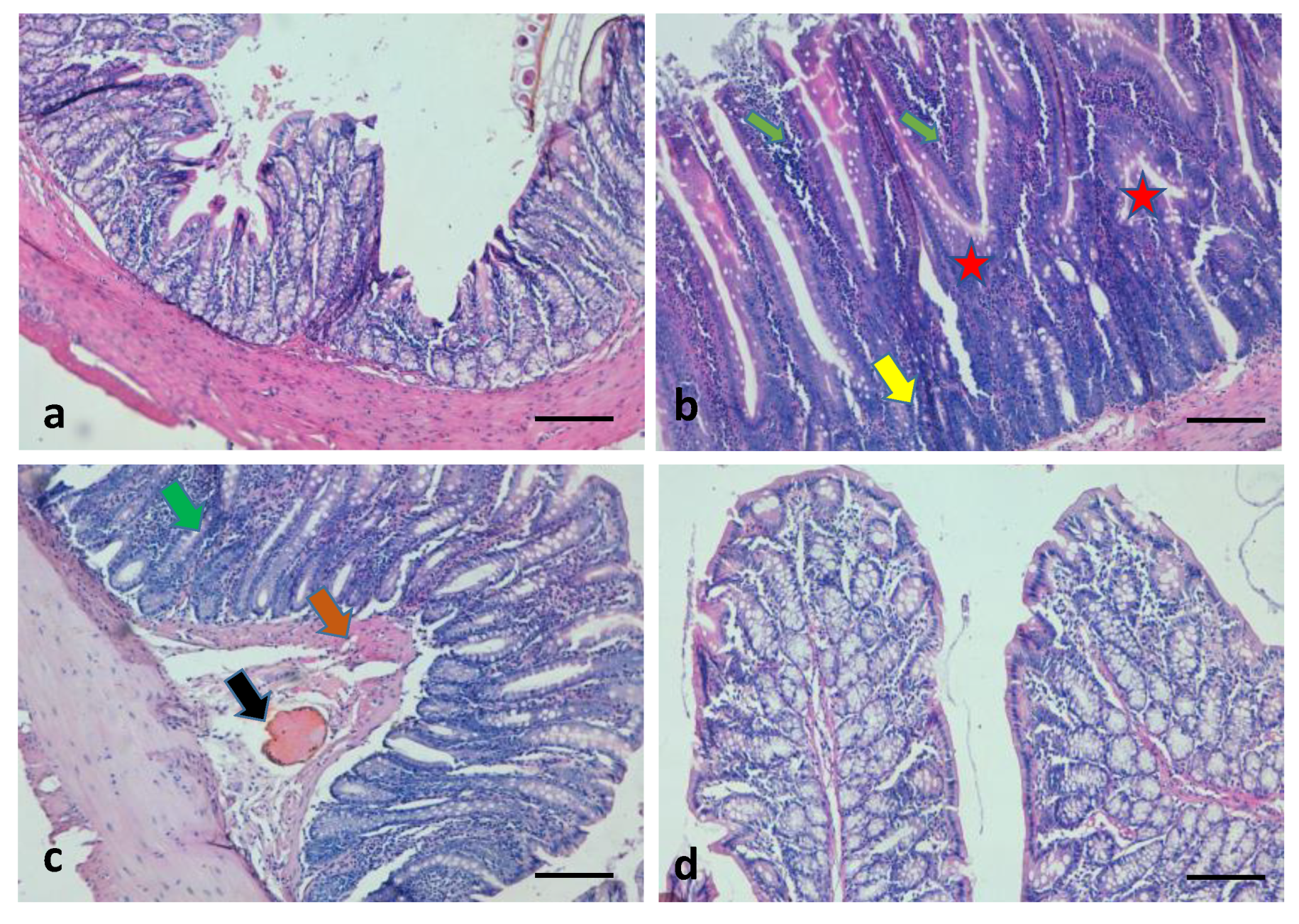
3.4. Role of 6-Gingerol on the Maintenance of Colon Tissue Architecture
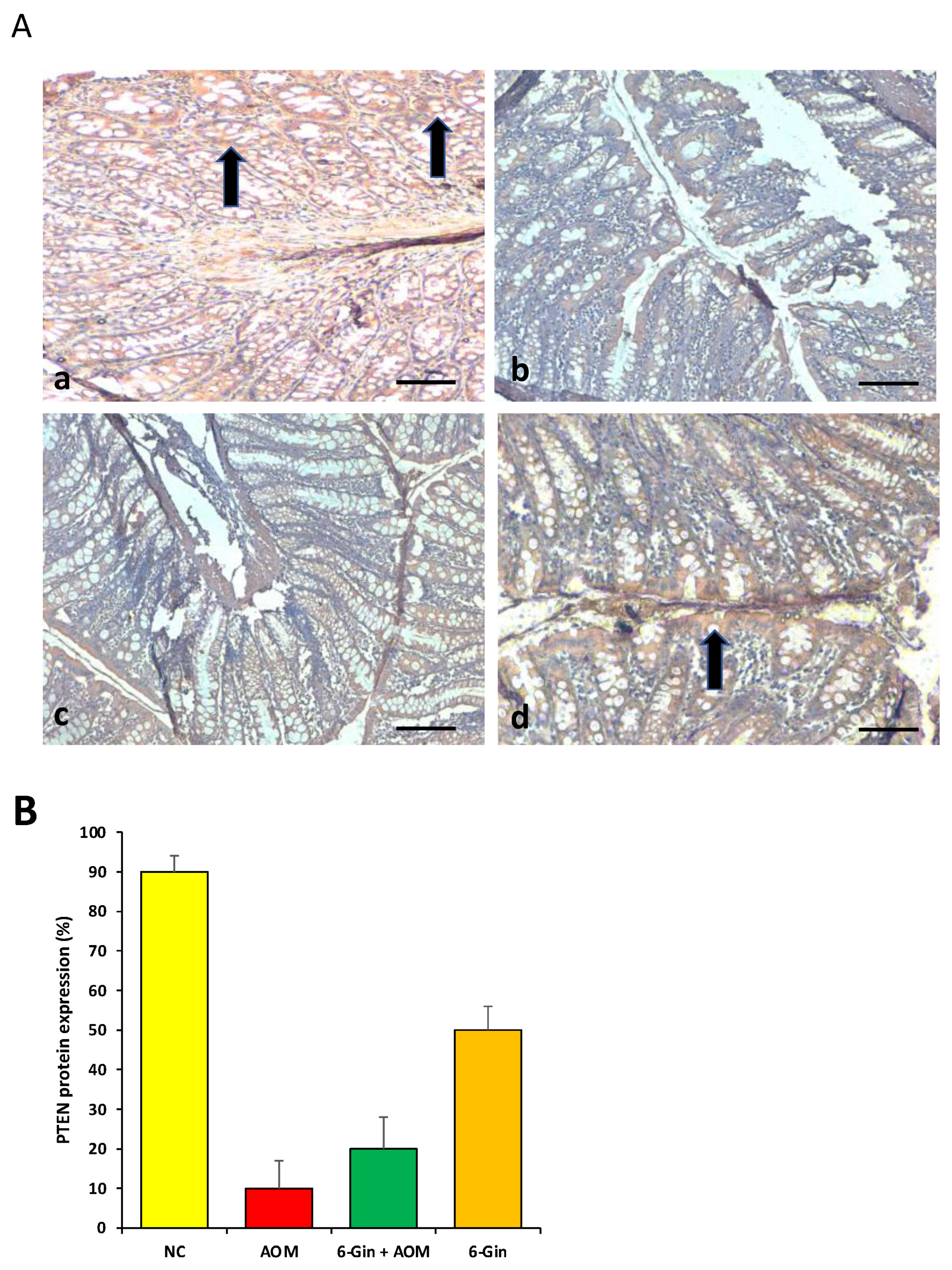
3.5. The Role of 6-Gingerol on PTEN Protein Expression
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radhakrishnan, E.K.; Bava, S.V.; Narayanan, S.S.; Nath, L.R.; Thulasidasan, A.K.; Soniya, E.V.; Anto, R.J. [6]-Gingerol induces caspase-dependent apoptosis and prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK/AP-1 signaling. PLoS ONE 2014, 9, e104401. [Google Scholar] [CrossRef] [PubMed]
- López, P.J.; Albero, J.S.; Rodríguez-Montes, J.A. Primary and secondary prevention of colorectal cancer. Clin. Med. Insights Gastroenterol. 2014, 7, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Al-Johar, D.; Shinwari, N.; Arif, J.; Al-Sanea, N.; Jabbar, A.A.; El-Sayed, R.A.; Mashhour, A.; Billedo, G.; El-Doush, I.; Al-Saleh, I. Role of Nigella sativa and a number of its antioxidant constituents towards azoxymethane-induced genotoxic effects and colon cancer in rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 1311–1323. [Google Scholar]
- Lijinsky, W.; Saavedra, J.E.; Reuber, M.D. Organ-specific carcinogenesis in rats by methyl and ethylazoxyalkanes. Cancer Res. 1985, 45, 76–79. [Google Scholar] [PubMed]
- Takahashi, M.; Wakabayashi, K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004, 95, 475–480. [Google Scholar] [CrossRef]
- O’Toole, S.M.; Pegg, A.E.; Swenberg, J.A. Repair of O6-methylguanine and O4-methylthymidine in F344 rat liver following treatment with 1,2-dimethylhydrazine and O6-benzylguanine. Cancer Res. 1993, 53, 3895–3898. [Google Scholar]
- Maduro, J.H.; Pras, E.; Willemse, P.H.; De Vries, E.G. Acute and long-term toxicity following radiotherapy alone or in combination with chemotherapy for locally advanced cervical cancer. Cancer Treat. Rev. 2003, 29, 471–488. [Google Scholar] [CrossRef]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal plants in the prevention and treatment of colon cancer. Oxidative Med. Cell. Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef]
- Huang, X.M.; Yang, Z.J.; Xie, Q.; Zhang, Z.K.; Zhang, H.; Ma, J.Y. Natural products for treating colorectal cancer: A mechanistic review. Biomed. Pharmacother. 2019, 117, 109142. [Google Scholar] [CrossRef]
- Krell, J.; Stebbing, J. Ginger: The root of cancer therapy? Lancet Oncol. 2012, 3, 235–236. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Lee, E.; Lee, J.M. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat. Res. /Fundam. Mol. Mech. Mutagen. 1998, 402, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.B.; Lin, C.C.; Tsay, G.J. 6-Gingerol inhibits growth of colon cancer cell LoVo via induction of G2/M arrest. Evid.-Based Complement. Altern. Med. 2012, 2012, 326096. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic substances. Mutat. Res. /Fundam. Mol. Mech. Mutagen. 1999, 428, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Kundu, J.K.; Shin, Y.K.; Park, J.H.; Cho, M.H.; Kim, T.Y.; Surh, Y.J. [6]-Gingerol inhibits COX-2 expression by blocking the activation of p38 MAP kinase and NF-κB in phorbol ester-stimulated mouse skin. Oncogene 2005, 24, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Min, J.K.; Kim, T.Y.; Lee, S.J.; Yang, H.O.; Han, S.; Kim, Y.M.; Kwon, Y.G. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 335, 300–308. [Google Scholar] [CrossRef]
- Lee, H.S.; Seo, E.Y.; Kang, N.E.; Kim, W.K. [6]-Gingerol inhibits metastasis of MDA-MB-231 human breast cancer cells. J. Nutr. Biochem. 2008, 19, 313–319. [Google Scholar] [CrossRef]
- Reddy, B.S.; Wang, C.X.; Kong, A.N.; Khor, T.O.; Zheng, X.; Steele, V.E.; Kopelovich, L.; Rao, C.V. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006, 66, 4542–4546. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-Gingerol, a major ingredient of ginger attenuates diethylnitrosamine-induced liver injury in rats through the modulation of oxidative stress and anti-inflammatory activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Rahmani, A.; Alzohairy, M.; Babiker, A.Y.; Rizvi, M.A.; Elkarimahmad, H.G. Clinicopathological significance of PTEN and bcl2 expressions in oral squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2012, 5, 965–971. [Google Scholar]
- Husain, N.E.; Babiker, A.Y.; Albutti, A.S.; Alsahli, M.A.; Aly, S.M.; Rahmani, A.H. Clinicopathological Significance of Vimentin and Cytokeratin Protein in the Genesis of Squamous Cell Carcinoma of Cervix. Obstet. Gynecol. Int. 2016, 2016, 8790120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arber, N.; Levin, B. Chemoprevention of colorectal cancer: Ready for routine use? Curr. Top. Med. Chem. 2005, 5, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Haniadka, R.; Pereira, M.M.; D’Souza, J.J.; Pallaty, P.L.; Bhat, H.P.; Popuri, S. Update on the chemopreventive effects of ginger and its phytochemicals. Crit. Rev. Food Sci. Nutr. 2011, 51, 499–523. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alnuqaydan, A.M.; Babiker, A.Y.; Almogbel, M.A.; Khan, A.A.; Husain Rahmani, A. 6-Gingerol, a Bioactive Compound of Ginger Attenuates Renal Damage in Streptozotocin-Induced Diabetic Rats by Regulating the Oxidative Stress and Inflammation. Pharmaceutics 2021, 13, 317. [Google Scholar] [CrossRef]
- Ju, S.A.; Park, S.M.; Lee, Y.S.; Bae, J.H.; Yu, R.; An, W.G.; Suh, J.H.; Kim, B.S. Administration of 6-gingerol greatly enhances the number of tumor-infiltrating lymphocytes in murine tumors. Int. J. Cancer 2012, 130, 2618–2628. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Ajayi, B.O.; Adedara, I.A.; Farombi, E.O. Pharmacological activity of 6-gingerol in dextran sulphate sodium-induced ulcerative colitis in BALB/c mice. Phytother. Res. 2015, 29, 566–572. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, Y.; Lin, B.; Tipoe, G.L.; Youdim, M.B.; Xing, F.; Liu, Y. A novel antioxidant multitarget iron chelator M30 protects hepatocytes against ethanol-induced injury. Oxidative Med. Cell. Longev. 2015, 2015, 607271. [Google Scholar] [CrossRef]
- Wang, T.; Di, G.; Yang, L.; Dun, Y.; Sun, Z.; Wan, J.; Peng, B.; Liu, C.; Xiong, G.; Zhang, C.; et al. Saponins from P anax japonicus attenuate D-galactose-induced cognitive impairment through its anti-oxidative and anti-apoptotic effects in rats. J. Pharm. Pharmacol. 2015, 67, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Cheung, F.; Tan, H.Y.; Wang, N.; Yuen, M.F.; Feng, Y. Hepatoprotective effects of Chinese medicinal herbs: A focus on anti-inflammatory and anti-oxidative activities. Int. J. Mol. Sci. 2016, 17, 465. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Davis, J.M.; McClellan, J.L.; Gordon, B.T.; Carmichael, M.D. Curcumin’s effect on intestinal inflammation and tumorigenesis in the ApcMin/+ mouse. J. Interferon. Cytokine Res. 2011, 31, 219–226. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Morazzoni, P.; Allegrini, P.; Faliva, M.A.; Naso, M.; Miccono, A.; Peroni, G.; Agosti, I.D.; Perna, S. The effect and safety of highly standardized Ginger (Zingiber officinale) and Echinacea (Echinacea angustifolia) extract supplementation on inflammation and chronic pain in NSAIDs poor responders. A pilot study in subjects with knee arthrosis. Nat. Prod. Res. 2017, 31, 1309–1313. [Google Scholar] [CrossRef]
- Joshi, D.; Srivastav, S.K.; Belemkar, S.; Dixit, V.A. Zingiber officinale and 6-gingerol alleviate liver and kidney dysfunctions and oxidative stress induced by mercuric chloride in male rats: A protective approach. Biomed. Pharmacother. 2017, 91, 645–655. [Google Scholar] [CrossRef]
- Yazdani, Y.; Farazmandfar, T.; Azadeh, H.; Zekavatian, Z. The prognostic effect of PTEN expression status in colorectal cancer development and evaluation of factors affecting it: miR-21 and promoter methylation. J. Biomed. Sci. 2016, 23, 1–8. [Google Scholar] [CrossRef]

| Group Number | Group Description | Short Name | Treatment Plan |
|---|---|---|---|
| 1 | Normal Control | NC | Rats received normal saline solution by oral gavage. |
| 2 | Disease control (Negative control) | AOM | Azoxymethane (15 mg/kg b.w.) was administered intraperitoneally in normal saline two times a week [18]. |
| 3 | 6-Gin Treatment + Disease control | 6-Gin + AOM | 6-Gingerol (50 mg/kg b.w.) was given intraperitoneally two times a week before the administration of AOM (15 mg/kg b.w.). |
| 4 | 6-Gin treatment only | 6-Gin | 6-Gingerol (50 mg/kg b.w.) was given intraperitoneally two times a week [19]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloliqi, A.A. Therapeutic Potential of 6-Gingerol in Prevention of Colon Cancer Induced by Azoxymethane through the Modulation of Antioxidant Potential and Inflammation. Curr. Issues Mol. Biol. 2022, 44, 6218-6228. https://doi.org/10.3390/cimb44120424
Aloliqi AA. Therapeutic Potential of 6-Gingerol in Prevention of Colon Cancer Induced by Azoxymethane through the Modulation of Antioxidant Potential and Inflammation. Current Issues in Molecular Biology. 2022; 44(12):6218-6228. https://doi.org/10.3390/cimb44120424
Chicago/Turabian StyleAloliqi, Abdulaziz A. 2022. "Therapeutic Potential of 6-Gingerol in Prevention of Colon Cancer Induced by Azoxymethane through the Modulation of Antioxidant Potential and Inflammation" Current Issues in Molecular Biology 44, no. 12: 6218-6228. https://doi.org/10.3390/cimb44120424
APA StyleAloliqi, A. A. (2022). Therapeutic Potential of 6-Gingerol in Prevention of Colon Cancer Induced by Azoxymethane through the Modulation of Antioxidant Potential and Inflammation. Current Issues in Molecular Biology, 44(12), 6218-6228. https://doi.org/10.3390/cimb44120424






