Beneficial Effects of RNS60 in Cardiac Ischemic Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Cardiac Hypoxia in Mice
2.2. Troponin T Concentration and Creatine Kinase Activity in Mouse Serum after the Hypoxic Event
2.3. Cardioplegic Arrest of the Rat Heart
2.4. The Mechanical Function of the Rat Heart
2.5. Sample Preparation and Metabolic Assays
Statistical Analysis
3. Results
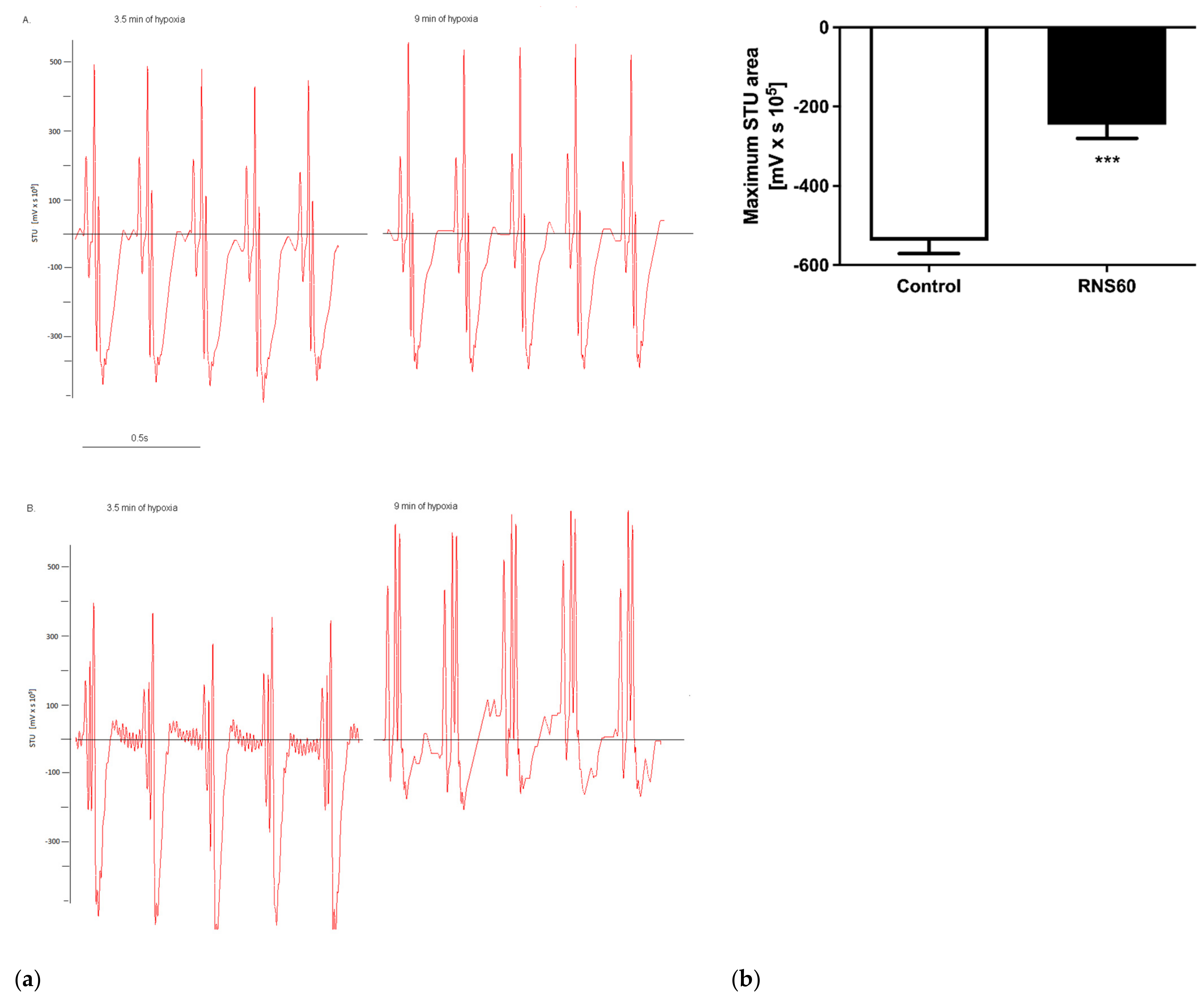
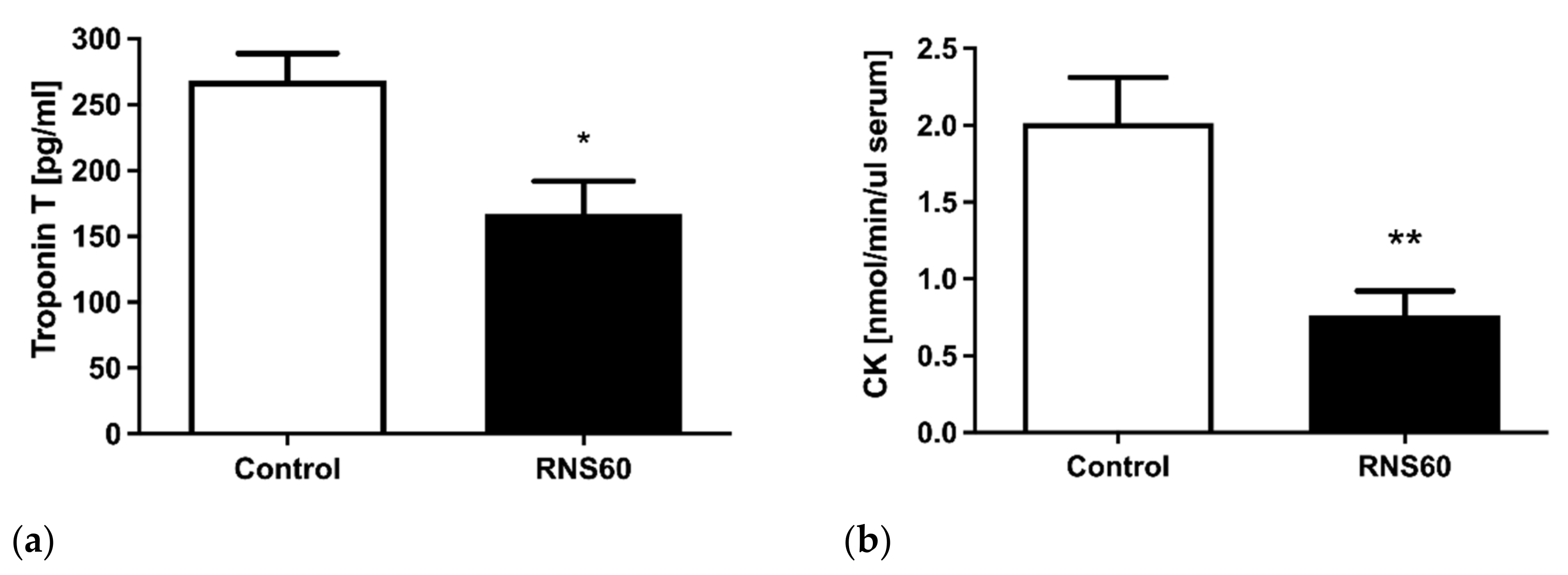
3.1. Electrocardiographic, Troponin T, and Creatine Kinase Activity Changes during and after Hypoxic Stress in ApoE−/−LDLr−/− Mice
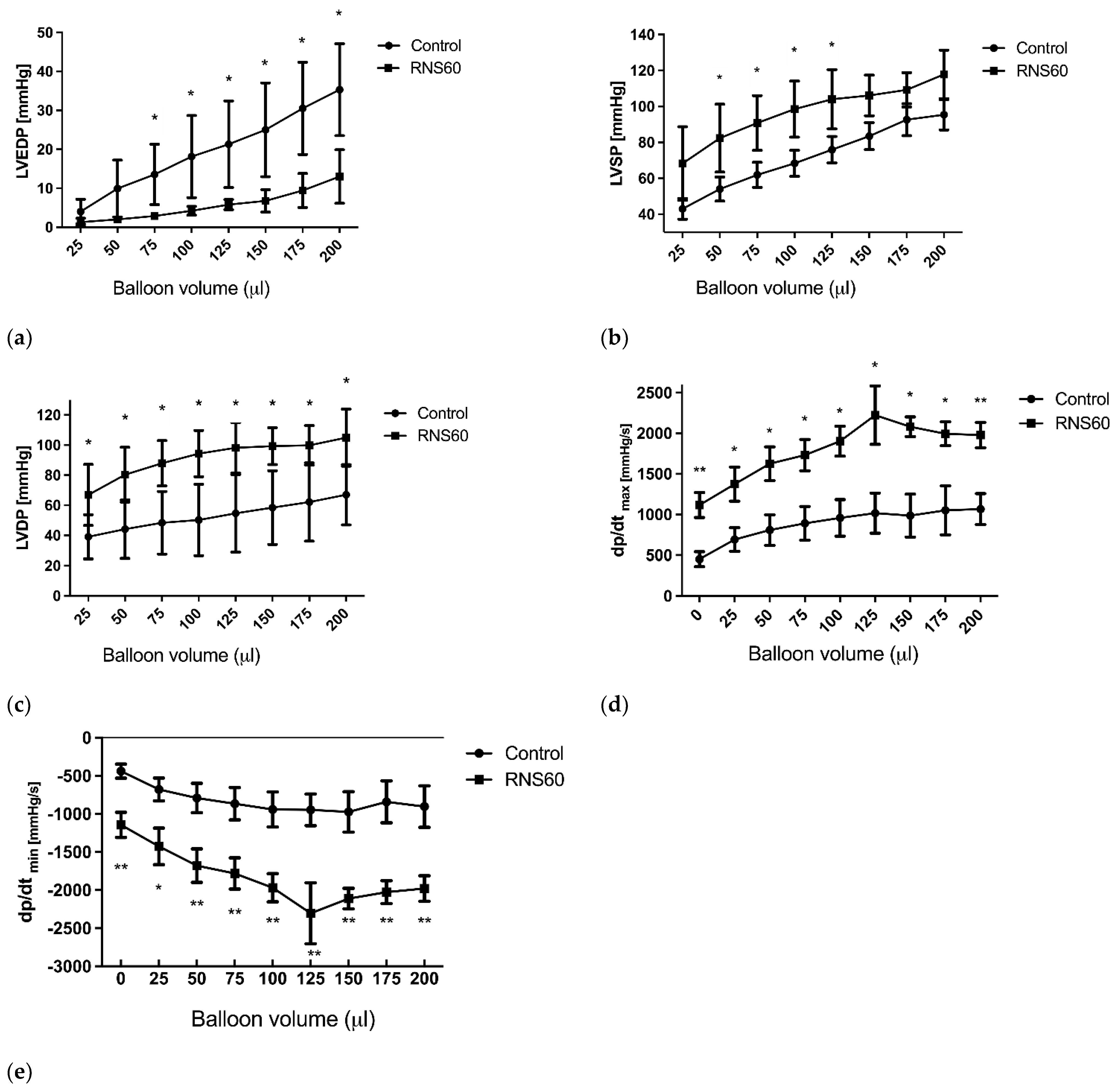
3.2. The Mechanical Function of the Rat Heart
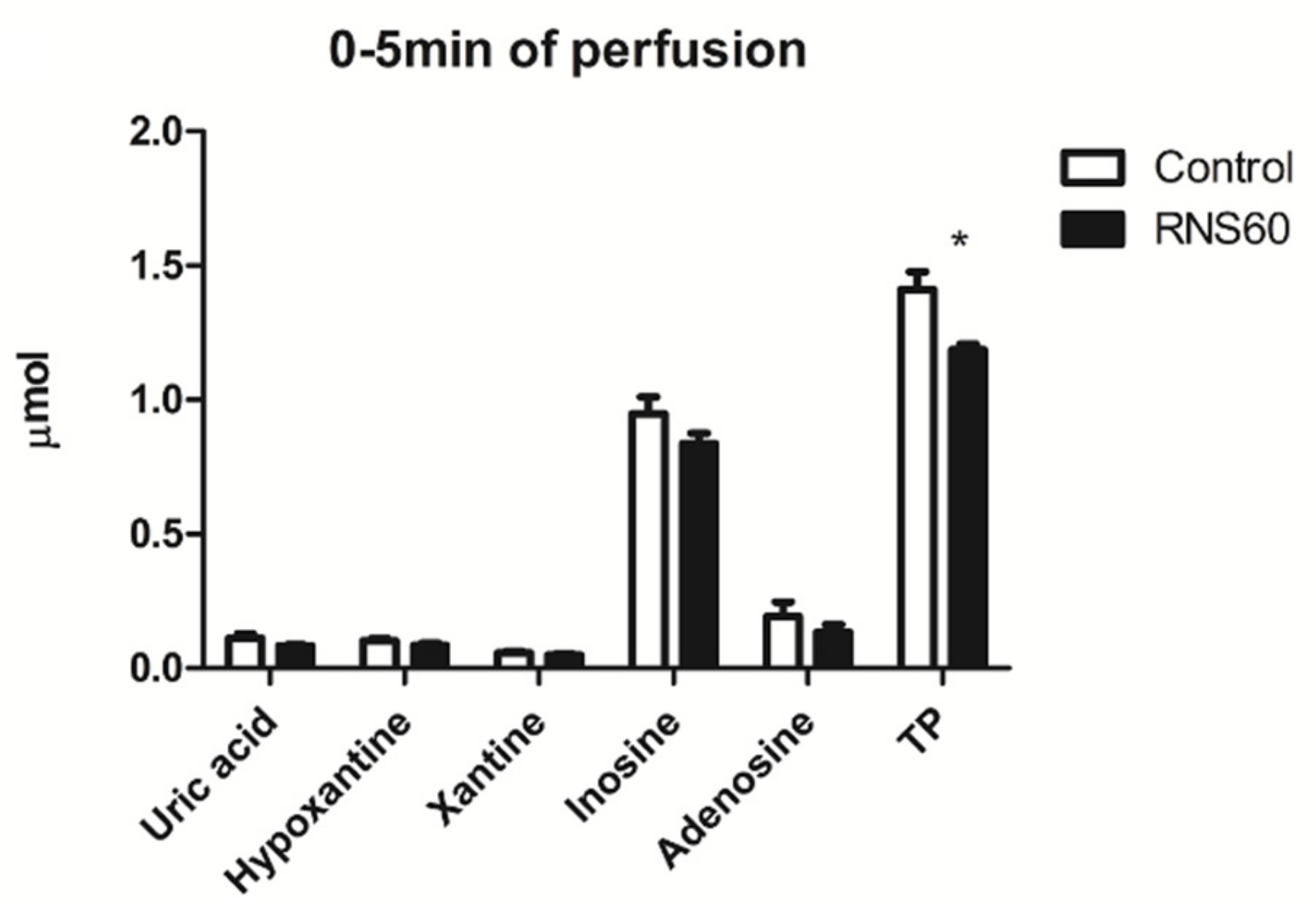
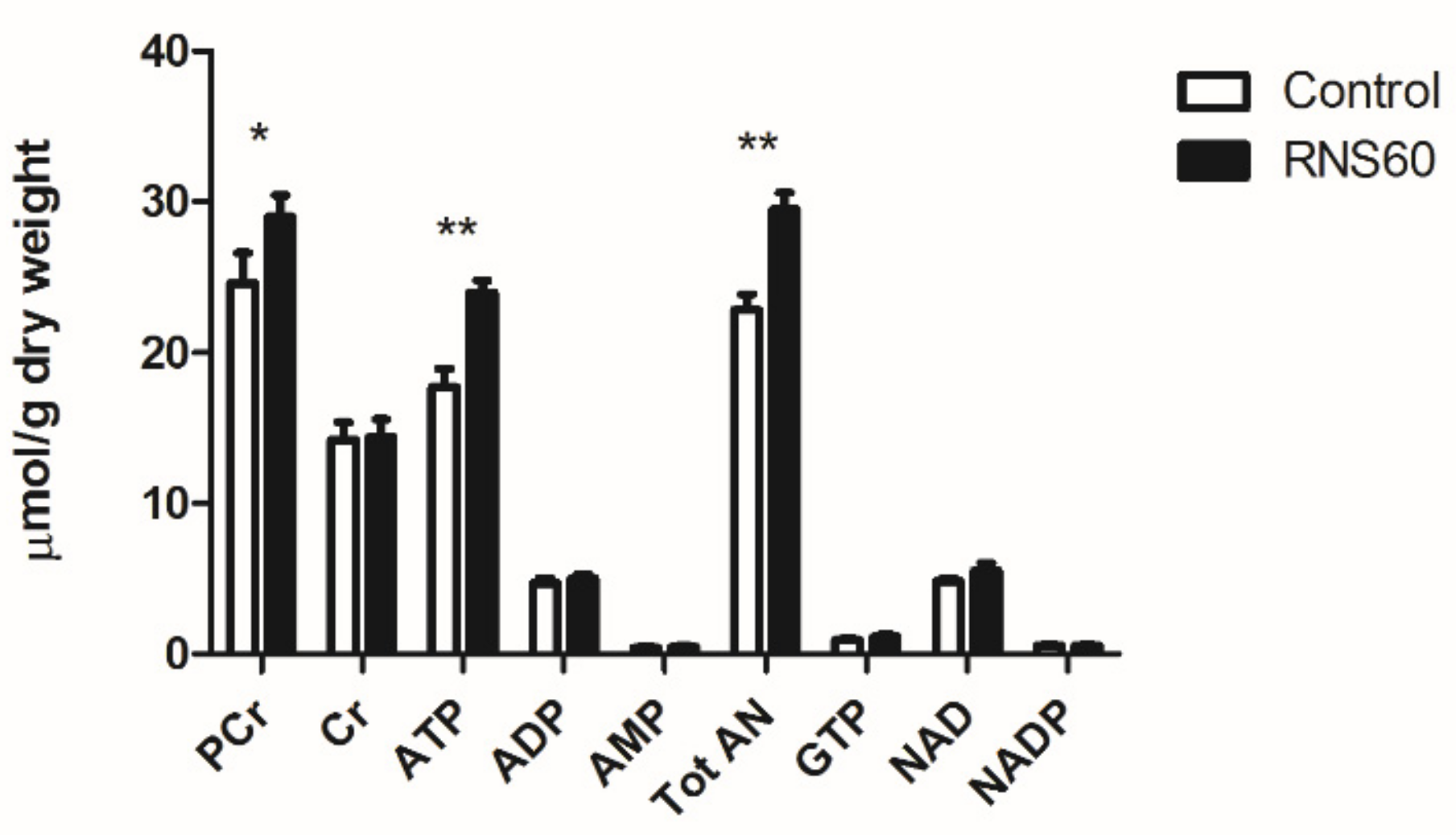
3.3. Metabolic Markers of Injury in Rat Heart
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorescu, D.; Griendling, K.K. Reactive Oxygen Species, Mitochondria, and NAD(P)H Oxidases in the Development and Progression of Heart Failure. Congest. Heart Fail. 2002, 8, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J. Oxidative Stress: The Paradox of Aerobic Life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Khasnavis, S.; Jana, A.; Roy, A.; Mazumder, M.; Bhushan, B.; Wood, T.; Ghosh, S.; Watson, R.; Pahan, K. Suppression of Nuclear Factor-KappaB Activation and Inflammation in Microglia by Physically Modified Saline. J. Biol. Chem. 2012, 287, 29529–29542. [Google Scholar] [CrossRef] [PubMed]
- Khasnavis, S.; Roy, A.; Ghosh, S.; Watson, R.; Pahan, K. Protection of Dopaminergic Neurons in a Mouse Model of Parkinson’s Disease by a Physically-Modified Saline Containing Charge-Stabilized Nanobubbles. J. Neuroimmune Pharmacol. 2014, 9, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Sims, B.; Powers, R.E.; Sabina, R.L.; Theibert, A.B. Elevated Adenosine Monophosphate Deaminase Activity in Alzheimer’s Disease Brain. Neurobiol. Aging 1998, 19, 385–391. [Google Scholar] [CrossRef]
- Mondal, S.; Martinson, J.A.; Ghosh, S.; Watson, R.; Pahan, K. Protection of Tregs, Suppression of Th1 and Th17 Cells, and Amelioration of Experimental Allergic Encephalomyelitis by a Physically-Modified Saline. PLoS ONE 2012, 7, e51869. [Google Scholar] [CrossRef]
- Choi, S.; Yu, E.; Kim, D.S.; Sugimori, M.; Llinas, R.R. RNS60, a Charge-Stabilized Nanostructure Saline Alters Xenopus Laevis Oocyte Biophysical Membrane Properties by Enhancing Mitochondrial ATP Production. Physiol. Rep. 2015, 3, e12261. [Google Scholar] [CrossRef]
- Kalmes, A.; Ghosh, S.; Watson, R. Abstract 17400: Charge-Stabilized Nanostructures Reduce Ischemia-Reperfusion Injury in A Pig Model in Vivo. Circulation 2011, 124, A17400. [Google Scholar]
- Kalmes, A.; Anderson-Haley, M.; Ghosh, S.; Watson, R. Abstract 18804: A Saline Containing Charge-Stabilized Nanostructures Inhibits Apoptosis of Cardiomyocytes Subjected to Ischemia/Reperfusion Injury. Circulation 2013, 128, A18804. [Google Scholar]
- Hemdahl, A.L.; Caligiuri, G.; Hansson, G.K.; Thoren, P. Electrocardiographic Characterization of Stress-Induced Myocardial Infarction in Atherosclerotic Mice. Acta Physiol. Scand. 2005, 184, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, G.; Levy, B.; Pernow, J.; Thoren, P.; Hansson, G.K. Myocardial Infarction Mediated by Endothelin Receptor Signaling in Hypercholesterolemic Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 6920–6924. [Google Scholar] [CrossRef]
- Zabielska, M.A.; Adamus, J.; Kowalski, R.; Gebicki, J.; Slominska, E.M.; Khapley, Z.; Smolenski, R.T. Cardioprotective Effect of N-Methylnicotinamide Salt of Pyruvate in Experimental Model of Cardiac Hypoxia. Pharmacol. Reports 2017, 70, 378–384. [Google Scholar] [CrossRef] [PubMed]
- de Groot, H.; Rauen, U. Ischemia-Reperfusion Injury: Processes in Pathogenetic Networks: A Review. Transplant. Proc. 2007, 39, 481–484. [Google Scholar] [CrossRef]
- Banner, N.R.; Thomas, H.L.; Curnow, E.; Hussey, J.C.; Rogers, C.A.; Bonser, R.S. The Importance of Cold and Warm Cardiac Ischemia for Survival After Heart Transplantation. Transplantation 2008, 86, 542–547. [Google Scholar] [CrossRef]
- Ackemann, J.; Gross, W.; Mory, M.; Schaefer, M.; Gebhard, M.-M. Celsior Versus Custodiol: Early Postischemic Recovery After Cardioplegia and Ischemia at 5 °C. Ann. Thorac. Surg. 2002, 74, 522–529. [Google Scholar] [CrossRef][Green Version]
- Edelman, J.J.B.; Seco, M.; Dunne, B.; Matzelle, S.J.; Murphy, M.; Joshi, P.; Yan, T.D.; Wilson, M.K.; Bannon, P.G.; Vallely, M.P.; et al. Custodiol for Myocardial Protection and Preservation: A Systematic Review. Ann. Cardiothorac. Surg. 2013, 2, 717–728. [Google Scholar]
- Smolenski, R.T.; Lachno, D.R.; Ledingham, S.J.; Yacoub, M.H. Determination of Sixteen Nucleotides, Nucleosides and Bases Using High-Performance Liquid Chromatography and Its Application to the Study of Purine Metabolism in Hearts for Transplantation. J. Chromatogr. 1990, 527, 414–420. [Google Scholar] [CrossRef]
- Rao, V.T.S.; Khan, D.; Jones, R.G.; Nakamura, D.S.; Kennedy, T.E.; Cui, Q.-L.; Rone, M.B.; Healy, L.M.; Watson, R.; Ghosh, S.; et al. Potential Benefit of the Charge-Stabilized Nanostructure Saline RNS60 for Myelin Maintenance and Repair. Sci. Rep. 2016, 6, 30020. [Google Scholar] [CrossRef]
- Roy, A.; Modi, K.K.; Khasnavis, S.; Ghosh, S.; Watson, R.; Pahan, K. Enhancement of Morphological Plasticity in Hippocampal Neurons by a Physically Modified Saline via Phosphatidylinositol-3 Kinase. PLoS ONE 2014, 9, e101883. [Google Scholar] [CrossRef] [PubMed]
- Hemdahl, A.L.; Falk, E.; Thoren, P.; Hansson, G.K. Thrombin Inhibitor Reduces Myocardial Infarction in ApoE-/- x LDLR-/- Mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H872–H877. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, W.; Katus, H.A.; Ravkilde, J.; Hamm, C.; Jørgensen, P.J.; Peheim, E.; Ljungdahl, L.; Löfdahl, P. S-Troponin T in Suspected Lschemic Myocardial Injury Compared with Mass and Catalytic Concentrations of S-Creatine Kinase Lsoenzyme MB. Clin. Chem. 1991, 37, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Jahania, M.S.; Sanchez, J.A.; Narayan, P.; Lasley, R.D.; Mentzer, R.M.; Demmy, T.L.; Biddle, J.S.; Bennet, L.E.; Walls, J.T.; Schmaltz, R.A.; et al. Heart Preservation for Transplantation: Principles and Strategies. Ann. Thorac. Surg. 1999, 68, 1983–1987. [Google Scholar] [CrossRef]
- Ogino, H.; Smolenski, R.T.; Zych, M.; Seymour, A.-M.L.; Yacoub, M.H. Influence of Preconditioning on Rat Heart Subjected to Prolonged Cardioplegic Arrest. Ann. Thorac. Surg. 1996, 62, 469–474. [Google Scholar] [CrossRef]
- Smolenski, R.T.; Raisky, O.; Slominska, E.M.; Abunasra, H.; Kalsi, K.K.; Jayakumar, J.; Suzuki, K.; Yacoub, M.H. Protection from Reperfusion Injury after Cardiac Transplantation by Inhibition of Adenosine Metabolism and Nucleotide Precursor Supply. Circulation 2001, 104, I246–I252. [Google Scholar] [CrossRef]
- Zabielska, M.A.; Borkowski, T.; Slominska, E.M.; Smolenski, R.T. Inhibition of AMP Deaminase as Therapeutic Target in Cardiovascular Pathology. Pharmacol. Reports 2015, 67, 682–688. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Zukowska, P.; Toczek, M.; Zabielska, M.; Lipinski, M.; Rybakowska, I.; Chlopicki, S.; Slominska, E.M.; Smolenski, R.T. Extracellular Nucleotide Catabolism in Aortoiliac Bifurcation of Atherosclerotic ApoE/LDLr Double Knock out Mice. Nucleosides Nucleotides Nucleic Acids 2014, 33, 323–328. [Google Scholar] [CrossRef]
- Zukowska, P.; Kutryb-Zajac, B.; Toczek, M.; Smolenski, R.T.; Slominska, E.M. The Role of Ecto-5′-Nucleotidase in Endothelial Dysfunction and Vascular Pathologies. Pharmacol. Rep. 2015, 67, 675–681. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Mateuszuk, L.; Zukowska, P.; Jasztal, A.; Zabielska, M.A.; Toczek, M.; Jablonska, P.; Zakrzewska, A.; Sitek, B.; Rogowski, J.; et al. Increased Activity of Vascular Adenosine Deaminase in Atherosclerosis and Therapeutic Potential of Its Inhibition. Cardiovasc. Res. 2016, 112, 590–605. [Google Scholar] [CrossRef]
- Katayama, O.; Ledingham, S.J.; Amrani, M.; Smolenski, R.T.; Lachno, D.R.; Jayakumar, J.; Yacoub, M.H. Functional and Metabolic Effects of Adenosine in Cardioplegia: Role of Temperature and Concentration. Ann. Thorac. Surg. 1997, 63, 449–455. [Google Scholar] [CrossRef]
- Stanley, W.C.; Chandler, M.P. Energy Metabolism in the Normal and Failing Heart: Potential for Therapeutic Interventions. Heart Fail. Rev. 2002, 7, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Kodde, I.F.; van der Stok, J.; Smolenski, R.T.; de Jong, J.W. Metabolic and Genetic Regulation of Cardiac Energy Substrate Preference. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Ingwall, J.S. Energy Metabolism in Heart Failure and Remodelling. Cardiovasc. Res. 2009, 81, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Guyton, R.A.; Dorsey, L.M.; Craver, J.M.; Bone, D.K.; Jones, E.L.; Murphy, D.A.; Hatcher, C.R. Improved Myocardial Recovery after Cardioplegic Arrest with an Oxygenated Crystalloid Solution. J. Thorac. Cardiovasc. Surg. 1985, 89, 877–887. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Garnier, A.; Veksler, V. Energy Metabolism in Heart Failure. J. Physiol. 2004, 555, 1–13. [Google Scholar] [CrossRef]
- Kalsi, K.K.; Smolenski, R.T.; Pritchard, R.D.; Khaghani, A.; Seymour, A.-M.L.; Yacoub, M.H. Energetics and Function of the Failing Human Heart with Dilated or Hypertrophic Cardiomyopathy. Eur. J. Clin. Investig. 1999, 29, 469–477. [Google Scholar] [CrossRef]
- Camelliti, P.; Al-Saud, S.A.; Smolenski, R.T.; Al-Ayoubi, S.; Bussek, A.; Wettwer, E.; Banner, N.R.; Bowles, C.T.; Yacoub, M.H.; Terracciano, C.M. Adult Human Heart Slices Are a Multicellular System Suitable for Electrophysiological and Pharmacological Studies. J. Mol. Cell. Cardiol. 2011, 51, 390–398. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabielska-Kaczorowska, M.A.; Wierzbicka, B.; Kalmes, A.; Slominska, E.M.; Yacoub, M.H.; Smolenski, R.T. Beneficial Effects of RNS60 in Cardiac Ischemic Injury. Curr. Issues Mol. Biol. 2022, 44, 4877-4887. https://doi.org/10.3390/cimb44100331
Zabielska-Kaczorowska MA, Wierzbicka B, Kalmes A, Slominska EM, Yacoub MH, Smolenski RT. Beneficial Effects of RNS60 in Cardiac Ischemic Injury. Current Issues in Molecular Biology. 2022; 44(10):4877-4887. https://doi.org/10.3390/cimb44100331
Chicago/Turabian StyleZabielska-Kaczorowska, Magdalena A., Barbara Wierzbicka, Andreas Kalmes, Ewa M. Slominska, Magdi H. Yacoub, and Ryszard T. Smolenski. 2022. "Beneficial Effects of RNS60 in Cardiac Ischemic Injury" Current Issues in Molecular Biology 44, no. 10: 4877-4887. https://doi.org/10.3390/cimb44100331
APA StyleZabielska-Kaczorowska, M. A., Wierzbicka, B., Kalmes, A., Slominska, E. M., Yacoub, M. H., & Smolenski, R. T. (2022). Beneficial Effects of RNS60 in Cardiac Ischemic Injury. Current Issues in Molecular Biology, 44(10), 4877-4887. https://doi.org/10.3390/cimb44100331




