Pro-Inflammatory and Pro-Apoptotic Effects of the Non-Protein Amino Acid L-Azetidine-2-Carboxylic Acid in BV2 Microglial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. MTT Assay
2.3. Griess Assay
2.4. RNA Extraction and cDNA Synthesis
2.5. Real Time Quantitative PCR Analysis
2.6. Protein Extraction and Western Blot Analyses
2.7. Flow Cytometry
2.8. Immunocytochemistry
2.9. Statistical Analysis
3. Results
3.1. Dose–Response and Time Course Study of the Effects of AZE on Cell Morphology, Viability and Nitric Oxide (NO) Release
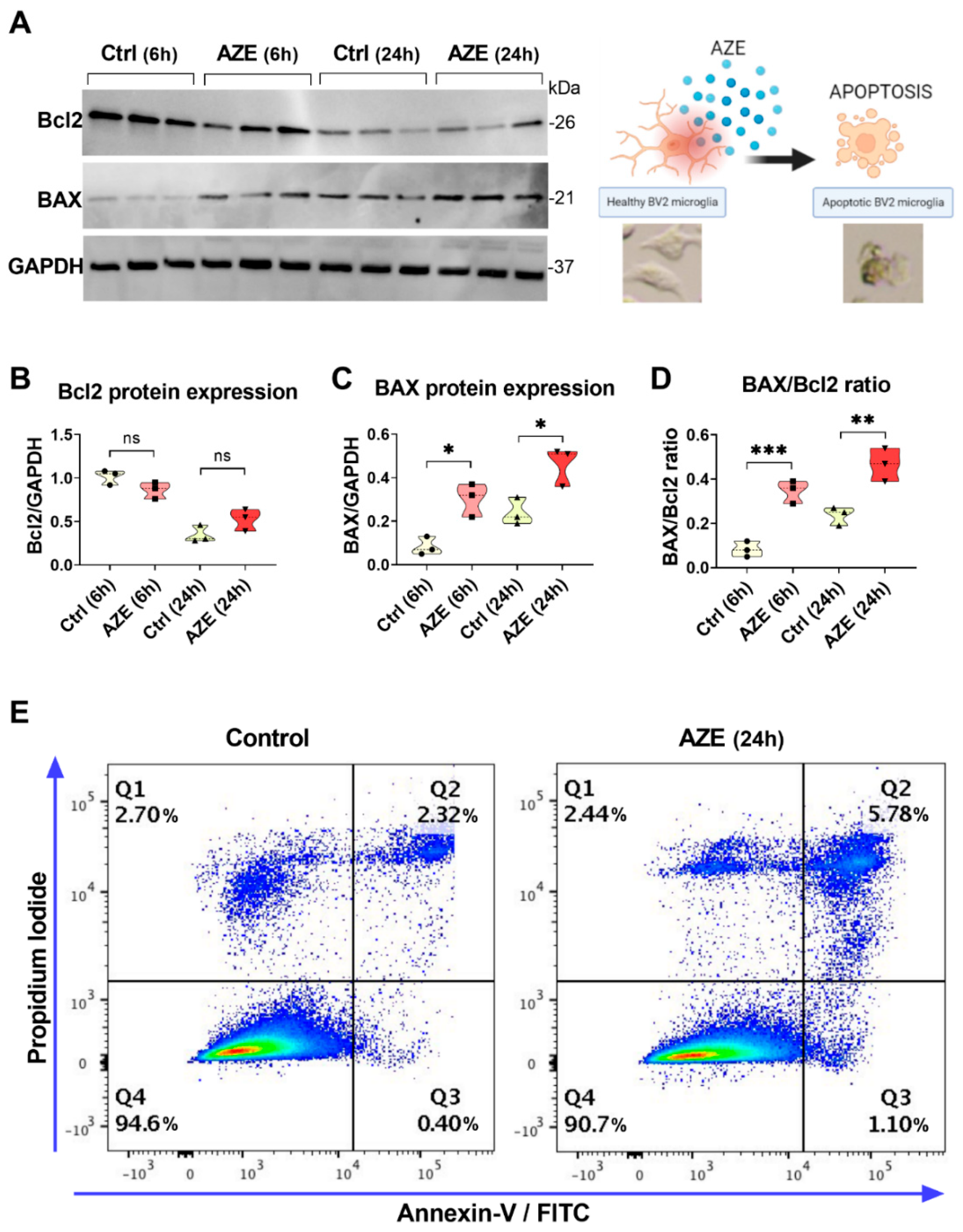
3.2. Effects of AZE Exposure on Bcl2 and BAX Protein Expression in Murine BV2 Microglial Cells
3.3. Effects of AZE Exposure on the mRNA Expression of Pro- and Anti-Inflammatory Markers in Murine BV2 Microglial Cells
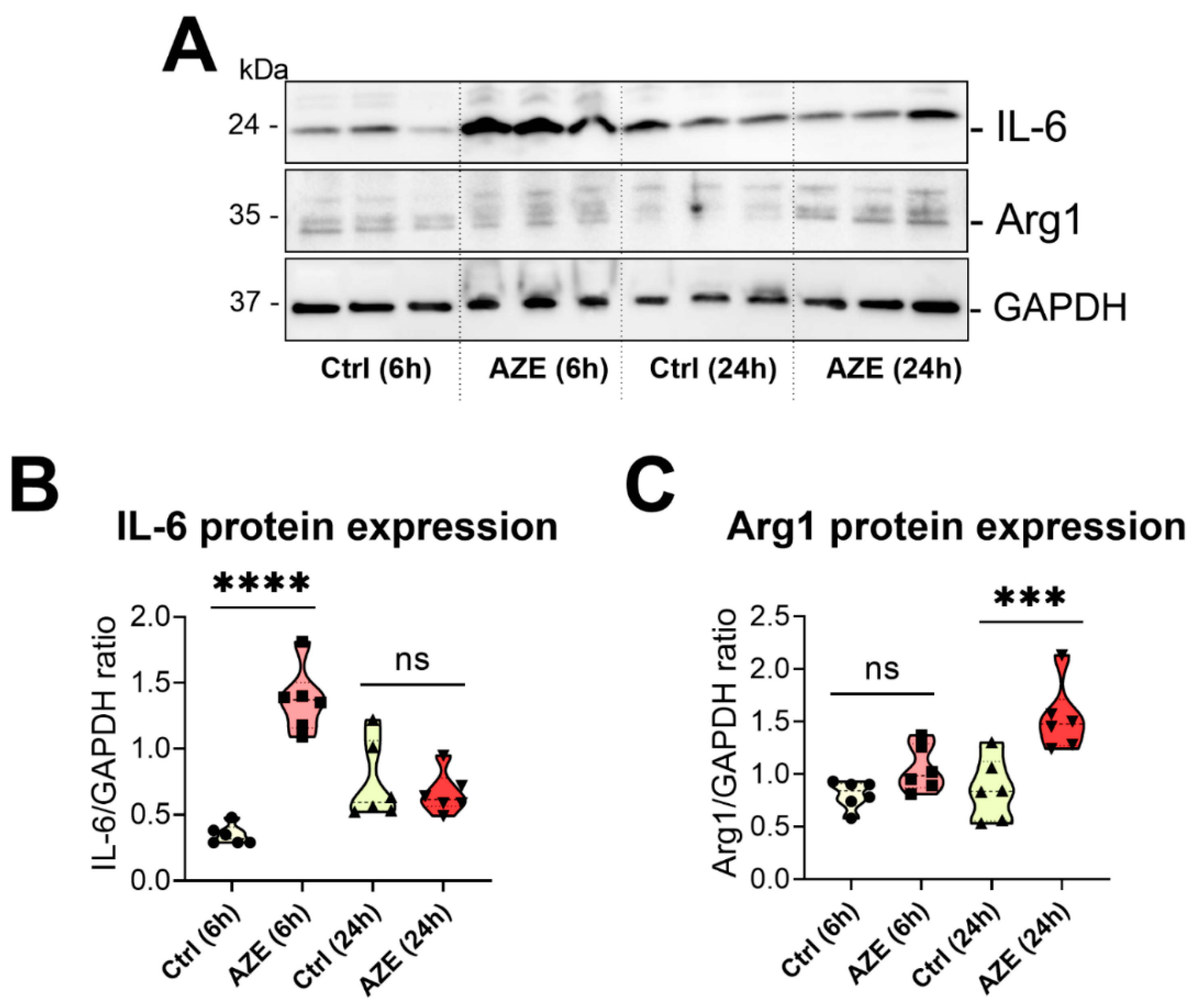
3.4. Effects of AZE Exposure on IL-6 and Arg1 Protein Expression in Murine BV2 Microglial Cells
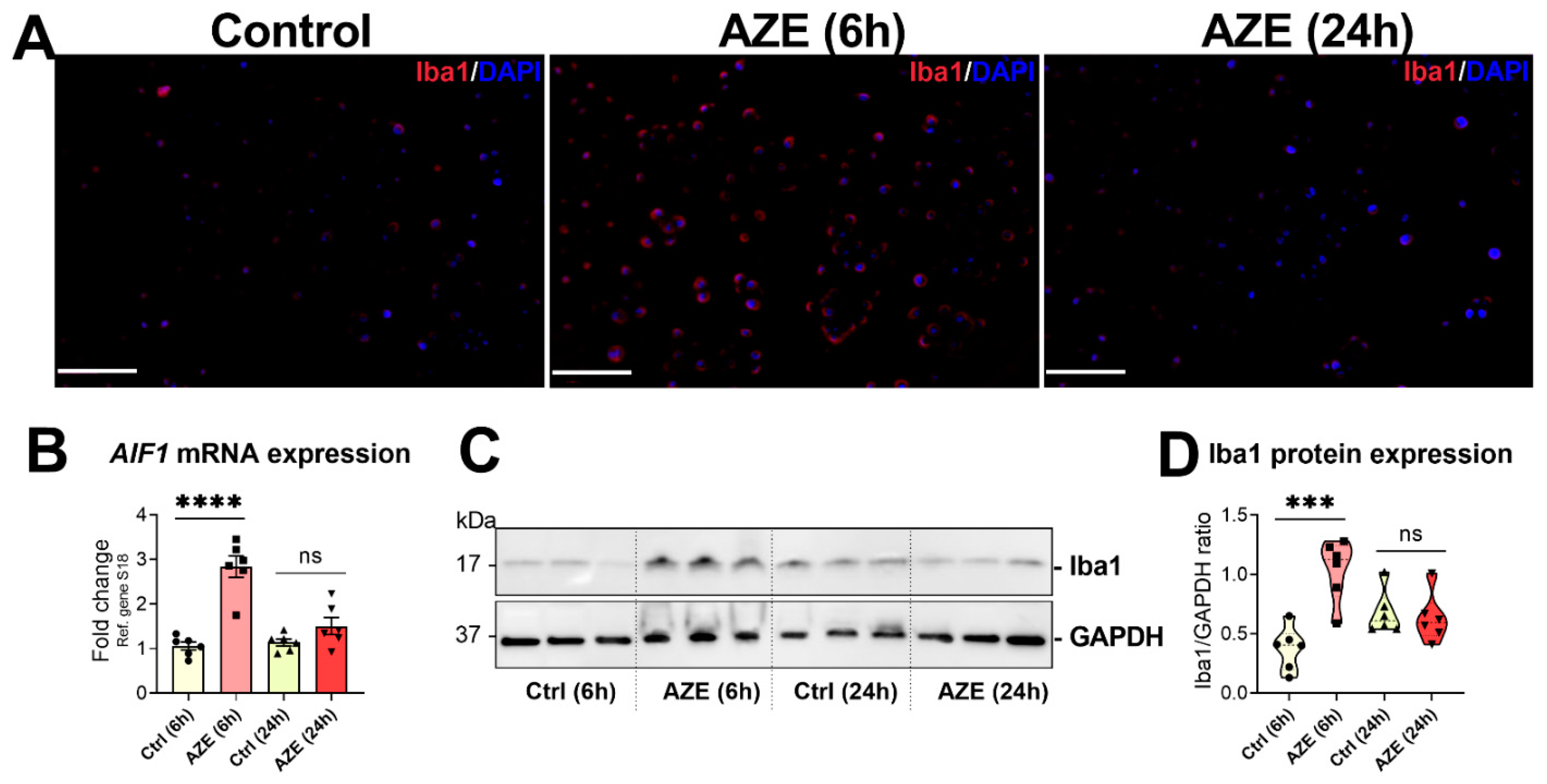
3.5. Effects of AZE Treatment on the Gene and Protein Expression of the Pro-Inflammatory Marker Allograft Inflammatory Factor 1 (AIF1)/Ionized Calcium-Binding Adapter Molecule 1 (Iba1)
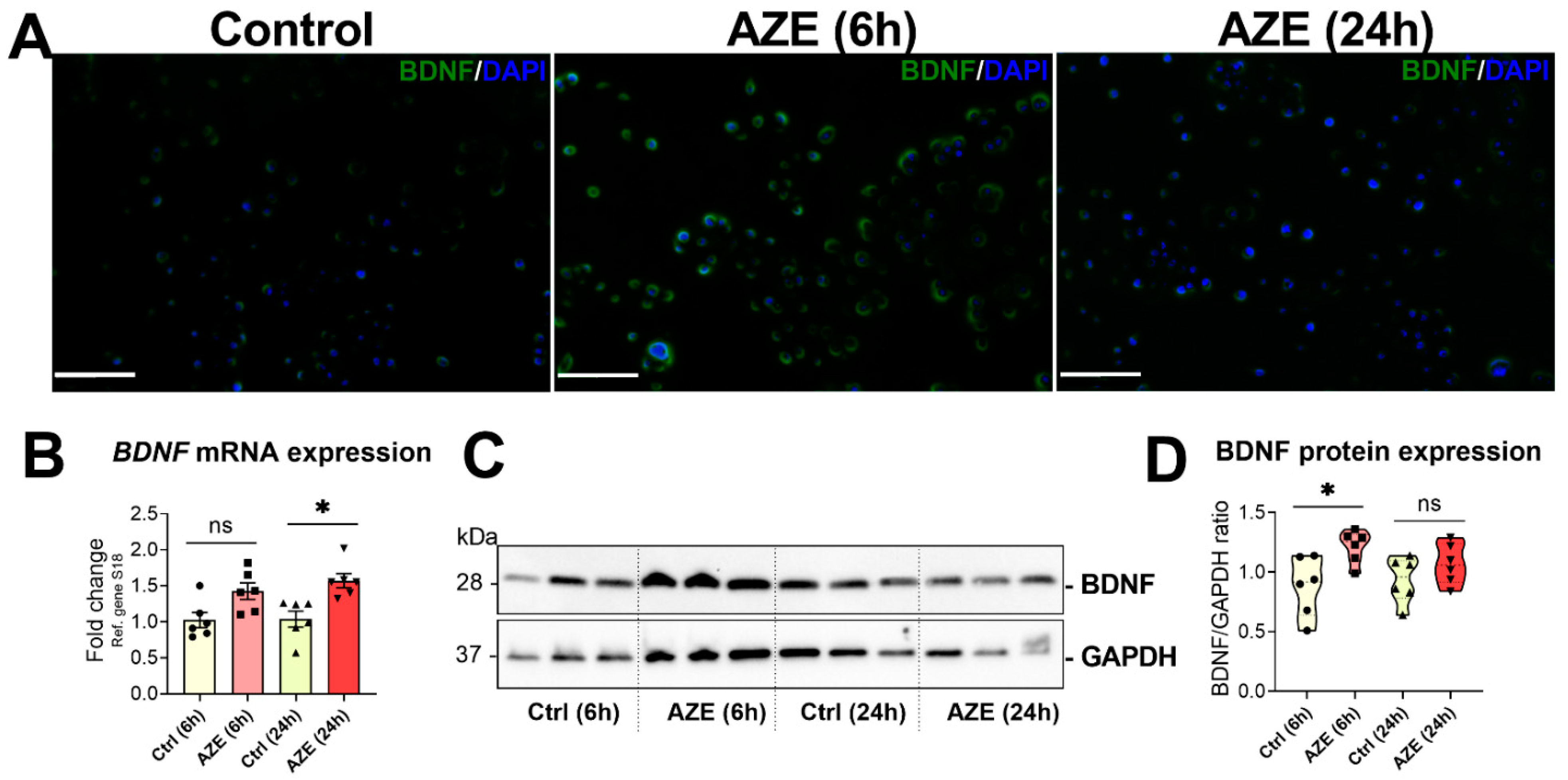
3.6. Effects of AZE Treatment on the Gene and Protein Expression of Brain-Derived Neurotrophic Factor (BDNF)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodgers, K.J. Non-protein amino acids and neurodegeneration: The enemy within. Exp. Neurol. 2014, 253, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Marchiosi, R.; Siqueira-Soares, R.D.C.; Barbosa de Lima, R.; Dantas dos Santos, W.; Ferrarese-Filho, O. The role of L-DOPA in plants. Plant Signal. Behav. 2014, 9, e28275. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E. Biologic effects of and clinical disorders caused by nonprotein amino acids. Medicine 2000, 79, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.; McLaughlin, T.; Winant, R.C.; Sanchez, A.; Eckart, M.; Krasinska, K.M.; Chien, A. Azetidine-2-carboxylic acid in the food chain. Phytochemistry 2009, 70, 100–104. [Google Scholar] [CrossRef]
- Rubenstein, E.; Zhou, H.; Krasinska, K.M.; Chien, A.; Becker, C.H. Azetidine-2-carboxylic acid in garden beets (Beta vulgaris). Phytochemistry 2006, 67, 898–903. [Google Scholar] [CrossRef]
- Rubenstein, E. Misincorporation of the proline analog azetidine-2-carboxylic acid in the pathogenesis of multiple sclerosis: A hypothesis. J. Neuropathol. Exp. Neurol. 2008, 67, 1035–1040. [Google Scholar] [CrossRef]
- Sobel, R.A.; Albertelli, M.; Hinojoza, J.R.; Eaton, M.J.; Grimes, K.V.; Rubenstein, E. Azetidine-2-Carboxylic Acid-Induced Oligodendrogliopathy: Relevance to the Pathogenesis of Multiple Sclerosis. J. Neuropathol. Exp. Neurol 2022, 81, 414–433. [Google Scholar] [CrossRef]
- Rone, M.B.; Cui, Q.-L.; Fang, J.; Wang, L.-C.; Zhang, J.; Khan, D.; Bedard, M.; Almazan, G.; Ludwin, S.K.; Jones, R.; et al. Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival. J. Neurosci. 2016, 36, 4698–4707. [Google Scholar] [CrossRef]
- Marzan, D.E.; Brügger-Verdon, V.; West, B.L.; Liddelow, S.; Samanta, J.; Salzer, J.L. Activated microglia drive demyelination via CSF1R signaling. Glia 2021, 69, 1583–1604. [Google Scholar] [CrossRef]
- Kinney, H.C.; Volpe, J.J. Myelination Events, in Volpe’s Neurology of the Newborn; Elsevier: Amsterdam, The Netherlands, 2018; pp. 176–188. [Google Scholar]
- Dikranian, K.; Cohen, R.; Mac Donald, C.; Pan, Y.; Brakefield, D.; Bayly, P.; Parsadanian, A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 2008, 211, 551–560. [Google Scholar] [CrossRef]
- Zhang, K.; Kaufman, R.J. Protein folding in the endoplasmic reticulum and the unfolded protein response. Handb. Exp. Pharmacol. 2006, 172, 69–91. [Google Scholar]
- Zhang, K.; Kaufman, R.J. The unfolded protein response: A stress signaling pathway critical for health and disease. Neurology 2006, 66 (Suppl. 1), S102–S109. [Google Scholar] [CrossRef] [PubMed]
- Fernández, D.; Geisse, A.; Bernales, J.I.; Lira, A.; Osorio, F. The Unfolded Protein Response in Immune Cells as an Emerging Regulator of Neuroinflammation. Front. Aging Neurosci. 2021, 13, 286. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Howell, O.W.; Rundle, J.L.; Garg, A.; Komada, M.; Brophy, P.J.; Reynolds, R. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J. Neuropathol. Exp. Neurol. 2010, 69, 1017–1033. [Google Scholar] [CrossRef]
- Broome, S.T.; Fisher, T.; Faiz, A.; Keay, K.; Musumeci, G.; Al-Badri, G.; Castorina, A. Assessing the Anti-Inflammatory Activity of the Anxiolytic Drug Buspirone Using CRISPR-Cas9 Gene Editing in LPS-Stimulated BV-2 Microglial Cells. Cells 2021, 10, 1312. [Google Scholar] [CrossRef]
- Giunta, S.; Castorina, A.; Adorno, A.; Mazzone, V.; Carnazza, M.L.; D’Agata, V. PACAP and VIP affect NF1 expression in rat malignant peripheral nerve sheath tumor (MPNST) cells. Neuropeptides 2010, 44, 45–51. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Maltese, A.; Castorina, A.; D’Agata, V.; Drago, F. Dopamine-3 receptor modulates intraocular pressure: Implications for glaucoma. Biochem. Pharmacol. 2012, 83, 680–686. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Castorina, A.; Waschek, J.A.; Marzagalli, R.; Cardile, V.; Drago, F. PACAP interacts with PAC1 receptors to induce tissue plasminogen activator (tPA) expression and activity in schwann cell-like cultures. PLoS ONE 2015, 10, e0117799. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Castorina, A.; Magro, G.; Cardile, V.; Castorina, S.; Ribatti, D. Enhanced expression of CD31/platelet endothelial cell adhesion molecule 1 (PECAM1) correlates with hypoxia inducible factor-1 alpha (HIF-1alpha) in human glioblastoma multiforme. Exp. Cell Res. 2015, 339, 407–416. [Google Scholar] [CrossRef]
- Artym, V.V.; Yamada, K.M.; Mueller, S.C. ECM degradation assays for analyzing local cell invasion. In Extracellular Matrix Protocols; Springer: Berlin/Heidelberg, Germany, 2009; pp. 211–219. [Google Scholar]
- Miki, S.; Suzuki, J.-I.; Takashima, M.; Ishida, M.; Kokubo, H.; Yoshizumi, M. S-1-Propenylcysteine promotes IL-10-induced M2c macrophage polarization through prolonged activation of IL-10R/STAT3 signaling. Sci. Rep. 2021, 11, 22469. [Google Scholar] [CrossRef] [PubMed]
- Broome, S.T.; Louangaphay, K.; Keay, K.A.; Leggio, G.M.; Musumeci, G.; Castorina, A. Dopamine: An immune transmitter. Neural Regen. Res. 2020, 15, 2173. [Google Scholar]
- Ding, H.; Chen, J.; Su, M.; Lin, Z.; Zhan, H.; Yang, F.; Li, W.; Xie, J.; Huang, Y.; Liu, X.; et al. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflamm. 2020, 17, 19. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Jansen, M.I.; Broome, S.T.; Castorina, A. Targeting the neurological comorbidities of multiple sclerosis: The beneficial effects of VIP and PACAP neuropeptides. J. Integr. Neurosci. 2022, 21, 33. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Wang, D.; Zhao, F.; Cao, Z.; Aschner, M.; Luo, W. The role of autophagy in modulation of neuroinflammation in microglia. Neuroscience 2016, 319, 155–167. [Google Scholar] [CrossRef]
- Lloyd, A.F.; Miron, V.E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 2019, 15, 447–458. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Nunn, P.B.; Bell, E.A.; Watson, A.A.; Nash, R.J. Toxicity of non-protein amino acids to humans and domestic animals. Nat. Prod. Commun 2010, 5, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Bowling, L.C.; Padula, M.P.; Bishop, D.P.; Mitrovic, S.M.; Guillemin, G.J.; Rodgers, K.J. Detection of the suspected neurotoxin β-methylamino-l-alanine (BMAA) in cyanobacterial blooms from multiple water bodies in Eastern Australia. Harmful Algae 2018, 74, 10–18. [Google Scholar] [CrossRef]
- Violi, J.P.; Mitrovic, S.M.; Colville, A.; Main, B.J.; Rodgers, K.J. Prevalence of β-methylamino-L-alanine (BMAA) and its isomers in freshwater cyanobacteria isolated from eastern Australia. Ecotoxicol. Environ. Saf. 2019, 172, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhou, H.; Vo, M.-N.; Shi, Y.; Nawaz, M.H.; Vargas-Rodriguez, O.; Diedrich, J.K.; Yates, J.R.; Kishi, S.; Musier-Forsyth, K.; et al. Double mimicry evades tRNA synthetase editing by toxic vegetable-sourced non-proteinogenic amino acid. Nat. Commun. 2017, 8, 2281. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Teo, G.; Krueger, S.; Rock, T.M.; Koh, H.W.; Choi, H.; Vogel, C. Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress. Mol. Syst. Biol. 2016, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Samardzic, K.; Rodgers, K.J. Cell death and mitochondrial dysfunction induced by the dietary non-proteinogenic amino acid L-azetidine-2-carboxylic acid (Aze). Amino Acids 2019, 51, 1221–1232. [Google Scholar] [CrossRef]
- Karunia, J.; Niaz, A.; Mandwie, M.; Broome, S.T.; Keay, K.A.; Waschek, J.A.; Al-Badri, G.; Castorina, A. PACAP and VIP Modulate LPS-Induced Microglial Activation and Trigger Distinct Phenotypic Changes in Murine BV2 Microglial Cells. Int. J. Mol. Sci. 2021, 22, 10947. [Google Scholar] [CrossRef]
- Anja Henn, S.L.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The Suitability of BV2 Cells as Alternative Model System for Primary Microglia Cultures or for Animal Experiments Examining Brain Inflammation. ALTEX Altern. Anim. Exp. 2009, 26, 83–94. [Google Scholar]
- Roy, A.; Fung, Y.K.; Liu, X.; Pahan, K. Up-regulation of microglial CD11b expression by nitric oxide. J. Biol. Chem. 2006, 281, 14971–14980. [Google Scholar] [CrossRef]
- Stöhr, D.; Jeltsch, A.; Rehm, M. TRAIL receptor signaling: From the basics of canonical signal transduction toward its entanglement with ER stress and the unfolded protein response. Int. Rev. Cell Mol. Biol. 2020, 351, 57–99. [Google Scholar] [PubMed]


| Gene | Forward Sequence 5′–3′ Reverse Sequence 3′–5′ | Tm (°C) | Product Size | Accession No. |
|---|---|---|---|---|
| IL-1β | GCTACCTGTGTCTTTCCCGT CATCTCGGAGCCTGTAGTGC | 59.68 60.25 | 164 | NM_008361.4 |
| Itgam | GAGCAGGGGTCATTCGCTAC GCTGGCTTAGATGCGATGGT | 60.53 60.53 | 94 | NM_001082960.1 |
| Il-6 | CCCCAATTTCCAATGCTCTCC CGCACTAGGTTTGCCGAGTA | 59.24 60.11 | 141 | NM_031168.2 |
| Cd68 | CTCCCACCACAAATGGCACT CTTGGACCTTGGACTAGGCG | 60.54 60.11 | 95 | NM_001291058.1 |
| Mhc-2a | CAAGCTGTCTTATCTCACCTTCA ATCTCAGGTTCCCAGTGTTTCA | 60.34 61.81 | 108 | NM_010378.3 |
| Mmp-9 | ATCATAGAGGAAGCCCATTACAG TTTGACGTCCAGAGAAGAAGAAA | 59.86 59.96 | 129 | NM_013599.4 |
| Nos2 | TACCAAAGTGACCTGAAAGAGG TCATCTTGTATTGTTGGGCTGA | 60.06 59.96 | 89 | NM_010927.4 |
| Il-10 | GCATGGCCCAGAAATCAAGG GAGAAATCGATGACAGCGCC | 59.54 59.42 | 91 | NM_010548.2 |
| Arg-1 | ACAAGACAGGGCTCCTTTCAG TTAAAGCCACTGCCGTGTTC | 59.93 59.05 | 105 | NM_007482.3 |
| Aif1 | ACGTTCAGCTACTCTGACTTTC GTTGGCCTCTTGTGTTCTTTG | 60.23 60.18 | 107 | NM_001361501.1 |
| Bdnf | CGAGTGGGTCACAGCGGCAG GCCCCTGCAGCCTTCCTTGG | 60.04 59.97 | 160 | NM_007540.4 |
| S18 | CCCTGAGAAGTTCCAGCACA GGTGAGGTCGATGTCTGCTT | 59.60 59.75 | 145 | NM_011296.2 |
| Antibody | Source | Predicted Band Size | Dilution |
|---|---|---|---|
| Bcl2 | ab182858, Abcam | 26 kDa | 1:2000 |
| BAX | ab32503, Abcam | 21 kDa | 1:1000 |
| Arg1 | GTX109242, GeneTex | 35 kDa | 1:1000 |
| BDNF | GTX132621, GeneTex | 28 kDa | 1:1000 (WB) 1:500 (IHC) |
| Iba1 | GTX100042, GeneTex | 17 kDa | 1:500 (WB) 1:250 (IHC) |
| iNOS | GTX60599, GeneTex | 32 kDa | 1:1000 |
| IL-6 | GTX110527, GeneTex | 24 kDa | 1:1000 (WB) |
| GAPDH | VPA00187, Bio-Rad | 37 kDa | 1:2000 |
| Goat anti Rabbit IgG HRP (Secondary) | STAR208P, Bio-Rad | 1:10,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piper, J.A.; Jansen, M.I.; Thomas Broome, S.; Rodgers, K.J.; Musumeci, G.; Castorina, A. Pro-Inflammatory and Pro-Apoptotic Effects of the Non-Protein Amino Acid L-Azetidine-2-Carboxylic Acid in BV2 Microglial Cells. Curr. Issues Mol. Biol. 2022, 44, 4500-4516. https://doi.org/10.3390/cimb44100308
Piper JA, Jansen MI, Thomas Broome S, Rodgers KJ, Musumeci G, Castorina A. Pro-Inflammatory and Pro-Apoptotic Effects of the Non-Protein Amino Acid L-Azetidine-2-Carboxylic Acid in BV2 Microglial Cells. Current Issues in Molecular Biology. 2022; 44(10):4500-4516. https://doi.org/10.3390/cimb44100308
Chicago/Turabian StylePiper, Jordan Allan, Margo Iris Jansen, Sarah Thomas Broome, Kenneth J. Rodgers, Giuseppe Musumeci, and Alessandro Castorina. 2022. "Pro-Inflammatory and Pro-Apoptotic Effects of the Non-Protein Amino Acid L-Azetidine-2-Carboxylic Acid in BV2 Microglial Cells" Current Issues in Molecular Biology 44, no. 10: 4500-4516. https://doi.org/10.3390/cimb44100308
APA StylePiper, J. A., Jansen, M. I., Thomas Broome, S., Rodgers, K. J., Musumeci, G., & Castorina, A. (2022). Pro-Inflammatory and Pro-Apoptotic Effects of the Non-Protein Amino Acid L-Azetidine-2-Carboxylic Acid in BV2 Microglial Cells. Current Issues in Molecular Biology, 44(10), 4500-4516. https://doi.org/10.3390/cimb44100308










