Human Blood Extracellular Vesicles Activate Transcription of NF-kB-Dependent Genes in A549 Lung Adenocarcinoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Blood Donors
2.3. Collection of Human Blood
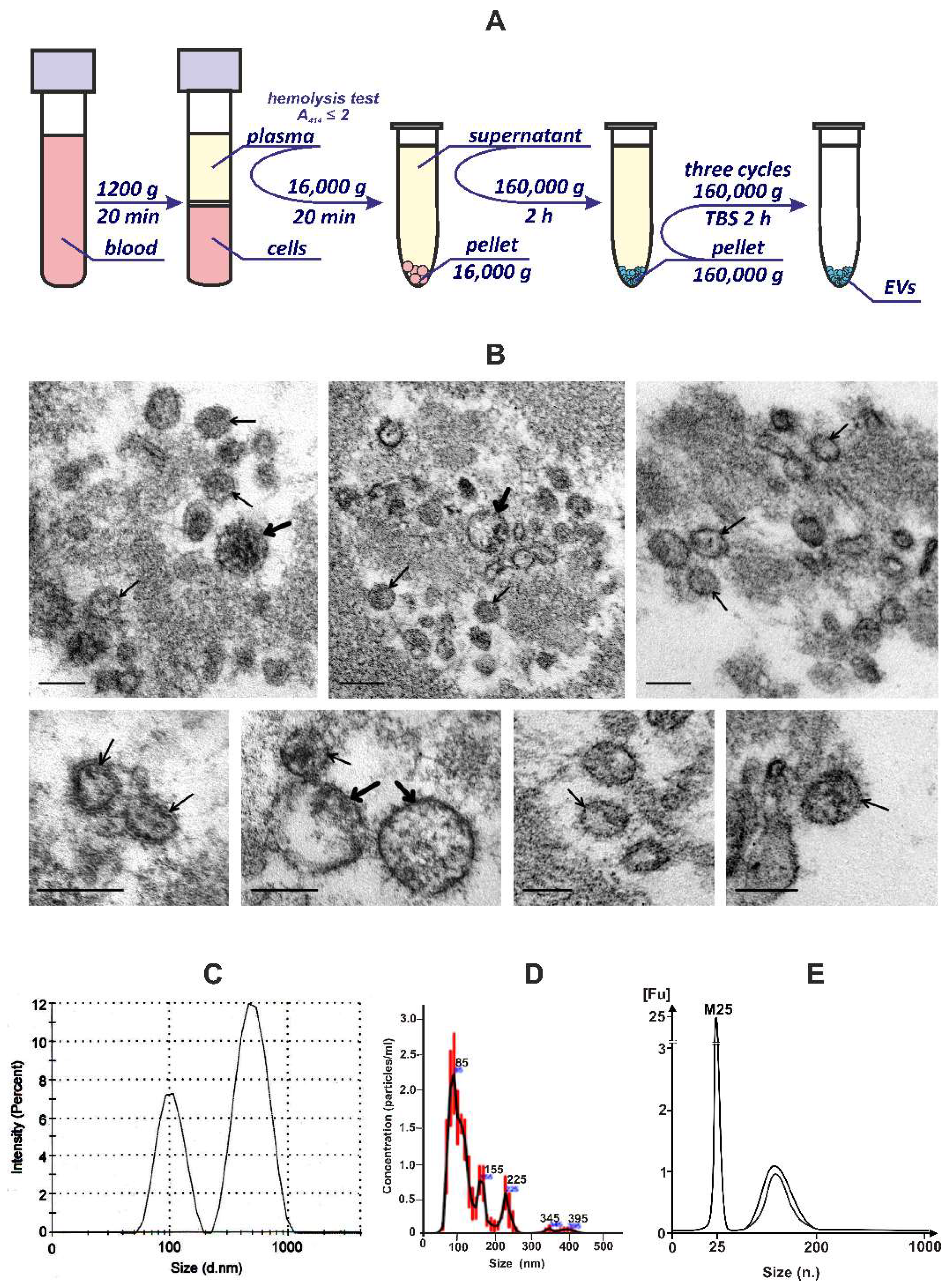
2.4. Plasma EVs Isolation and Purification
2.5. Dynamic Light Scattering Analysis (DLS)
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. Transmission Electron Microscopy (TEM)
2.8. Flow Cytometry Analysis
2.9. Cell Culture
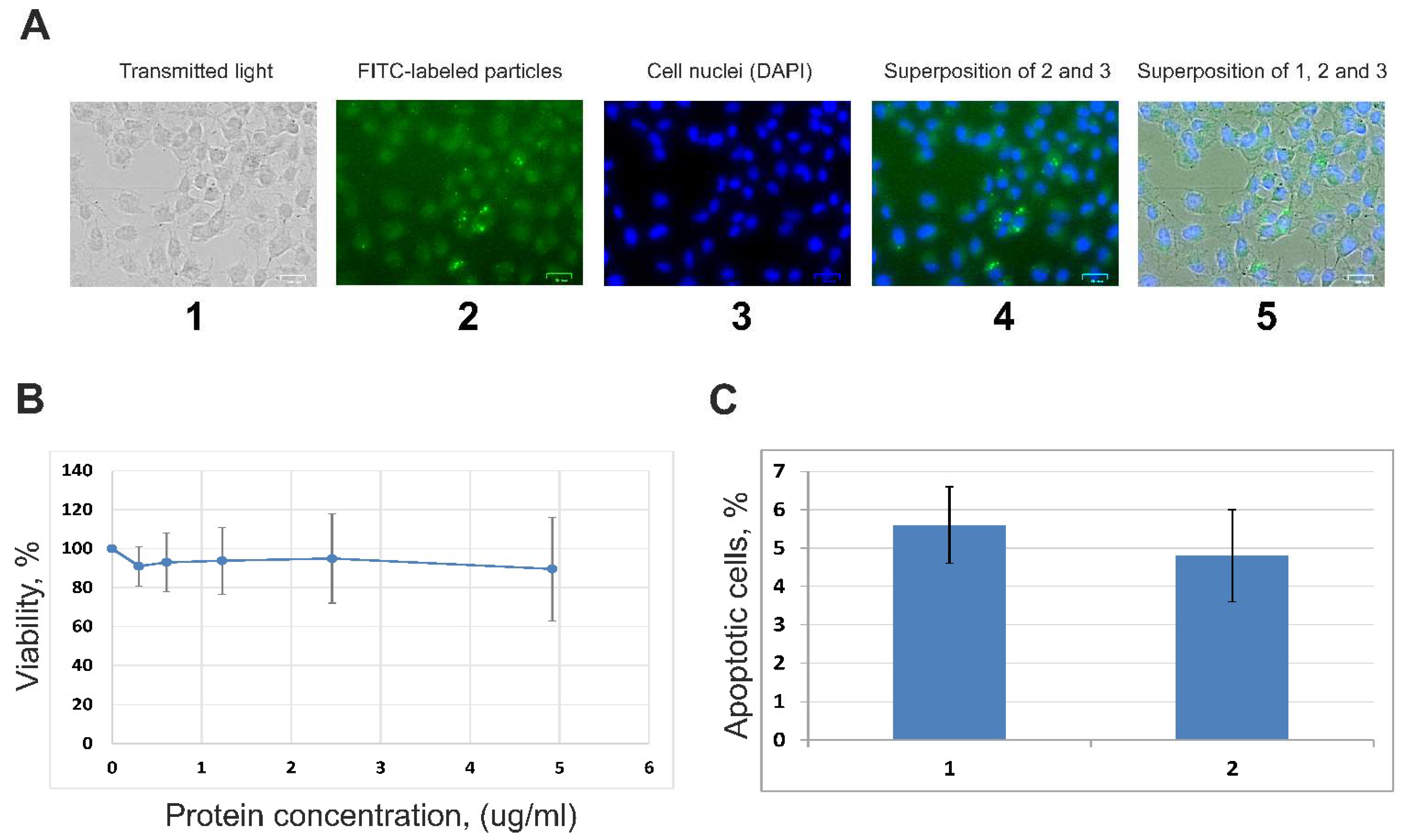
2.10. Fluorescence Microscopy Analysis
2.11. Viability of A549 Cells (MTT Assay)
2.12. Proapoptotic Changes of A549 Cells (FCM)
2.13. RNAs Extraction, cDNA Library Construction, Illumina Sequencing, and Differential Gene Expression Analysis
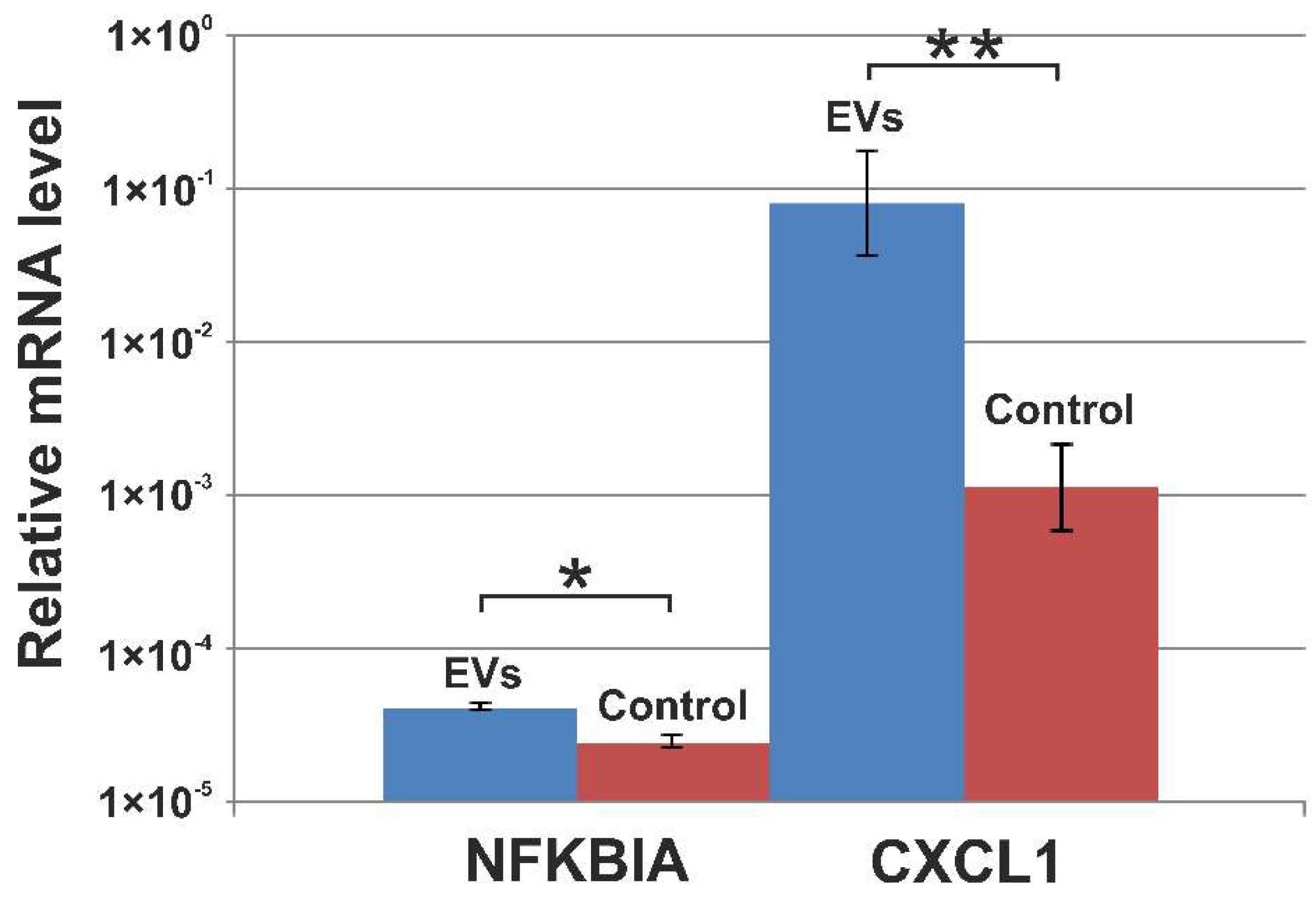
2.14. Validation of mRNA Transcripts with qRT-PCR
3. Results
3.1. Isolation and Characterization of EVs from the Blood Plasma of Healthy Donors
3.2. Internalization of EVs by A549 Cells and Influence of Vesicles on the Cells’ Viability
3.3. RNAs of Human Blood Extracellular Vesicles
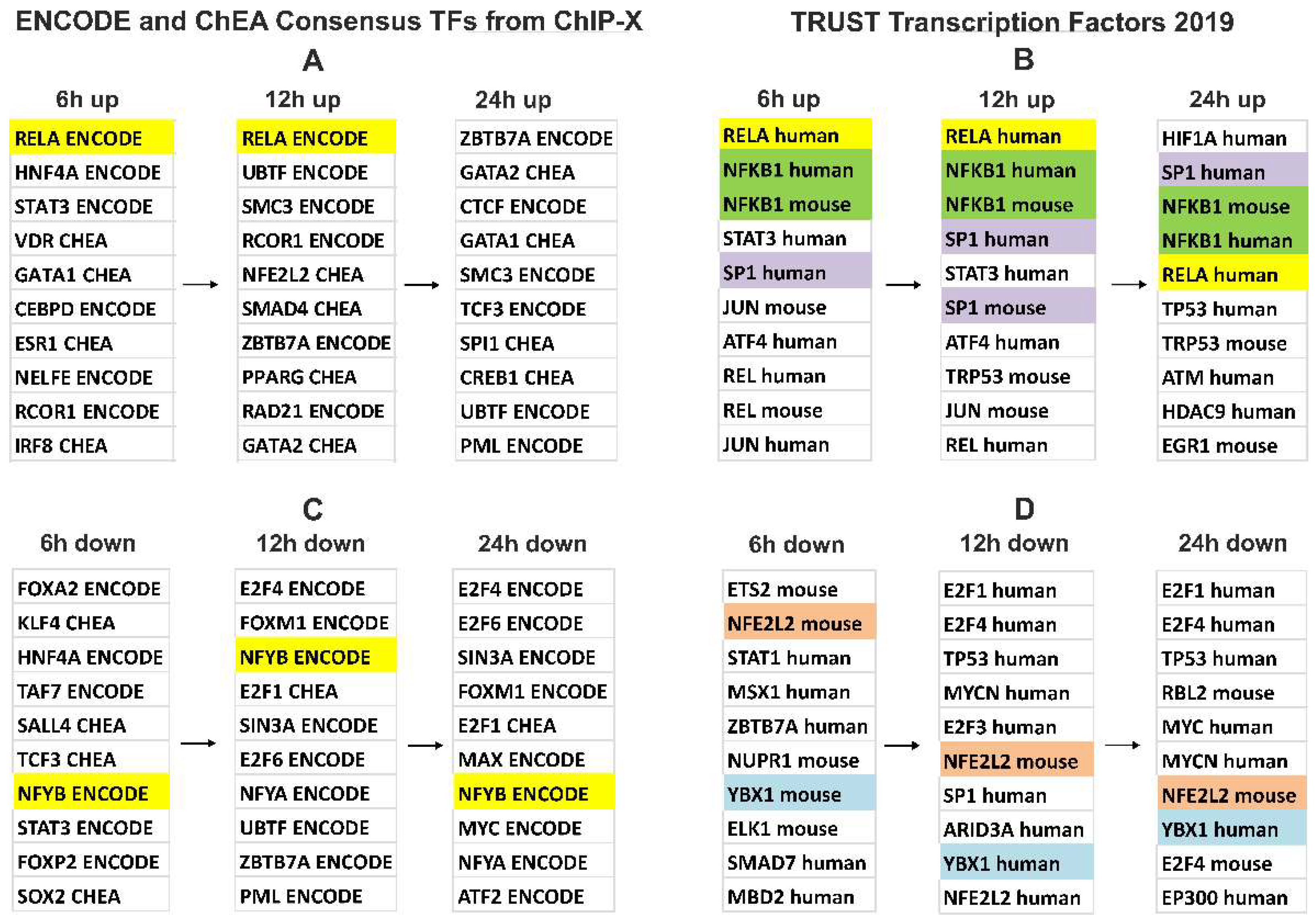
3.4. Gene Expression Changes in A549 Cells Treated with Blood EVs
3.5. Verification of NGS Data with qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Rajasingh, S.; Drosos, N.; Zhou, Z.; Dawn, B.; Rajasingh, J. Exosomes: New Molecular Targets of Diseases. Acta Pharmacol. Sin. 2018, 39, 501–513. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Giannessi, F.; Percario, Z.A.; Affabris, E. An Emerging Interplay between Extracellular Vesicles and Cytokines. Cytokine Growth Factor Rev. 2020, 51, 49–60. [Google Scholar] [CrossRef]
- Fitzner, D.; Schnaars, M.; Van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective Transfer of Exosomes from Oligodendrocytes to Microglia by Macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.G.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C.; et al. Endocytosis, Intracellular Sorting, and Processing of Exosomes by Dendritic Cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef]
- Montecalvo, A.; Larregina, A.T.; Shufesky, W.J.; Stolz, D.B.; Sullivan, M.L.G.; Karlsson, J.M.; Baty, C.J.; Gibson, G.A.; Erdos, G.; Wang, Z.; et al. Mechanism of Transfer of Functional MicroRNAs between Mouse Dendritic Cells via Exosomes. Blood 2012, 119, 756–766. [Google Scholar] [CrossRef]
- Escrevente, C.; Keller, S.; Altevogt, P.; Costa, J. Interaction and Uptake of Exosomes by Ovarian Cancer Cells. BMC Cancer 2011, 11, 108. [Google Scholar] [CrossRef]
- Souza, A.G.; Colli, L.M. Extracellular Vesicles and Interleukins: Novel Frontiers in Diagnostic and Therapeutic for Cancer. Front. Immunol. 2022, 13, 836922. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, K.K.; Zhang, J.; Xiao, B.; Huang, Z.; Ju, C.; Sun, J.; Zhang, F.; Lv, X.-B.; Huang, G. The Decade of Exosomal Long RNA Species: An Emerging Cancer Antagonist. Mol. Cancer 2018, 17, 75. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in Extracellular Vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, e1413. [Google Scholar] [CrossRef] [PubMed]
- Quesenberry, P.J.; Aliotta, J.; Deregibus, M.C.; Camussi, G. Role of Extracellular RNA-Carrying Vesicles in Cell Differentiation and Reprogramming. Stem Cell Res. Ther. 2015, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borràs, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed]
- Rodosthenous, R.S.; Hutchins, E.; Reiman, R.; Yeri, A.S.; Srinivasan, S.; Whitsett, T.G.; Ghiran, I.; Silverman, M.G.; Laurent, L.C.; Van Keuren-Jensen, K.; et al. Profiling Extracellular Long RNA Transcriptome in Human Plasma and Extracellular Vesicles for Biomarker Discovery. iScience 2020, 23, 101182. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Kirschner, M.B.; Kao, S.C.; Edelman, J.J.; Armstrong, N.J.; Vallely, M.P.; van Zandwijk, N.; Reid, G. Haemolysis during Sample Preparation Alters MicroRNA Content of Plasma. PLoS ONE 2011, 6, e24145. [Google Scholar] [CrossRef]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyl-2H-Tetrazolium Bromide (MTT) Assay When Compared to Three Commonly Used Cell Enumeration Assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-Seq Reveals Unannotated Transcripts and Isoform Switching during Cell Differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.W.; Mullikin, J.C. QoRTs: A Comprehensive Toolset for Quality Control and Data Processing of RNA-Seq Experiments. BMC Bioinform. 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Al Amir Dache, Z.; Otandault, A.; Tanos, R.; Pastor, B.; Meddeb, R.; Sanchez, C.; Arena, G.; Lasorsa, L.; Bennett, A.; Grange, T.; et al. Blood Contains Circulating Cell-free Respiratory Competent Mitochondria. FASEB J. 2020, 34, 3616–3630. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Spakova, T.; Janockova, J.; Rosocha, J. Characterization and Therapeutic Use of Extracellular Vesicles Derived from Platelets. Int. J. Mol. Sci. 2021, 22, 9701. [Google Scholar] [CrossRef]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and Phenotyping of Cellular Vesicles Using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef]
- Arraud, N.; Linares, R.; Tan, S.; Gounou, C.; Pasquet, J.M.; Mornet, S.; Brisson, A.R. Extracellular Vesicles from Blood Plasma: Determination of Their Morphology, Size, Phenotype and Concentration. J. Thromb. Haemost. 2014, 12, 614–627. [Google Scholar] [CrossRef]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-like Vesicles, and Apoptotic Bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes Provide a Protective and Enriched Source of MiRNA for Biomarker Profiling Compared to Intracellular and Cell-Free Blood. J. Extracell. Vesicles 2014, 3, 23743. [Google Scholar] [CrossRef] [PubMed]
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal MicroRNA: A Diagnostic Marker for Lung Cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Li, D.; Rahman, I. Small RNA-Sequence Analysis of Plasma-Derived Extracellular Vesicle MiRNAs in Smokers and Patients with Chronic Obstructive Pulmonary Disease as Circulating Biomarkers. J. Extracell. Vesicles 2019, 8, 1684816. [Google Scholar] [CrossRef]
- Bortoluzzi, S.; Lovisa, F.; Gaffo, E.; Mussolin, L. Small RNAs in Circulating Exosomes of Cancer Patients: A Minireview. High-Throughput 2017, 6, 13. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Chen, B.; Zhao, J.; Yu, S.; Tang, Y.; Zheng, Q.; Li, Y.; Wang, P.; He, X.; et al. ExoRBase: A Database of CircRNA, LncRNA and MRNA in Human Blood Exosomes. Nucleic Acids Res. 2018, 46, D106–D112. [Google Scholar] [CrossRef]
- Preußer, C.; Hung, L.H.; Schneider, T.; Schreiner, S.; Hardt, M.; Moebus, A.; Santoso, S.; Bindereif, A. Selective Release of CircRNAs in Platelet-Derived Extracellular Vesicles. J. Extracell. Vesicles 2018, 7, 1424473. [Google Scholar] [CrossRef]
- Kumar, S.R.; Kimchi, E.T.; Manjunath, Y.; Gajagowni, S.; Stuckel, A.J.; Kaifi, J.T. RNA Cargos in Extracellular Vesicles Derived from Blood Serum in Pancreas Associated Conditions. Sci. Rep. 2020, 10, 2800. [Google Scholar] [CrossRef]
- Huang, X.; Yuan, T.; Tschannen, M.; Sun, Z.; Jacob, H.; Du, M.; Liang, M.M.; Dittmar, R.L.; Liu, Y.; Liang, M.M.; et al. Characterization of Human Plasma-Derived Exosomal RNAs by Deep Sequencing. BMC Genom. 2013, 14, 319. [Google Scholar] [CrossRef]
- Amorim, M.G.; Valieris, R.; Drummond, R.D.; Pizzi, M.P.; Freitas, V.M.; Sinigaglia-Coimbra, R.; Calin, G.A.; Pasqualini, R.; Arap, W.; Silva, I.T.; et al. A Total Transcriptome Profiling Method for Plasma-Derived Extracellular Vesicles: Applications for Liquid Biopsies. Sci. Rep. 2017, 7, 14395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Yu, S.; Wang, Z.; He, X.; Su, Y.; Guo, T.; Sheng, H.; Chen, J.; Zheng, Q.; et al. Extracellular Vesicles Long RNA Sequencing Reveals Abundant MRNA, CircRNA, and LncRNA in Human Blood as Potential Biomarkers for Cancer Diagnosis. Clin. Chem. 2019, 65, 798–808. [Google Scholar] [CrossRef]
- Sun, S.-C. The Noncanonical NF-ΚB Pathway. Immunol. Rev. 2012, 246, 125–140. [Google Scholar] [CrossRef]
- Perkins, N.D. Integrating Cell-Signalling Pathways with NF-KappaB and IKK Function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Schweiger, M.W.; Li, M.; Giovanazzi, A.; Fleming, R.L.; Tabet, E.I.; Nakano, I.; Würdinger, T.; Chiocca, E.A.; Tian, T.; Tannous, B.A. Extracellular Vesicles Induce Mesenchymal Transition and Therapeutic Resistance in Glioblastomas through NF-ΚB/STAT3 Signaling. Adv. Biosyst. 2020, 4, 1900312. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mao, J.-H.; Wang, B.-Y.; Wang, L.-X.; Wen, H.-Y.; Xu, L.-J.; Fu, J.-X.; Yang, H. Exosomal MiR-1910-3p Promotes Proliferation, Metastasis, and Autophagy of Breast Cancer Cells by Targeting MTMR3 and Activating the NF-ΚB Signaling Pathway. Cancer Lett. 2020, 489, 87–99. [Google Scholar] [CrossRef]
- Li, J.; Tan, M.; Xiang, Q.; Zhou, Z.; Yan, H. Thrombin-Activated Platelet-Derived Exosomes Regulate Endothelial Cell Expression of ICAM-1 via MicroRNA-223 during the Thrombosis-Inflammation Response. Thromb. Res. 2017, 154, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Bretz, N.P.; Ridinger, J.; Rupp, A.K.; Rimbach, K.; Keller, S.; Rupp, C.; Marmé, F.; Umansky, L.; Umansky, V.; Eigenbrod, T.; et al. Body Fluid Exosomes Promote Secretion of Inflammatory Cytokines in Monocytic Cells via Toll-Like Receptor Signaling. J. Biol. Chem. 2013, 288, 36691–36702. [Google Scholar] [CrossRef]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs Bind to Toll-like Receptors to Induce Prometastatic Inflammatory Response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef]
- Ye, W.; Tang, X.; Yang, Z.; Liu, C.; Zhang, X.; Jin, J.; Lyu, J. Plasma-Derived Exosomes Contribute to Inflammation via the TLR9-NF-ΚB Pathway in Chronic Heart Failure Patients. Mol. Immunol. 2017, 87, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Biemmi, V.; Milano, G.; Ciullo, A.; Cervio, E.; Burrello, J.; Dei Cas, M.; Paroni, R.; Tallone, T.; Moccetti, T.; Pedrazzini, G.; et al. Inflammatory Extracellular Vesicles Prompt Heart Dysfunction via TRL4-Dependent NF-ΚB Activation. Theranostics 2020, 10, 2773–2790. [Google Scholar] [CrossRef] [PubMed]


| Gene | Sequences |
|---|---|
| GAPDH | F: GAAGGTGAAGGTCGGAGT |
| R: GAAGATGGTGATGGGATTTC | |
| NFKBIA | F: TGTCTACACTTAGCCTCTATC |
| R: TCTGTGAACTCCGTGAACTC | |
| CXCL1 | F: AGGCAGGGGAATGTATGTGC |
| R: AAGCCCCTTTGTTCTAAGCCA |
| Markers | Content, % |
|---|---|
| CD63 | 51.4 ± 12 |
| CD3 | 51 ± 22 |
| СD79а | 5.0 ± 3.5 |
| CD41a | 43.6 ± 11 |
| RNA Class | Reads × 10−3 (Ave) | Сontribution (%) |
|---|---|---|
| rRNA | 2792 | 72 |
| tRNA, U1-U12 snRNA, YRNA, scRNA, transcribed genomic repeats | 90 | 2.33 |
| Mitochondrial transcripts | 25 | 0.66 |
| Human genome transcripts (hg19) including RefGene RNA (mRNAs, lncRNAs, snoRNAs, and others) | 968 | 25 |
| Total | 3875 | 100 |
| Genes | Annotation |
|---|---|
| Upregulated | |
| CCL2 | C-C motif chemokine ligand 2 |
| CXCL1 | C-X-C motif chemokine ligand 1 |
| CXCL2 | C-X-C motif chemokine ligand 2 |
| CXCL3 | C-X-C motif chemokine ligand 3 |
| CXCL5 | C-X-C motif chemokine ligand 5 |
| CXCL8 | C-X-C motif chemokine ligand 8 |
| IL32 | interleukin 32 |
| NFKB1 | nuclear factor kappa B subunit 1 |
| NFKB2 | nuclear factor kappa B subunit 2 |
| NFKBIA | NFKB inhibitor alpha |
| RELA | RELA proto-oncogene, NF-kB subunit |
| RELB | RELB proto-oncogene, NF-kB subunit |
| RELT | RELT TNF receptor |
| TNFAIP1 | TNF alpha-induced protein 1 |
| TNFAIP2 | TNF alpha-induced protein 2 |
| TNFAIP3 | TNF alpha-induced protein 3 |
| TNFAIP8 | TNF Alpha-induced protein 8 |
| TNFRSF10B | TNF receptor superfamily member 10b |
| TNFRSF10D | TNF receptor superfamily member 10d |
| TNFRSF12A | TNF receptor superfamily member 12A |
| TNFRSF1A | TNF receptor superfamily member 1A |
| TNFRSF9 | TNF receptor superfamily member 9 |
| TNIP1 | TNFAIP3 interacting protein 1 |
| Downregulated | |
| ATF5 | activating transcription factor 5 |
| CHD4 | chromodomain helicase DNA binding protein 4 |
| CNBP | CCHC-type zinc finger nucleic acid binding protein |
| CSRP1 | cysteine and glycine-rich protein 1 |
| ETV4 | variant transcription factor 4 |
| KANK2 | KN motif and ankyrin repeat domains 2 |
| TRIM28 | tripartite motif containing 28 |
| UBTF | upstream binding transcription factor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savinovskaya, Y.I.; Nushtaeva, A.A.; Savelyeva, A.V.; Morozov, V.V.; Ryabchikova, E.I.; Kuligina, E.V.; Richter, V.A.; Semenov, D.V. Human Blood Extracellular Vesicles Activate Transcription of NF-kB-Dependent Genes in A549 Lung Adenocarcinoma Cells. Curr. Issues Mol. Biol. 2022, 44, 6028-6045. https://doi.org/10.3390/cimb44120411
Savinovskaya YI, Nushtaeva AA, Savelyeva AV, Morozov VV, Ryabchikova EI, Kuligina EV, Richter VA, Semenov DV. Human Blood Extracellular Vesicles Activate Transcription of NF-kB-Dependent Genes in A549 Lung Adenocarcinoma Cells. Current Issues in Molecular Biology. 2022; 44(12):6028-6045. https://doi.org/10.3390/cimb44120411
Chicago/Turabian StyleSavinovskaya, Yulya I., Anna A. Nushtaeva, Anna V. Savelyeva, Vitaliy V. Morozov, Elena I. Ryabchikova, Elena V. Kuligina, Vladimir A. Richter, and Dmitriy V. Semenov. 2022. "Human Blood Extracellular Vesicles Activate Transcription of NF-kB-Dependent Genes in A549 Lung Adenocarcinoma Cells" Current Issues in Molecular Biology 44, no. 12: 6028-6045. https://doi.org/10.3390/cimb44120411
APA StyleSavinovskaya, Y. I., Nushtaeva, A. A., Savelyeva, A. V., Morozov, V. V., Ryabchikova, E. I., Kuligina, E. V., Richter, V. A., & Semenov, D. V. (2022). Human Blood Extracellular Vesicles Activate Transcription of NF-kB-Dependent Genes in A549 Lung Adenocarcinoma Cells. Current Issues in Molecular Biology, 44(12), 6028-6045. https://doi.org/10.3390/cimb44120411







