Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples Collection
2.2. Cells Culture
2.3. Extraction of Macrophages
2.4. Total RNA Extraction and RT-qPCR
2.5. Gene Oncology (GO) Analysis, Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis
2.6. Prussian Blue Staining
2.7. Intracellular Ferrous Iron Staining
2.8. Analysis of Lipid Peroxidation
2.9. Malondialdehyde (MDA) Content Assay
2.10. Phagocytosis Assay
2.11. Cell Viability Measurements
2.12. Statistical Analysis
3. Results
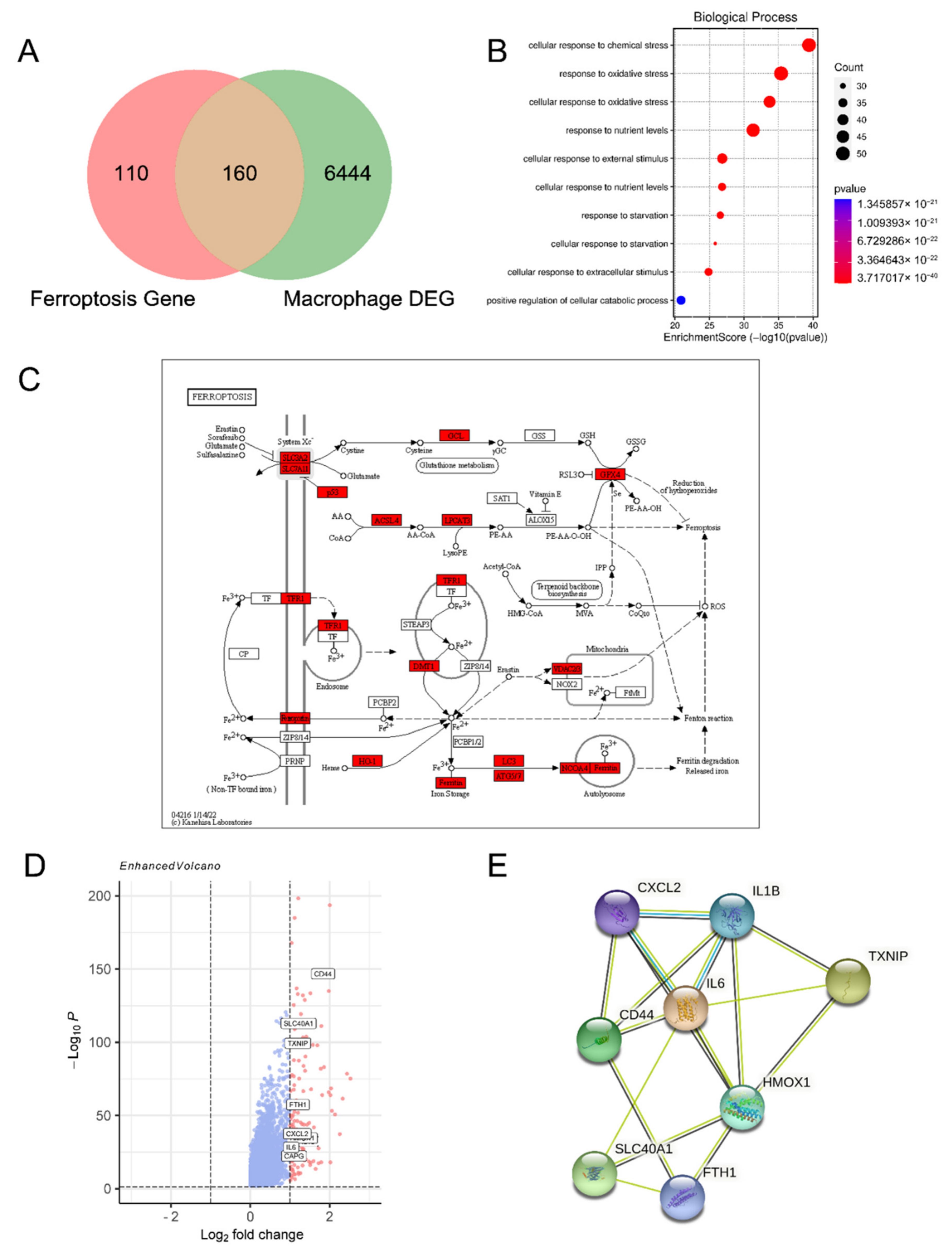
3.1. Ferroptosis-Related Genes Are Enriched in Macrophages of Endometriosis Lesions
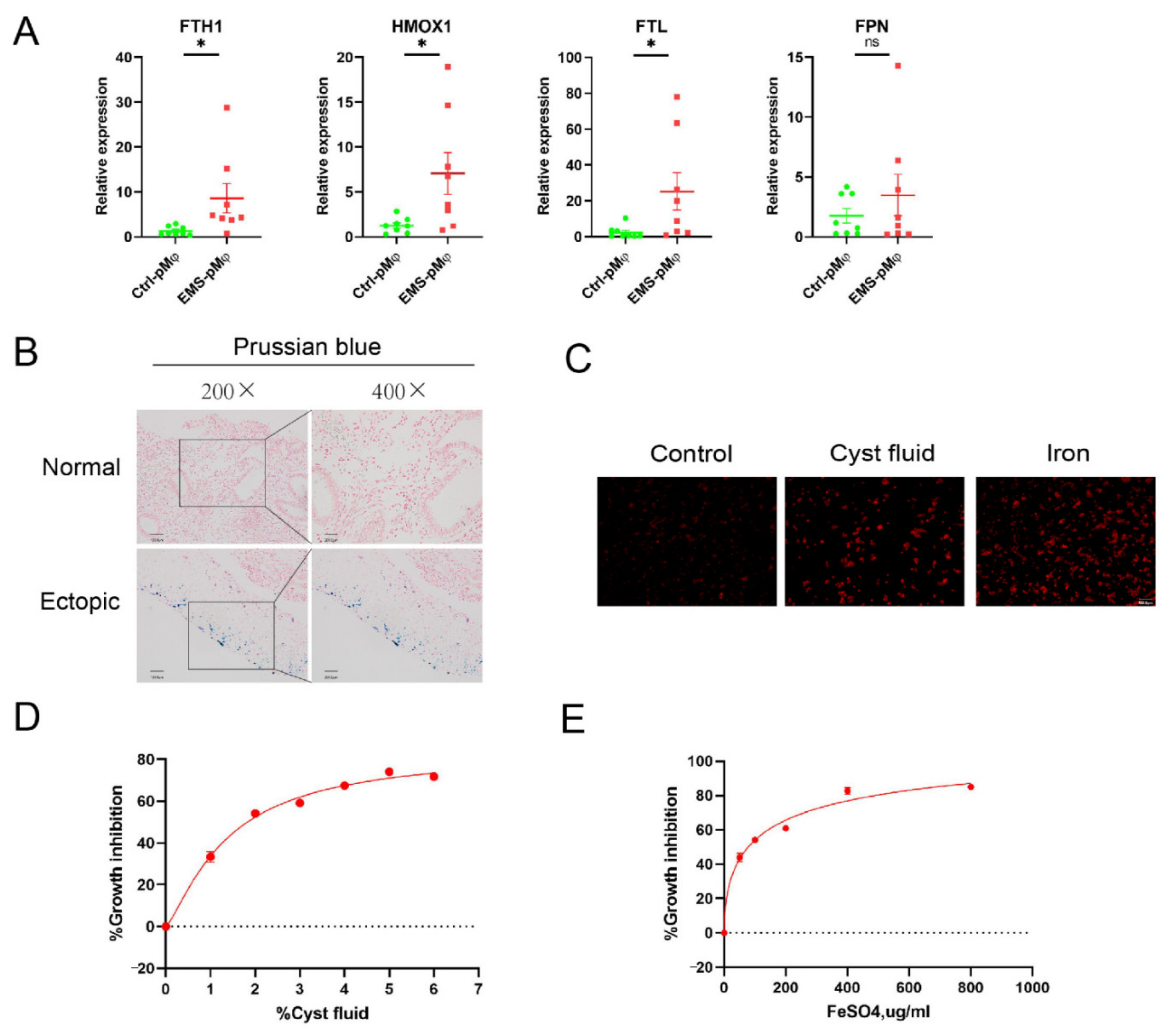
3.2. Cyst Fluid Induces Iron Accumulation and Cell Death in Macrophage
3.3. Cyst Fluid Triggers Ferroptosis in Macrophages
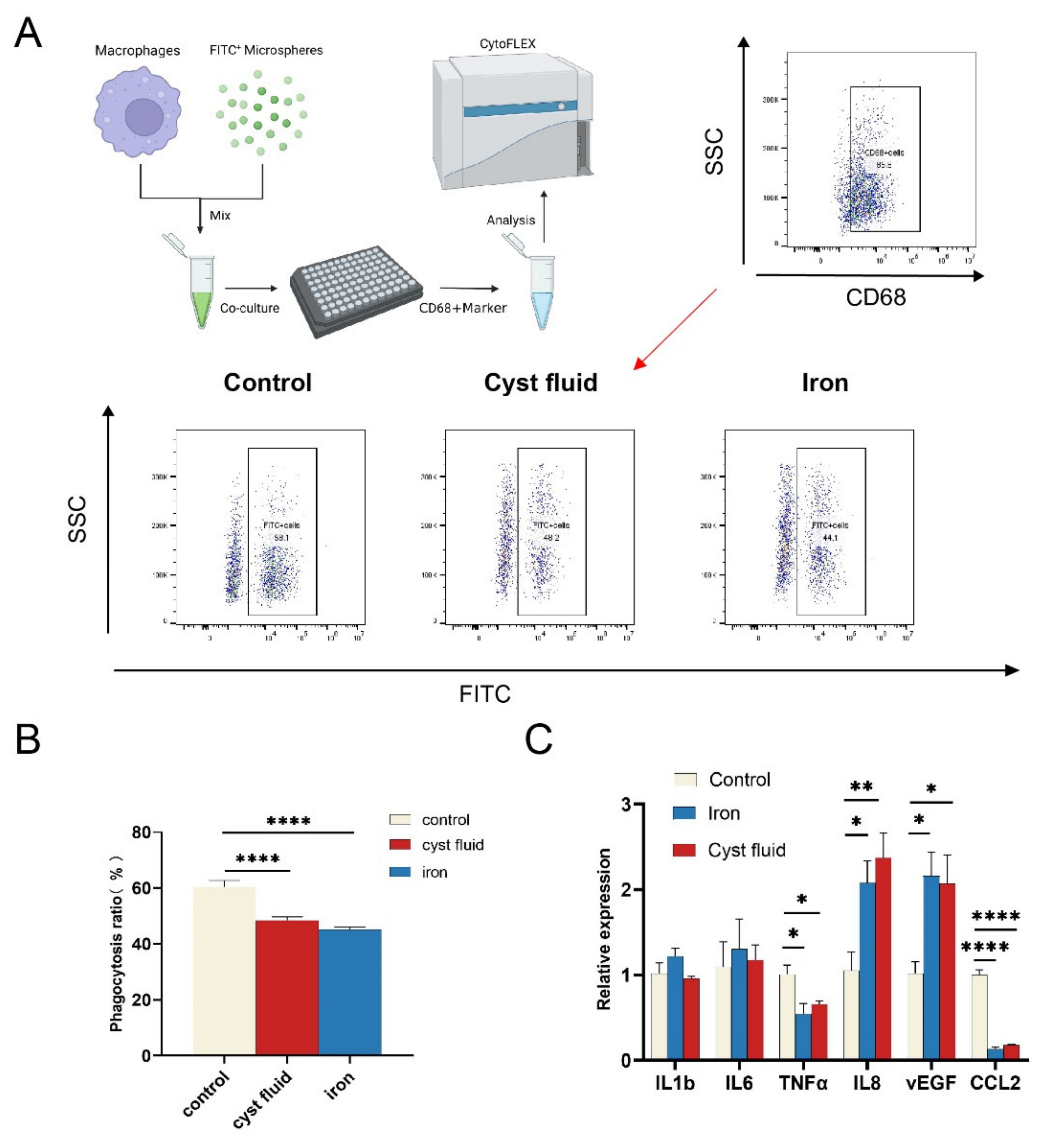
3.4. Ferroptosis Reduces the Phagocytic Function of Macrophage
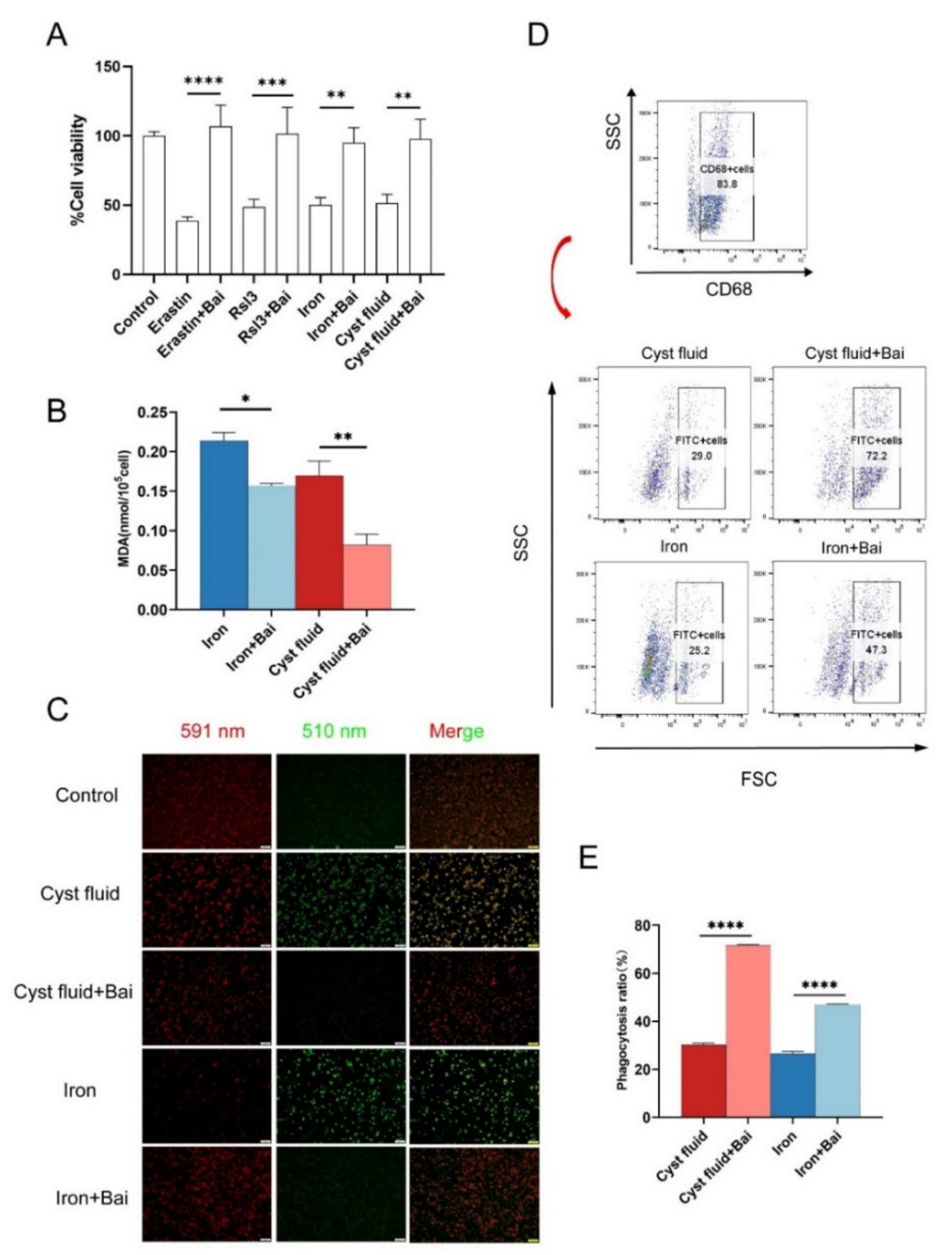
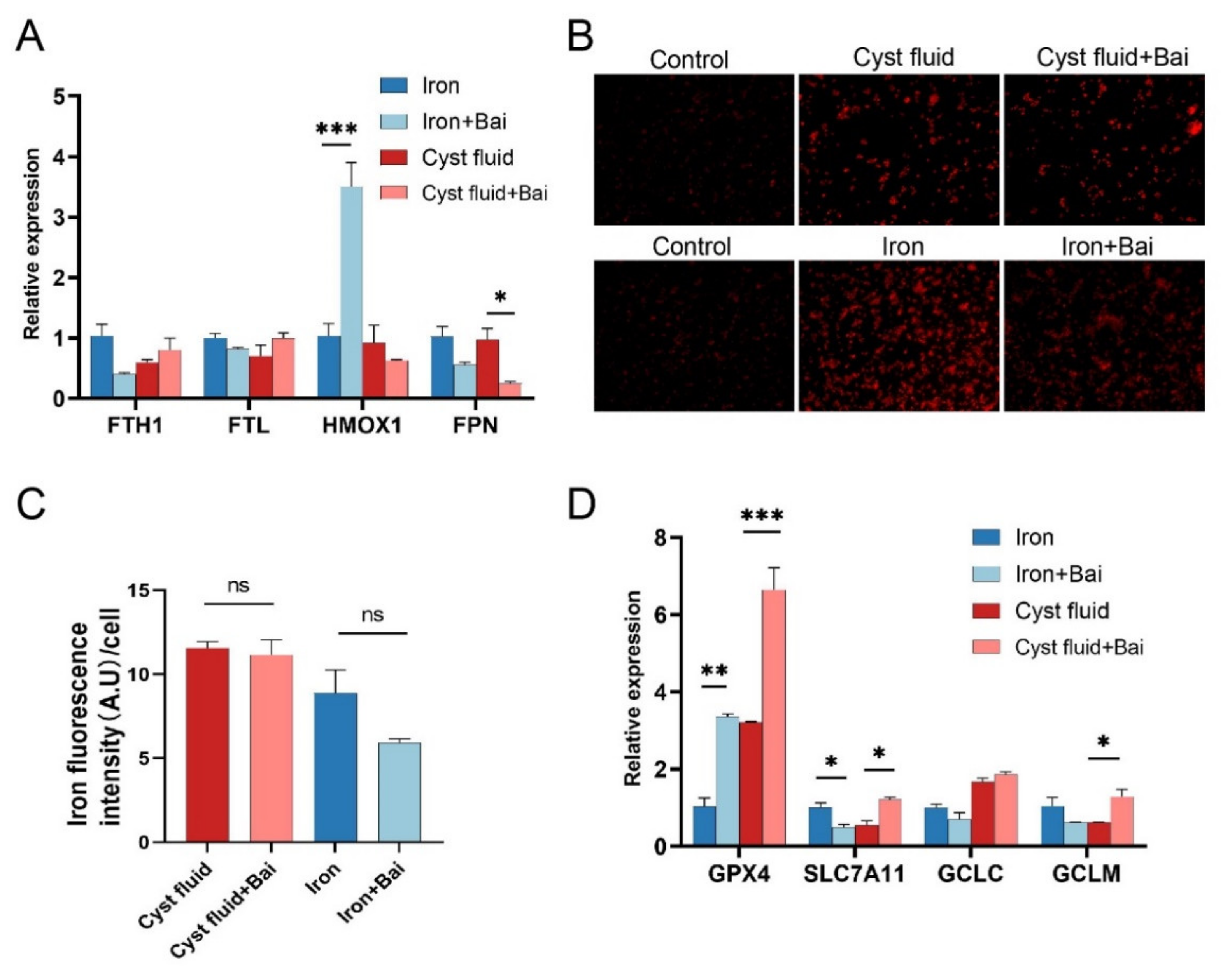
3.5. Baicalein Protects Macrophages against Ferroptosis and Rescues Phagocytosis
3.6. Baicalein Increases GPX4 Expression and Inhibits Ferroptosis in Macrophage
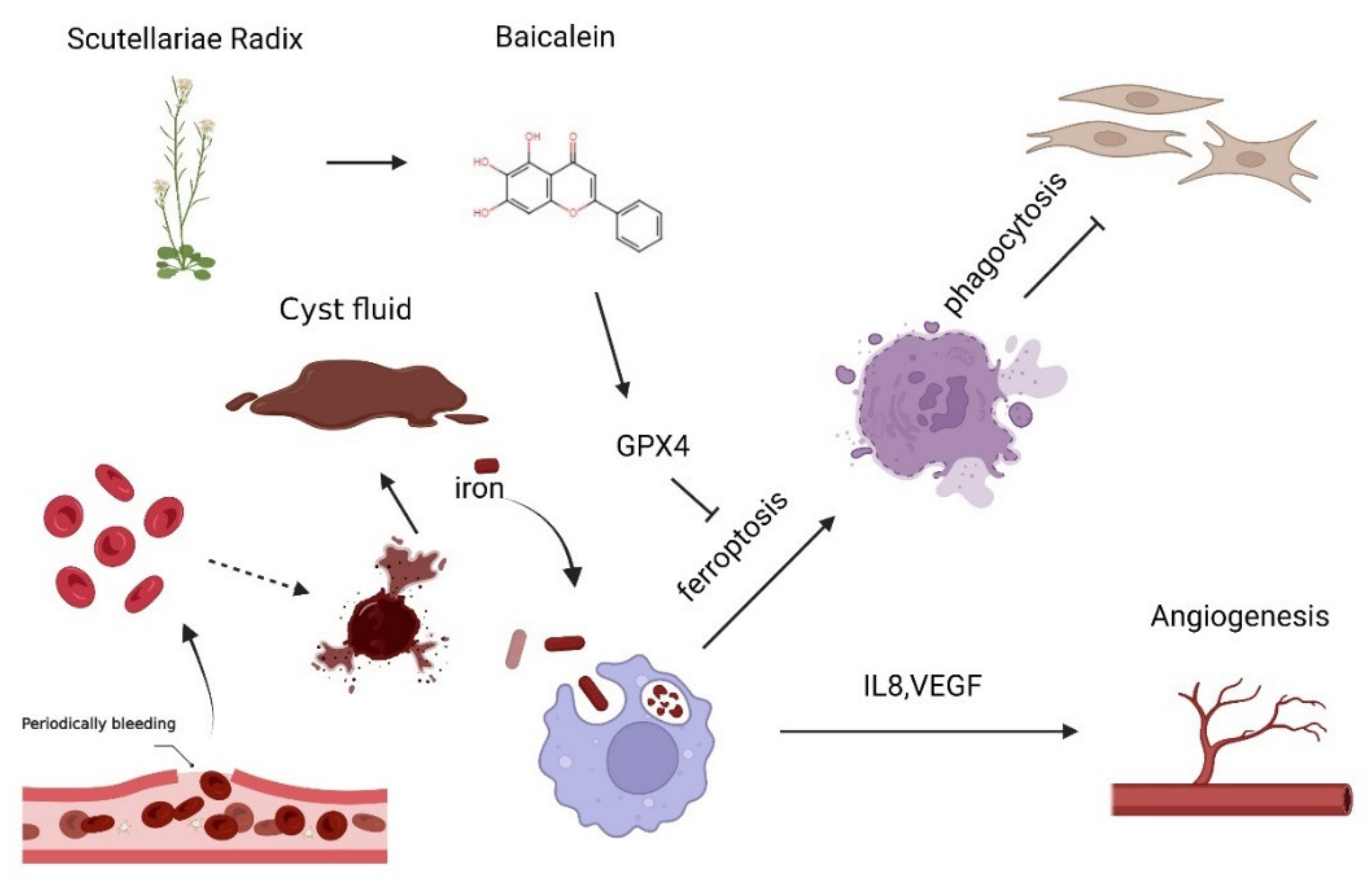
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mehedintu, C.; Plotogea, M.N.; Ionescu, S.; Antonovici, M. Endometriosis still a challenge. J. Med. Life 2014, 7, 349–357. [Google Scholar] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Attar, R.; Attar, E. Experimental treatments of endometriosis. Women’s Health 2015, 11, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Huang, Q.; Fu, X.; Zhang, L.; Gao, F.; Jin, Z.; Wu, L.; Shu, C.; Zhang, X.; et al. Iron overload in endometriosis peritoneal fluid induces early embryo ferroptosis mediated by HMOX1. Cell Death Discov. 2021, 7, 355. [Google Scholar] [CrossRef]
- Ni, Z.; Li, Y.; Song, D.; Ding, J.; Mei, S.; Sun, S.; Cheng, W.; Yu, J.; Zhou, L.; Kuang, Y.; et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis. 2022, 13, 579. [Google Scholar] [CrossRef]
- Li, G.; Lin, Y.; Zhang, Y.; Gu, N.; Yang, B.; Shan, S.; Liu, N.; Ouyang, J.; Yang, Y.; Sun, F.; et al. Endometrial stromal cell ferroptosis promotes angiogenesis in endometriosis. Cell Death Discov. 2022, 8, 29. [Google Scholar] [CrossRef]
- Králíčková, M.; Vetvicka, V. Immunological aspects of endometriosis: A review. Ann. Transl. Med. 2015, 3, 153. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Hu, W.; Tang, L.; Sheng, Y.; Wei, C.; Li, M.; Zhu, X. Elevated heme impairs macrophage phagocytosis in endometriosis. Reproduction 2019, 158, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Tsang, P.W.; Chau, K.; Yang, H. Baicalein exhibits inhibitory effect on the energy-dependent efflux pump activity in non-albicans Candida fungi. J. Chemother. 2015, 27, 61–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef]
- Xie, Y.; Song, X.; Sun, X.; Huang, J.; Zhong, M.; Lotze, M.T.; Zeh, H.J.R.; Kang, R.; Tang, D. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016, 473, 775–780. [Google Scholar] [CrossRef]
- Kenny, E.M.; Fidan, E.; Yang, Q.; Anthonymuthu, T.S.; New, L.A.; Meyer, E.A.; Wang, H.; Kochanek, P.M.; Dixon, C.E.; Kagan, V.E.; et al. Ferroptosis Contributes to Neuronal Death and Functional Outcome After Traumatic Brain Injury. Crit. Care Med. 2019, 47, 410–418. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, M.; Li, X.; Zheng, C.; Wang, A.; Feng, J.; Hu, X.; Chang, S.; Zhang, H. Hematopoietic protection and mechanisms of ferrostatin-1 on hematopoietic acute radiation syndrome of mice. Int. J. Radiat. Biol. 2021, 97, 464–473. [Google Scholar] [CrossRef]
- Jin, Z.; Huang, J.; Zhu, Z. Baicalein reduces endometriosis by suppressing the viability of human endometrial stromal cells through the nuclear factor-κB pathway in vitro. Exp. Ther. Med. 2017, 14, 2992–2998. [Google Scholar] [CrossRef]
- Ke, J.; Yang, J.; Li, J.; Xu, Z.; Li, M.; Zhu, Z. Baicalein inhibits FURIN-MT1-MMP-mediated invasion of ectopic endometrial stromal cells in endometriosis possibly by reducing the secretion of TGFB1. Am. J. Reprod. Immunol. 2021, 85, e13344. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Draghici, S.; Khatri, P.; Tarca, A.L.; Amin, K.; Done, A.; Voichita, C.; Georgescu, C.; Romero, R. A systems biology approach for pathway level analysis. Genome Res. 2007, 17, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, L.; Zhan, H.; Mo, Y.; Ren, Z.; Shao, A.; Lin, J. Single-cell transcriptomic analysis of endometriosis provides insights into fibroblast fates and immune cell heterogeneity. Cell Biosci. 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Chang, K.; Sun, H. Immunosuppressive macrophages induced by IDO1 promote the growth of endometrial stromal cells in endometriosis. Mol. Med. Rep. 2017, 15, 2255–2260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chuang, P.; Lin, Y.; Wu, M.; Wing, L.C.; Shoji, Y.; Tsai, S. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am. J. Pathol. 2010, 176, 850–860. [Google Scholar] [CrossRef]
- Weng, L.; Hou, S.; Lei, S.; Peng, H.; Li, M.; Zhao, D. Estrogen-regulated CD200 inhibits macrophage phagocytosis in endometriosis. J. Reprod. Immunol. 2020, 138, 103090. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Ito, Y.; Hattori, K.; Inoue, T.; Hosono, K.; Honda, M.; Numao, A.; Amano, H.; Shibuya, M.; Unno, N.; et al. VEGF Receptor 1-Expressing Macrophages Recruited from Bone Marrow Enhances Angiogenesis in Endometrial Tissues. Sci. Rep. 2019, 9, 7037. [Google Scholar] [CrossRef]
- Haber, E.; Danenberg, H.D.; Koroukhov, N.; Ron-El, R.; Golomb, G.; Schachter, M. Peritoneal macrophage depletion by liposomal bisphosphonate attenuates endometriosis in the rat model. Hum. Reprod. 2009, 24, 398–407. [Google Scholar] [CrossRef]
- Ioannidou, E.; Moschetta, M.; Shah, S.; Parker, J.S.; Ozturk, M.A.; Pappas-Gogos, G.; Sheriff, M.; Rassy, E.; Boussios, S. Angiogenesis and Anti-Angiogenic Treatment in Prostate Cancer: Mechanisms of Action and Molecular Targets. Int. J. Mol. Sci. 2021, 22, 9926. [Google Scholar] [CrossRef]
- Berbic, M.; Schulke, L.; Markham, R.; Tokushige, N.; Russell, P.; Fraser, I.S. Macrophage expression in endometrium of women with and without endometriosis. Hum. Reprod. 2009, 24, 325–332. [Google Scholar] [CrossRef]
- Revythis, A.; Limbu, A.; Mikropoulos, C.; Ghose, A.; Sanchez, E.; Sheriff, M.; Boussios, S. Recent Insights into PARP and Immuno-Checkpoint Inhibitors in Epithelial Ovarian Cancer. Int. J. Environ. Res. Public Health 2022, 19, 8577. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Y.; Wu, X.; Zhang, Q.; Chen, S.; Ma, X.; Yu, M. Ironing Out the Details: How Iron Orchestrates Macrophage Polarization. Front. Immunol. 2021, 12, 669566. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Chen, T.; Buang, N.; Olona, A.; Ko, J.; Prendecki, M.; Costa, A.S.H.; Nikitopoulou, E.; Tronci, L.; Pusey, C.D.; et al. Acute Iron Deprivation Reprograms Human Macrophage Metabolism and Reduces Inflammation In Vivo. Cell Rep. 2019, 28, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Kroner, A.; Greenhalgh, A.D.; Zarruk, J.G.; Passos Dos Santos, R.; Gaestel, M.; David, S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 2014, 83, 1098–1116. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, B.D.; Wright, C.B.; Kim, Y.; Yasuma, T.; Yasuma, R.; Li, S.; Fowler, B.J.; Bastos-Carvalho, A.; Kerur, N.; Uittenbogaard, A.; et al. Iron Toxicity in the Retina Requires Alu RNA and the NLRP3 Inflammasome. Cell Rep. 2015, 11, 1686–1693. [Google Scholar] [CrossRef]
- Ward, R.J.; Wilmet, S.; Legssyer, R.; Leroy, D.; Toussaint, L.; Crichton, R.R.; Pierreux, C.; Hue, L.; Piette, J.; Srai, S.K.; et al. Effects of marginal iron overload on iron homeostasis and immune function in alveolar macrophages isolated from pregnant and normal rats. Biometals Int. J. Role Metal Ions Biol. Biochem. Med. 2009, 22, 211–223. [Google Scholar] [CrossRef]
- Gan, Z.; Wang, Q.; Li, J.; Wang, X.; Wang, Y.; Du, H. Iron Reduces M1 Macrophage Polarization in RAW264.7 Macrophages Associated with Inhibition of STAT1. Mediat. Inflamm. 2017, 2017, 8570818. [Google Scholar] [CrossRef]
- Aerbajinai, W.; Ghosh, M.C.; Liu, J.; Kumkhaek, C.; Zhu, J.; Chin, K.; Rouault, T.A.; Rodgers, G.P. Glia maturation factor-γ regulates murine macrophage iron metabolism and M2 polarization through mitochondrial ROS. Blood Adv. 2019, 3, 1211–1225. [Google Scholar] [CrossRef]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Kroemer, G.; Klionsky, D.J.; Zeh, H.J.; Kang, R.; Wang, J.; Tang, D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 2020, 16, 2069–2083. [Google Scholar] [CrossRef]
- Kapralov, A.A.; Yang, Q.; Dar, H.H.; Tyurina, Y.Y.; Anthonymuthu, T.S.; Kim, R.; St Croix, C.M.; Mikulska-Ruminska, K.; Liu, B.; Shrivastava, I.H.; et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 2020, 16, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J. Membrane redox state and apoptosis: Death by peroxide. Cell Metab. 2008, 8, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Schneider, M.; Förster, H.; Roth, S.; Wirth, E.K.; Culmsee, C.; Plesnila, N.; Kremmer, E.; Rådmark, O.; Wurst, W.; et al. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008, 8, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Li, H.; Zhang, X.; Liu, X.; Song, Y. Baicalein inhibits RLS3-induced ferroptosis in melanocytes. Biochem. Biophys. Res. Commun. 2021, 561, 65–72. [Google Scholar] [CrossRef]
- Drefs, M.; Thomas, M.N.; Guba, M.; Angele, M.K.; Werner, J.; Conrad, M.; Steib, C.J.; Holdt, L.M.; Andrassy, J.; Khandoga, A.; et al. Modulation of Glutathione Hemostasis by Inhibition of 12/15-Lipoxygenase Prevents ROS-Mediated Cell Death after Hepatic Ischemia and Reperfusion. Oxidative Med. Cell. Longev. 2017, 2017, 8325754. [Google Scholar] [CrossRef]
- Probst, L.; Dächert, J.; Schenk, B.; Fulda, S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem. Pharmacol. 2017, 140, 41–52. [Google Scholar] [CrossRef]

| Variables Mean (Full Ranges) | EMS (n = 8) | CP (n = 8) | CE (n = 8) | p Value (EMS vs. CP) | p Value (EMS vs. CE) |
|---|---|---|---|---|---|
| Age, years | 31 (25–39) | 29.5 (21–44) | 30.25 (23–35) | 0.7053 | 0.7429 |
| Gravidity, n | 0.875 (0–2) | 0.625 (0–2) | 0.25 (0–1) | 0.5797 | 0.0851 |
| Parity, n | 0.5 (0–1) | 0.375 (0–1) | 0.25 (0–1) | 0.5773 | 0.4383 |
| BMI, kg/m2 | 21.94 (17.99–25.64) | 21.19 (18.14–27.85) | 23.90 (19.95–30.84) | 0.6059 | 0.2349 |
| Phase (proliferative phase/secretory phase) | 8/0 | 5/3 | 8/0 | – | – |
| Gene Name | Forward and Reverse Primer | Tm (℃) | Product Length (bp) |
|---|---|---|---|
| ACTB | F: 5′CTACCTCATGAAGATCCTCACC3′ | 60.8 | 250 |
| R: 5′AGTTGAAGGTAGTTTCGTGGAT3′ | 60.2 | ||
| HMOX1 | F: 5′AAGACTGCGTTCCTGCTCAAC3′ | 62.9 | 247 |
| R: 5′AAAGCCCTACAGCAACTGTCG3′ | 62.6 | ||
| FTL | F: 5′CAGCCTGGTCAATTTGTACCT3′ | 60.0 | 114 |
| R: 5′GCCAATTCGCGGAAGAAGTG3′ | 62.0 | ||
| FTH1 | F: 5′CCCCCATTTGTGTGACTTCAT3′ | 60.2 | 180 |
| R:5′GCCCGAGGCTTAGCTTTCATT3′ | 62.8 | ||
| SLC40A1 | F: 5′CTACTTGGGGAGATCGGATGT3′ | 60.4 | 176 |
| R: 5′CTGGGCCACTTTAAGTCTAGC3′ | 60.1 | ||
| GPX4 | F: 5′GAGGCAAGACCGAAGTAAACTAC3′ | 60.6 | 100 |
| R: 5′CCGAACTGGTTACACGGGAA3′ | 61.8 | ||
| SLC7A11 | F: 5′TCTCCAAAGGAGGTTACCTGC3′ | 61.1 | 123 |
| R: 5′AGACTCCCCTCAGTAAAGTGAC3′ | 60.5 | ||
| GCLC | F: 5′GGAGGAAACCAAGCGCCAT3′ | 62.7 | 79 |
| R: 5′CTTGACGGCGTGGTAGATGT3′ | 61.9 | ||
| GCLM | F: 5′TGTCTTGGAATGCACTGTATCTC3′ | 60.0 | 239 |
| R: 5′CCCAGTAAGGCTGTAAATGCTC3′ | 60.7 | ||
| IL1b | F: 5′ATGATGGCTTATTACAGTGGCAA3′ | 60.0 | 132 |
| R: 5′GTCGGAGATTCGTAGCTGGA3′ | 60.8 | ||
| IL6 | F: 5′ACTCACCTCTTCAGAACGAATTG3′ | 60.2 | 149 |
| R: 5′CCATCTTTGGAAGGTTCAGGTTG3′ | 61.3 | ||
| TNFα | F: 5′CCTCTCTCTAATCAGCCCTCTG3′ | 60.8 | 220 |
| R: 5′GAGGACCTGGGAGTAGATGAG3′ | 60.2 | ||
| IL8 | F: 5′TTTTGCCAAGGAGTGCTAAAGA3′ | 60.1 | 194 |
| R: 5′AACCCTCTGCACCCAGTTTTC3′ | 62.5 | ||
| CCL2 | F: 5′CAGCCAGATGCAATCAATGCC3′ | 62.3 | 190 |
| R: 5′TGGAATCCTGAACCCACTTCT3′ | 60.4 | ||
| VEGFA | F: 5′AGGGCAGAATCATCACGAAGT3′ | 61.2 | 75 |
| R: 5′AGGGTCTCGATTGGATGGCA3′ | 62.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Z.-H.; Li, S.-Q.; Ke, J.-Y.; Wang, Y.; Zhao, M.-Z.; Li, J.; Li, M.-Q.; Zhu, Z.-L. Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis. Curr. Issues Mol. Biol. 2022, 44, 6189-6204. https://doi.org/10.3390/cimb44120422
Yi Z-H, Li S-Q, Ke J-Y, Wang Y, Zhao M-Z, Li J, Li M-Q, Zhu Z-L. Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis. Current Issues in Molecular Biology. 2022; 44(12):6189-6204. https://doi.org/10.3390/cimb44120422
Chicago/Turabian StyleYi, Zhi-Hui, Shu-Qing Li, Jun-Ya Ke, Yun Wang, Ming-Zhi Zhao, Jing Li, Ming-Qing Li, and Zhi-Ling Zhu. 2022. "Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis" Current Issues in Molecular Biology 44, no. 12: 6189-6204. https://doi.org/10.3390/cimb44120422
APA StyleYi, Z.-H., Li, S.-Q., Ke, J.-Y., Wang, Y., Zhao, M.-Z., Li, J., Li, M.-Q., & Zhu, Z.-L. (2022). Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis. Current Issues in Molecular Biology, 44(12), 6189-6204. https://doi.org/10.3390/cimb44120422






