Proposed Mechanism for Emodin as Agent for Methicillin Resistant Staphylococcus Aureus: In Vitro Testing and In Silico Study
Abstract
1. Introduction
2. Materials and Methods
Antimicrobial Assay
3. Molecular Modeling
4. Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marks, K.J.; Whitaker, M.; Anglin, O.; Milucky, J.; Patel, K.; Pham, H.; Chai, S.J.; Kirley, P.D.; Armistead, I.; McLafferty, S. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19—COVID-NET, 14 States, July 2021–January 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 271. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J. COVID-19 will continue but the end of the pandemic is near. Lancet 2022, 399, 417–419. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S. Changes in prevalence of health care–associated infections in US hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S. Vital signs: Epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214. [Google Scholar] [CrossRef]
- Purrello, S.; Garau, J.; Giamarellos, E.; Mazzei, T.; Pea, F.; Soriano, A.; Stefani, S. Methicillin-resistant Staphylococcus aureus infections: A review of the currently available treatment options. J. Glob. Antimicrob. Resist. 2016, 7, 178–186. [Google Scholar] [CrossRef]
- Chambers, H.F.; DeLeo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Bhakdi, S.; Tranum-Jensen, J. Alpha-toxin of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 1991, 55, 733–751. [Google Scholar] [CrossRef]
- Meesters, C.; Brack, A.; Hellmann, N.; Decker, H. Structural characterization of the α-hemolysin monomer from Staphylococcus aureus. Protein Struct. Funct. Bioinform. 2009, 75, 118–126. [Google Scholar] [CrossRef]
- Gouaux, E. α-Hemolysin fromStaphylococcus aureus: An archetype of β-barrel, channel-forming toxins. J. Struct. Biol. 1998, 121, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Hilliard, J.J.; Shi, Y.; Tkaczyk, C.; Cheng, L.I.; Yu, X.; Datta, V.; Ren, S.; Feng, H.; Zinsou, R.; et al. Assessment of an anti-alpha-toxin monoclonal antibody for prevention and treatment of Staphylococcus aureus-induced pneumonia. Antimicrob. Agents Chemother. 2014, 58, 1108–1117. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Meng, X.-M.; Li, B.; Wang, C.-Q.; Wang, S.-Q.; Wang, T.-L.; Tian, Y.-M. Inhibition of alpha-hemolysin expression by resveratrol attenuates Staphylococcus aureus virulence. Microb. Pathog. 2019, 127, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.M.; Elokely, K.M.; Atef, A.; Mohammad, A.-E.I.; Jacob, M.; Radwan, M.M.; Doerksen, R.J.; Cutler, S.J.; Ross, S.A. Asphodosides AE, anti-MRSA metabolites from Asphodelus microcarpus. Phytochemistry 2014, 105, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.M.; Elokely, K.M.; Atef, A.; Abd Elsalam, I.M.; Jacob, M.; Cutler, S.J.; Doerksen, R.J.; Ross, S.A. Isolation and characterization of new secondary metabolites from Asphodelus microcarpus. Med. Chem. Res. 2014, 23, 3510–3515. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Peng, W.; Li, X.; Liu, M.; Li, B.; Qin, R.; Jiang, W.; Cen, Y.; Pan, X.; Yan, Z. Emodin is identified as the active component of ether extracts from Rhizoma Polygoni Cuspidati, for anti-MRSA activity. Can. J. Physiol. Pharmacol. 2015, 93, 485–493. [Google Scholar] [CrossRef]
- Chalothorn, T.; Rukachaisirikul, V.; Phongpaichit, S.; Pannara, S.; Tansakul, C. Synthesis and antibacterial activity of emodin and its derivatives against methicillin-resistant Staphylococcus aureus. Tetrahedron Lett. 2019, 60, 151004. [Google Scholar] [CrossRef]
- Ji, X.; Liu, X.; Peng, Y.; Zhan, R.; Xu, H.; Ge, X. Comparative analysis of methicillin-sensitive and resistant Staphylococcus aureus exposed to emodin based on proteomic profiling. Biochem. Biophys. Res. Commun. 2017, 494, 318–324. [Google Scholar] [CrossRef]
- Bharate, S.B.; Khan, S.I.; Yunus, N.A.; Chauthe, S.K.; Jacob, M.R.; Tekwani, B.L.; Khan, I.A.; Singh, I.P. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorg. Med. Chem. 2007, 15, 87–96. [Google Scholar] [CrossRef]
- Liu, J.; Kozhaya, L.; Torres, V.J.; Unutmaz, D.; Lu, M. Structure-based discovery of a small-molecule inhibitor of methicillin-resistant Staphylococcus aureus virulence. J. Biol. Chem. 2020, 295, 5944–5959. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput.-Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2018-4: Protein Preparation Wizard; Epik, Schrödinger, LLC: New York, NY, USA, 2016; Impact, Schrödinger, LLC: New York, NY, USA, 2016; Prime, Schrödinger, LLC: New York, NY, USA, 2018.
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Schrödinger Release 2018-4: Glide; Schrödinger, LLC: New York, NY, USA, 2018.
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Schrödinger Release 2018-4: LigPrep; Schrödinger, LLC: New York, NY, USA, 2018.
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins Struct. Funct. Bioinform. 2004, 55, 351–367. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.P.; Friesner, R.A.; Xiang, Z.; Honig, B. On the role of the crystal environment in determining protein side-chain conformations. J. Mol. Biol. 2002, 320, 597–608. [Google Scholar] [CrossRef]
- Schrödinger Release 2018-4: Prime; Schrödinger, LLC: New York, NY, USA, 2018.
- Farid, R.; Day, T.; Friesner, R.A.; Pearlstein, R.A. New insights about HERG blockade obtained from protein modeling, potential energy mapping, and docking studies. Bioorg. Med. Chem. 2006, 14, 3160–3173. [Google Scholar] [CrossRef]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an induced fit receptor structure in virtual screening. Chem. Biol. Drug Des. 2006, 67, 83–84. [Google Scholar] [CrossRef]
- Schrödinger Release 2018-4: Induced Fit Docking Protocol; Glide, Schrödinger, LLC: New York, NY, USA, 2018; Prime, Schrödinger, LLC: New York, NY, USA, 2018.
- Horn, H.W.; Swope, W.C.; Pitera, J.W.; Madura, J.D.; Dick, T.J.; Hura, G.L.; Head-Gordon, T. Development of an improved four-site water model for biomolecular simulations: TIP4P-Ew. J. Chem. Phys. 2004, 120, 9665–9678. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of SC’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 43. [Google Scholar]
- Melchionna, S.; Ciccotti, G.; Lee Holian, B. Hoover NPT dynamics for systems varying in shape and size. Mol. Phys. 1993, 78, 533–544. [Google Scholar] [CrossRef]
- Martyna, G.J.; Tobias, D.J.; Klein, M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1994, 101, 4177–4189. [Google Scholar] [CrossRef]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Toukmaji, A.Y.; Board Jr, J.A. Ewald summation techniques in perspective: A survey. Comput. Phys. Commun. 1996, 95, 73–92. [Google Scholar] [CrossRef]
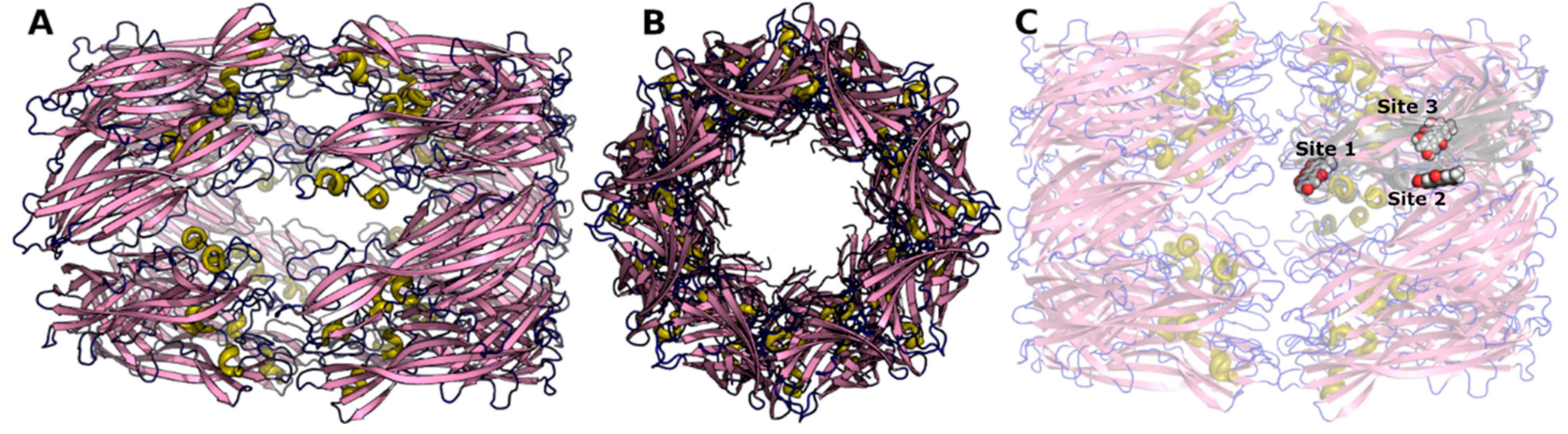

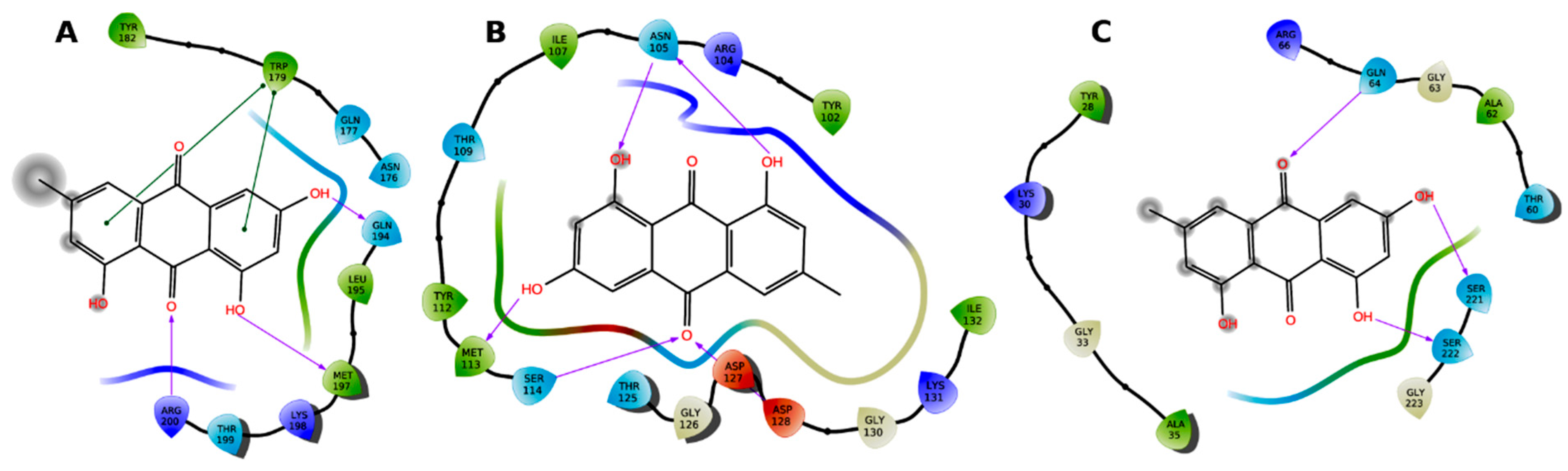
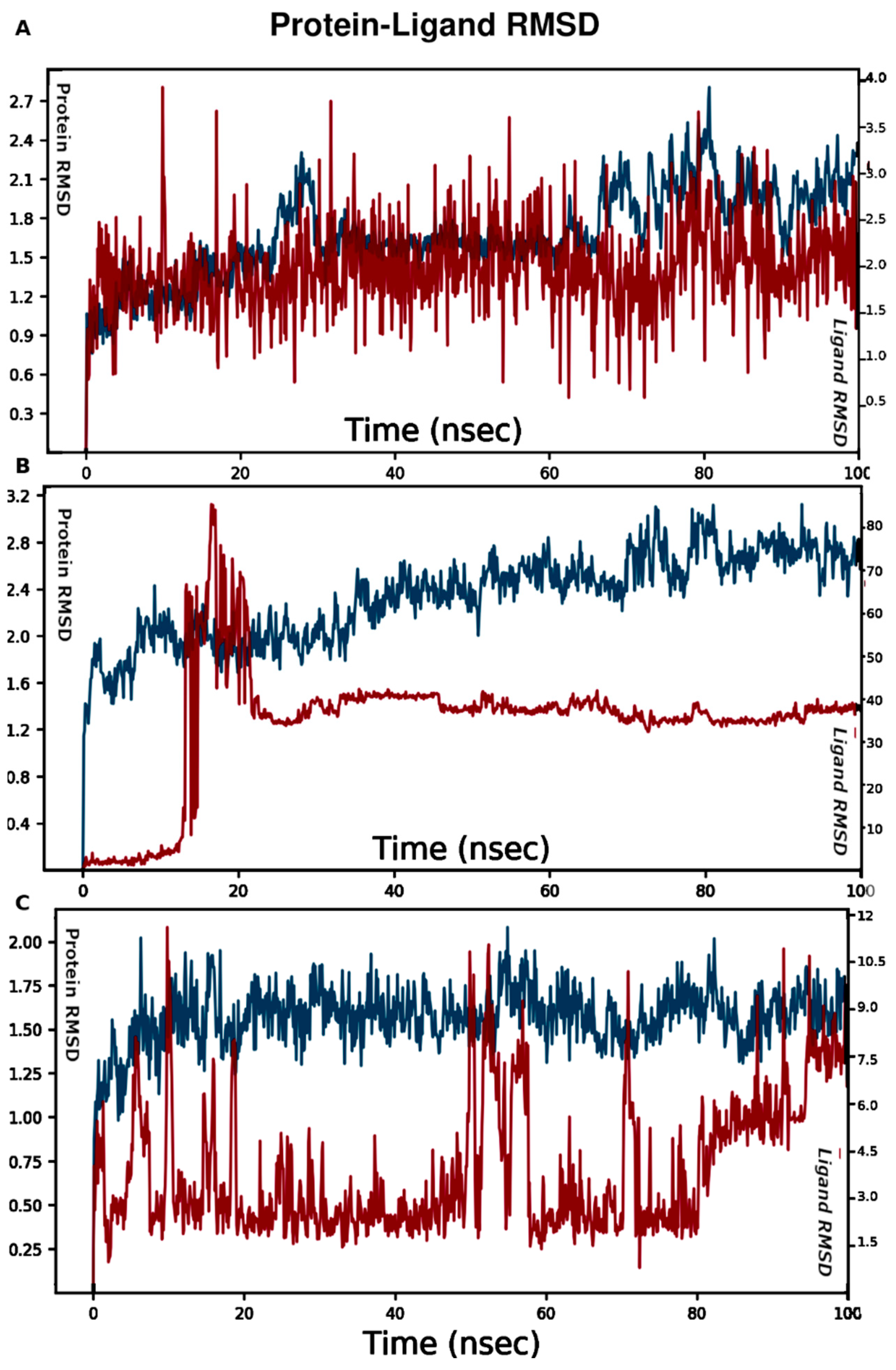
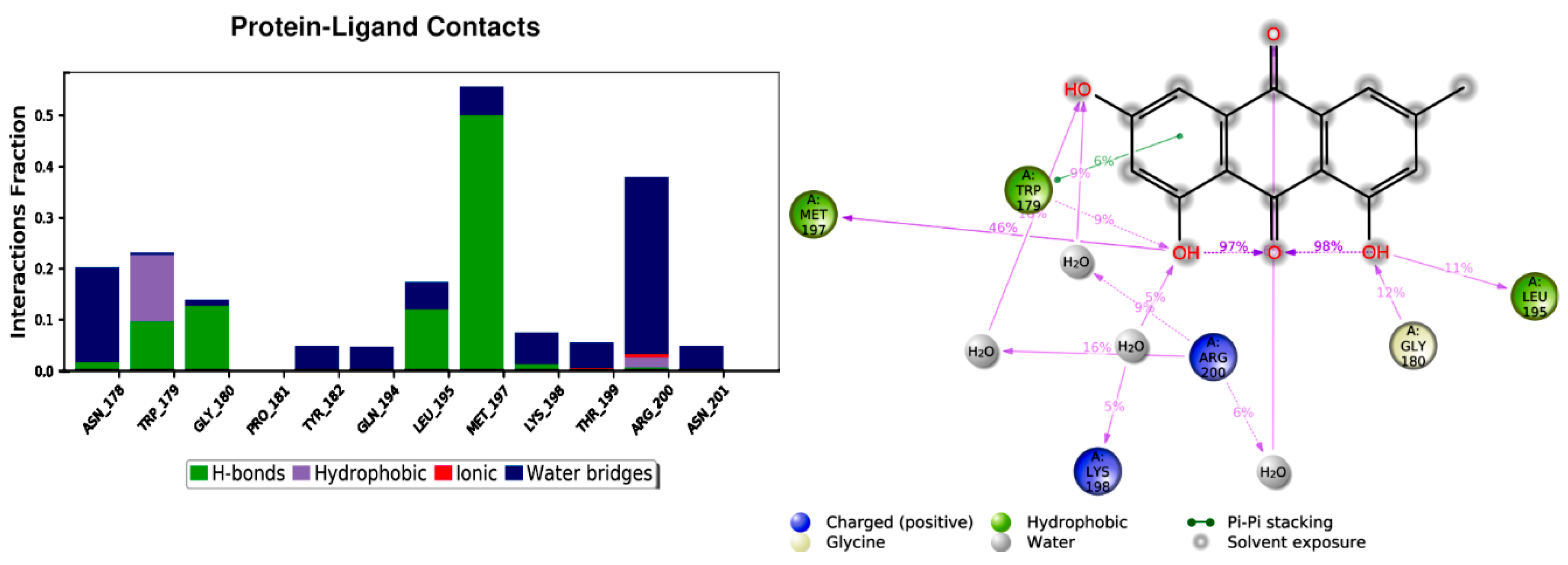

| Binding Site | Simulation Time (ns) | No of Atoms | No of Water Atoms | Counter Ion Conc. mM |
|---|---|---|---|---|
| Site 1 | 100 | 46,683 | 14,022 | 3.890 |
| Site 2 | 100 | 35,658 | 10,347 | 5.272 |
| Site 3 | 100 | 40,926 | 12,103 | 4.507 |
| Compound 1 IC50 (μg/mL) | Compound 2 IC50 (μg/mL) | Ciprofloxacin * IC50 (μg/mL) | |
|---|---|---|---|
| Staphylococcus aureus | 4.2 | NA | 0.1 |
| methicillin-resistant S. aureus | 6.3 | NA | 0.1 |
| Test concentrations (µg/mL) | 20, 4, 0.8 | 20, 4, 0.8 | 20, 4, 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoneim, M.M.; Al-Serwi, R.H.; El-Sherbiny, M.; El-ghaly, E.-s.M.; Hamad, A.E.; Abdelgawad, M.A.; Ragab, E.A.; Bukhari, S.I.; Bukhari, K.; Elokely, K.; et al. Proposed Mechanism for Emodin as Agent for Methicillin Resistant Staphylococcus Aureus: In Vitro Testing and In Silico Study. Curr. Issues Mol. Biol. 2022, 44, 4490-4499. https://doi.org/10.3390/cimb44100307
Ghoneim MM, Al-Serwi RH, El-Sherbiny M, El-ghaly E-sM, Hamad AE, Abdelgawad MA, Ragab EA, Bukhari SI, Bukhari K, Elokely K, et al. Proposed Mechanism for Emodin as Agent for Methicillin Resistant Staphylococcus Aureus: In Vitro Testing and In Silico Study. Current Issues in Molecular Biology. 2022; 44(10):4490-4499. https://doi.org/10.3390/cimb44100307
Chicago/Turabian StyleGhoneim, Mohammed M., Rasha Hamed Al-Serwi, Mohamed El-Sherbiny, El-sayed M. El-ghaly, Amal E. Hamad, Mohamed A. Abdelgawad, Ehab A. Ragab, Sarah I. Bukhari, Khulud Bukhari, Khaled Elokely, and et al. 2022. "Proposed Mechanism for Emodin as Agent for Methicillin Resistant Staphylococcus Aureus: In Vitro Testing and In Silico Study" Current Issues in Molecular Biology 44, no. 10: 4490-4499. https://doi.org/10.3390/cimb44100307
APA StyleGhoneim, M. M., Al-Serwi, R. H., El-Sherbiny, M., El-ghaly, E.-s. M., Hamad, A. E., Abdelgawad, M. A., Ragab, E. A., Bukhari, S. I., Bukhari, K., Elokely, K., & Nael, M. A. (2022). Proposed Mechanism for Emodin as Agent for Methicillin Resistant Staphylococcus Aureus: In Vitro Testing and In Silico Study. Current Issues in Molecular Biology, 44(10), 4490-4499. https://doi.org/10.3390/cimb44100307






