RETRACTED: Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Analyses
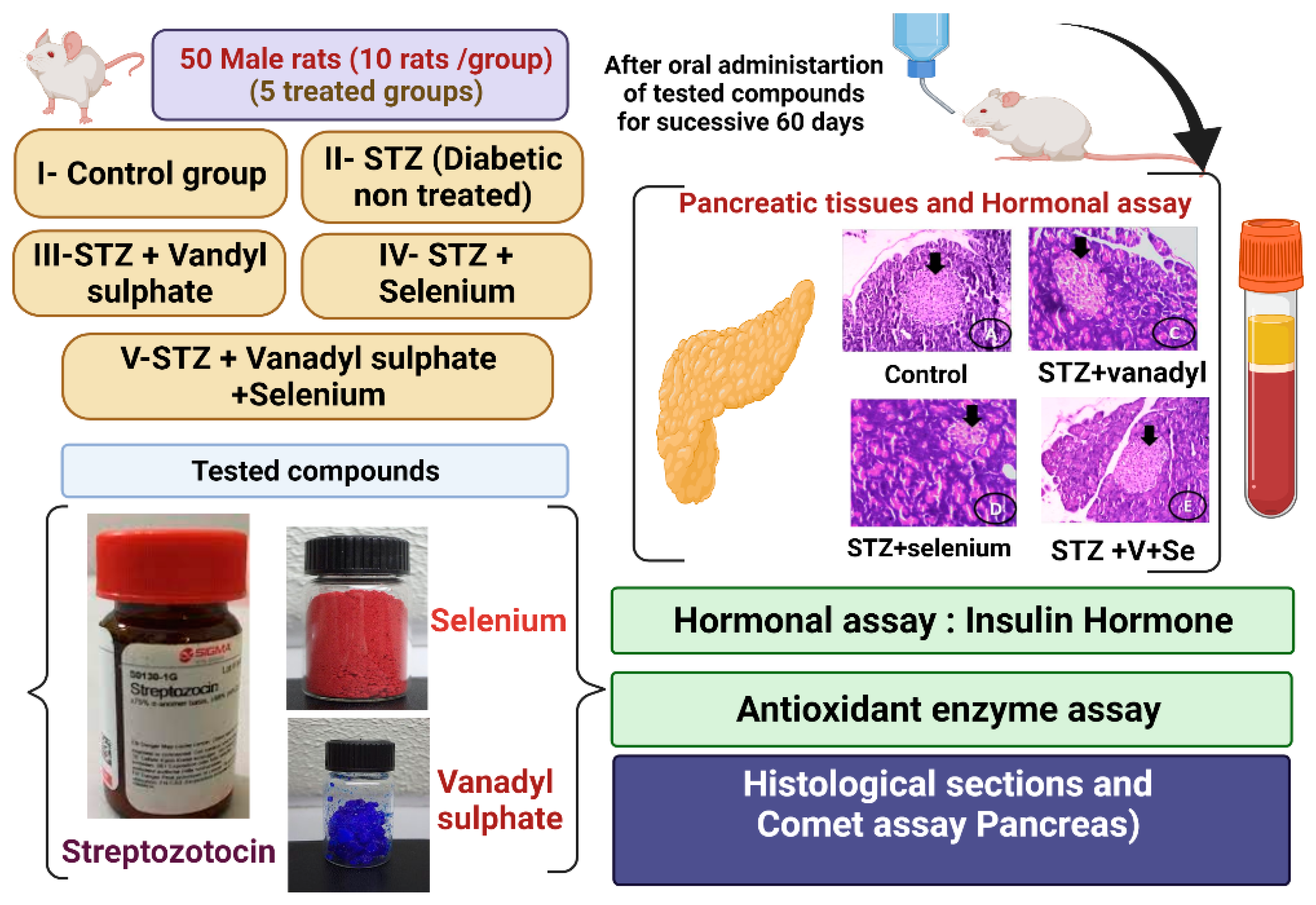
2.2. Experimental Animals
2.3. Animals and Experimental Design
2.4. Experimental Induction of Diabetes Mellitus
2.5. Blood Collection
2.6. Determination of the Blood Glucose Level
2.7. Measurements of Serum Insulin, C-Peptide, and HbA1c
2.8. Preparation of Pancreatic Tissue Homogenates
2.9. Determination of Oxidative Stress Markers
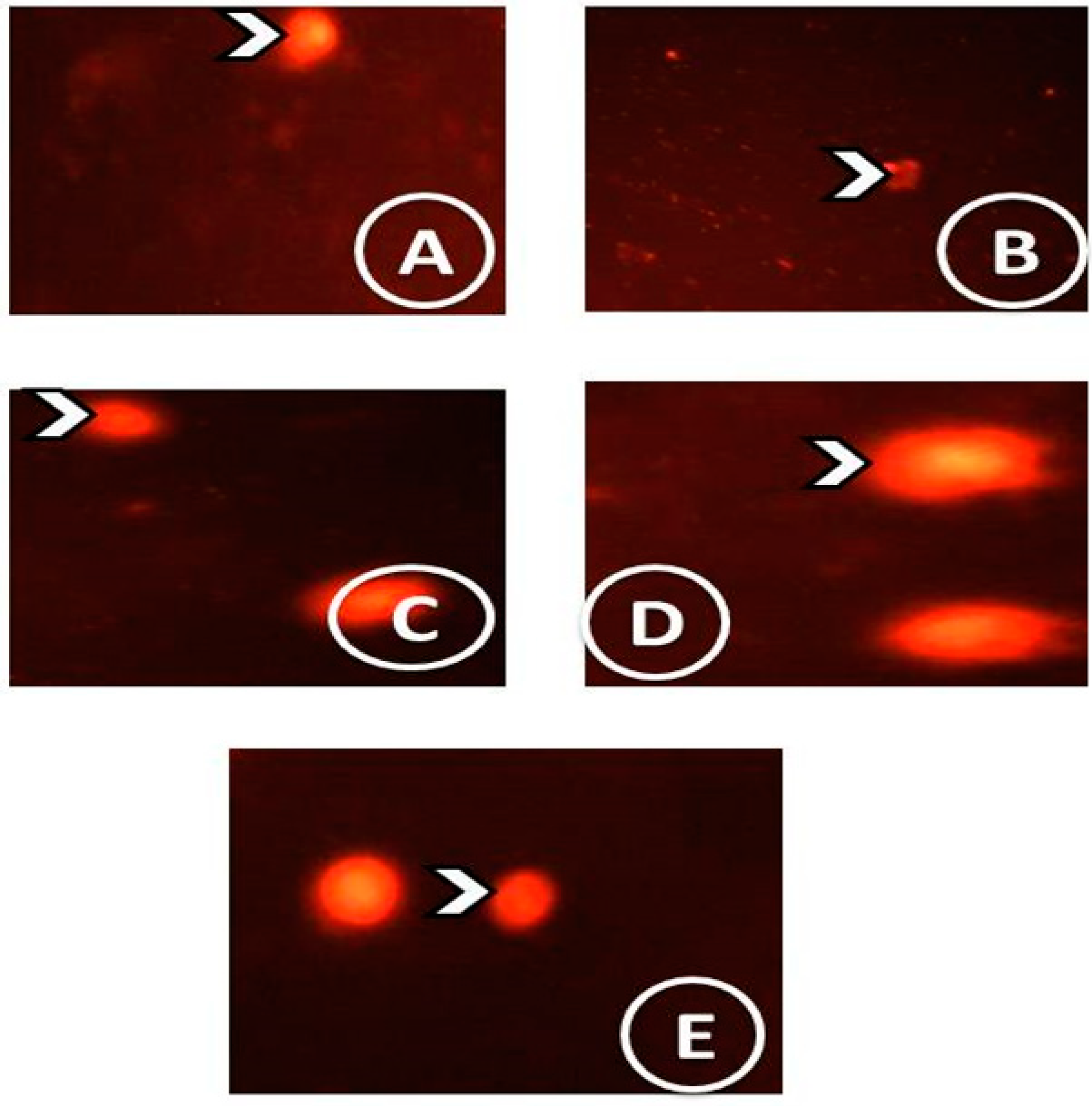
2.10. Single-Cell Gel Electrophoresis (SCGE) (Comet Assay)
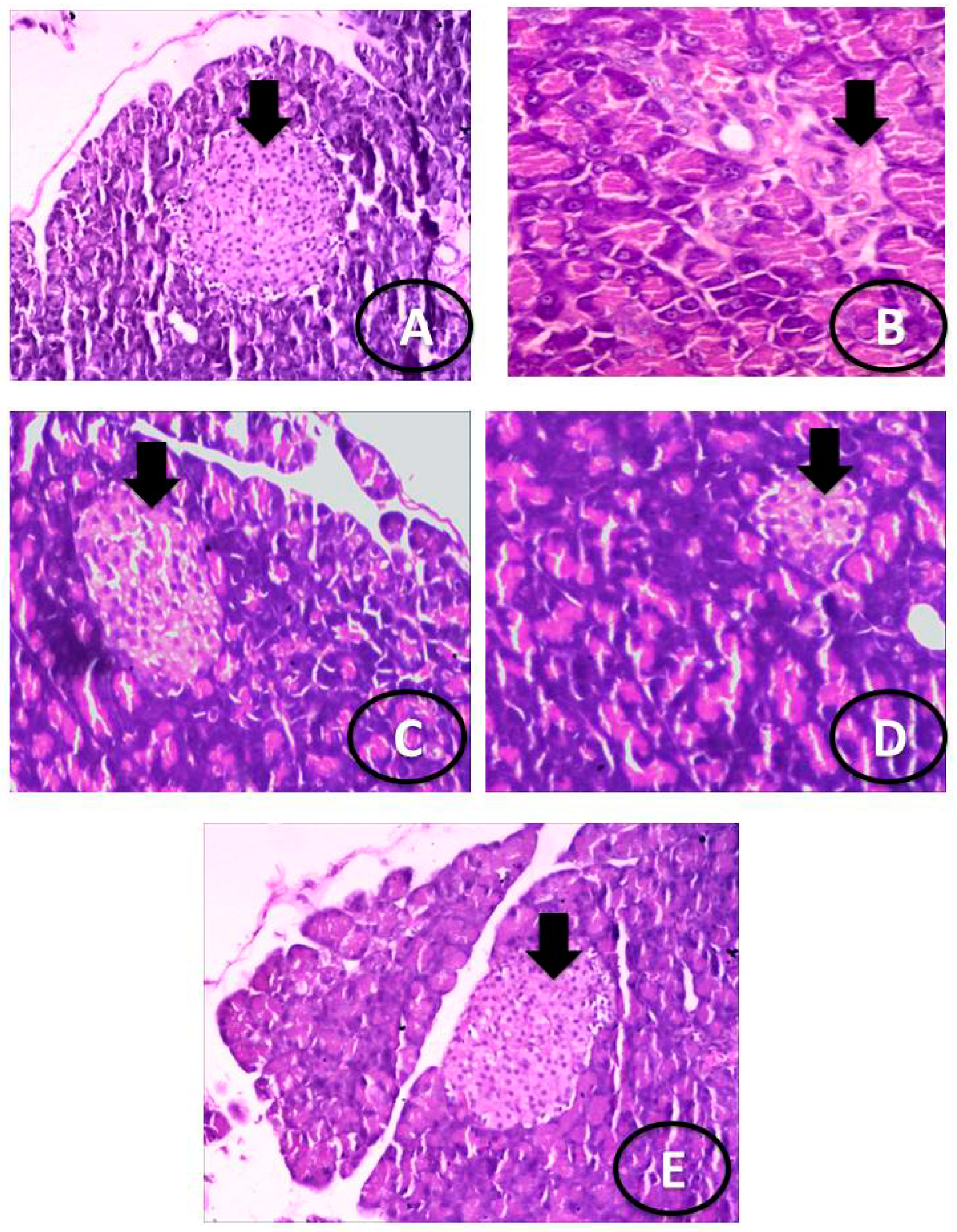
2.11. Histological Analysis of the Pancreas Tissues
2.12. Statistical Analysis
3. Results
3.1. Blood Glucose Level, Insulin Hormone, and Fasting C-Peptide Serum
3.2. Oxidative Stress Enzyme Biomarkers
3.3. Histological Examination
3.4. Comet Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Song, L.; Strange, C.; Dong, X.; Wang, H. Therapeutic Effects of Adipose Stem Cells from Diabetic Mice for the Treatment of Type 2 Diabetes. Am. Soc. Gene Cell Ther. 2018, 26, 1921–1930. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Palmiero, G.; Cesaro, A.; Vetrano, E.; Pafundi, P.; Galiero, R.; Caturano, A.; Moscarella, E.; Gragnano, F.; Salvatore, T.; Rinaldi, L.; et al. Impact of SGLT2 Inhibitors on Heart Failure: From Pathophysiology to Clinical Effects. Int. J. Mol. Sci. 2021, 22, 5863. [Google Scholar] [CrossRef]
- Sasso, F.C.; Pafundi, P.C.; Simeon, V.; De Nicola, L.; Chiodini, P.; Galiero, R.; Rinaldi, L.; Nevola, R.; Salvatore, T.; Sardo, C.; et al. Efcacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Sasso et al. Cardiovasc. Diabetol. 2021, 20, 145. [Google Scholar] [CrossRef]
- Hamza, R.Z.; EL-Megharbel, S.M.; Altalhi, T.; Gobouri, A.A.; Alrogi, A.A. Hypolipidemic and hepatoprotective synergistic effects of selenium nanoparticles and vitamin. E against acrylamide induced hepatic alterations in male albino mice. Appl. Organomet. Chem. 2020, 34, e5458. [Google Scholar] [CrossRef]
- Pessoa, J.C. Thirty years through vanadium chemistry. J. Inorg. Biochem. 2015, 147, 4–24. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C. Antidiabetic, Chemical, and Physical Properties of Organic Vanadates as Presumed Transition-State Inhibitors for Phosphatases. J. Org. Chem. 2015, 80, 11899–11915. [Google Scholar] [CrossRef]
- Domingo, J.L.; Gómez, M. Vanadium compounds for the treatment of human diabetes mellitus: A scientific curiosity? A review of thirty years of research. Food Chem. Toxicol. 2016, 95, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Rezvani, A.R.; Ghasemi, K.; Graiff, C. Glycine and metformin as new counter ions for mono and dinuclear vanadium (V)-dipicolinic acid complexes based on the insulin-enhancing anions: Synthesis, spectroscopic characterization, and crystal structure. J. Mol. Struct. 2018, 1154, 319. [Google Scholar] [CrossRef]
- Carpéné, C.; Garcia-Vicente, S.; Serrano, M.; Marti, L.; Belles, C.; Royo, M.; Galitzky, J.; Zorzano, A.; Testar, X. Insulin-mimetic compound hexaquis (benzylammonium) decavanadate is antilipolytic in human fat cells. World J. Diabetes 2017, 8, 143. [Google Scholar] [CrossRef]
- Al-Harbi, M.S.; Hamza, R.Z. Potential Ameliorative Effects of Selenium and Chromium Supplementation Against Toxicity and Oxidative Stress in Streptozotocin Diabetic Rats. Int. J. Pharmacol. 2016, 12, 483–495. [Google Scholar] [CrossRef]
- Abuelzahab, H.; Hamza, R.; Montaser, M.; El-Mahdi, M.M.; Al-Harthi, W.A. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol. Environ. Saf. 2019, 173, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zeng, C.; Gong, Q.Y.; Yang, H.B.; Li, X.X.; Lei, G.H.; Yang, T.B. The association between dietary selenium intake and diabetes: A cross-sectional study among middle-aged and older adults. Nutr. J. 2015, 14, 18. [Google Scholar] [CrossRef]
- Tsave, O.; Yavropoulou, M.; Kafantari, M.; Gabriel, C.; Yovos, J.; Salifoglou, A. Comparative assessment of metal-specific adipogenic activity in zinc and vanadium-citrates through associated gene expression. J. Inorg. Biochem. 2018, 186, 217–227. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Motaan, S.E.; Malik, N. Protective and Antioxidant Role of Selenium Nanoparticles and Vitamin C Against Acrylamide Induced Hepatotoxicity in Male Mice. Int. J. Pharmacol. 2019, 15, 664–674. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLOS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z.; Gobouri, A.A.; Refat, M.S. Synthesis of new antidiabetic agent by complexity between vanadyl (II) sulfate and vitamin B1: Structural, characterization, anti-DNA damage, structural alterations and antioxidative damage studies. Appl. Organomet. Chem. 2019, 33, e4892. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z.; Refat, M.S. Synthesis, spectroscopic, structural and thermal characterizations of vanadyl (IV) adenine complex prospective as antidiabetic drug agent Spectrochim. Acta Part A 2015, 135, 850. [Google Scholar] [CrossRef]
- Johri, S.; Shukla, S.; Sharma, P. Role of chelating agents and antioxidants in beryllium induced toxicity. Indian J. Exp. Boil. 2002, 40, 575–582. [Google Scholar]
- Litwack, G.; Bothwell, J.W.; Williams, J.N.; Elvehjem, C.A. A Colorimetric Assay for Xanthine Oxidase in Rat Liver Homogenates. J. Biol. Chem. 1953, 200, 303. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef]
- Suzuki, H.; Ota, S.; Sasagawa, T.; Sakatani, T. Fujikura, Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 1983, 132, 345. [Google Scholar] [CrossRef]
- Litwack, G.; Bothwell, J.W.; Williams, J.N.; Elvehjem, C.A. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. J. Biol. Chem. 1953, 200, 303. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351. [Google Scholar] [CrossRef]
- Beers, J.R.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 6th ed.; Oxford & IBH Co.: Bombay/New Delhi, India, 1967. [Google Scholar]
- Hamza, R.Z.; Al-Baqami, N.M.; Khojah, E.; Mansour, A.M.A.; Al-Motaani, S.E.; Al-Salmi, F.A.; El-Megharbel, S.M. Possible Antioxidant and Antidiabetic Effects of Combretum Molle Extract in a Diabetes Mellitus Experimental Model in Male Rats. Nat. Prod. Commun. 2021, 16, 1–10. [Google Scholar]
- West, E.; Simon, O.R.; Morrison, E.Y. Streptozotocin alters pancreatic beta-cell responsiveness to glucose within six hours of injection into rats. West Indian Med. J. 1996, 45, 60. [Google Scholar]
- Treviño, S.; Diaz, A. Vanadium and insulin: Partners in metabolic regulation. J. Inorg. Biochem. 2020, 208, 111094. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Al-Salmi, F.A.; Al-Harthi, S.; Alsolami, K.; Hamza, R.Z. Chitosan/Selenium Nanoparticles Attenuate Diclofenac Sodium-Induced Testicular Toxicity in Male Rats. Crystals 2021, 11, 1477. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are Oxidative Stress−Activated Signaling Pathways Mediators of Insulin Resistance and β-Cell Dysfunction? Diabetes 2003, 52, 1. [Google Scholar] [CrossRef]
- Pepato, M.T.; Khalil, N.M.; Giocondo, M.P.; Brunetti, I.L. Vanadium and its Complexes: The Renewed Interest in its Biochemistry. Lat. Am. J. Pharm. 2008, 27, 468–476. [Google Scholar]
- El-Megharbel, S.M.; Al-Salmi, F.A.; Refat, M.S.; Hamza, R.Z. Selenium/Chitosan-Folic Acid Metal Complex Ameliorates Hepatic Damage and Oxidative Injury in Male Rats Exposed to Sodium Fluoride. Crystals 2021, 11, 1354. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Protection against reactive oxygen species by selenoproteins. Biochim. Biophys. Acta 2009, 1790, 1478–1485. [Google Scholar] [CrossRef]
- Refat, M.S.; Hamza, R.Z.; Adam, A.M.A.; Saad, H.A.; Gobouri, A.A.; Al-Harbi, F.S.; Al-Salmi, F.A.; Altalhi, T.; El-Megharbel, S.M. Quercetin/Zinc complex and stem cells: A new drug therapy to ameliorate glycometabolic control and pulmonary dysfunction in diabetes mellitus: Structural characterization and genetic studies. PLoS ONE 2021, 16, e0246265. [Google Scholar] [CrossRef]
- Ithayarasi, A.P.; Devi, C.S. Effect of alpha-tocopherol on lipid peroxidation in iso-proterenol induced myocardial infarction in rats. Indian J. Physiol. Pharmacol. 1997, 41, 369–376. [Google Scholar]
- El-Demerdash, F.M. Effects of selenium and mercury on the enzymatic activitiesand lipid peroxidation in brain, liver, and blood of rats. J. Env. Sci. Health B 2001, 36, 489–499. [Google Scholar] [CrossRef]
- Thompson, K.H.; Orvig, C. Vanadium in diabetes: 100 years from phase 0 to phaseI. J. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Stróżyk, A.; Osica, Z.; Przybylak, J.D.; Kołodziej, M.; Zalewski, B.M. Effectiveness and safety of selenium supplementation for type 2 diabetes mellitus in adults: A systematic review of randomised controlled trials. J. Hum. Nutr. Diet. 2019, 32, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Diab, A.E.A.A. Testicular protective and antioxidant effects of selenium nanoparticles on Monosodium glutamate-induced testicular structure alterations in male mice. Toxicol. Rep. 2020, 7, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Utsuyama, M.; Takiwwa, T.; Thurlbeck, W.M. Changes in lung morphologic features and elasticity caused by streptozotocin-induced diabetes mellitus in growing rats. Am. Rev. Respir. Dis. 1983, 128, 125–131. [Google Scholar] [CrossRef]
- Parkman, J.K.; Sklioutovskaya-Lopez, K.; Menikdiwela, K.R.; Freeman, L.; Moustaid-Moussa, N.; Kim, J.H. Effects of high fat diets and supplemental tart cherry and fish oil on obesity and type 2 diabetes in male and female C57BL/6J and TALLYHO/Jng mice. J. Nutr. Biochem. 2021, 94, 108644. [Google Scholar] [CrossRef] [PubMed]
| Groups | Parameters | |||
|---|---|---|---|---|
| Blood Glucose (mg/dl) | Insulin Hormone (uIU/mL) | HbA1C (mmol/mol) | Fasting Serum C-Peptide (ng/mL) | |
| Control group | 84.01 ± 3.25 e | 25.36 ± 1.25 a,b | 3.02 ± 0.65 d | 4.18 ± 0.69 a |
| STZ group | 371.25 ± 4.02 a | 4.40 ± 0.24 d | 9.51 ± 1.36 a,b | 0.52 ± 0.05 d |
| STZ plus VOSO4 group | 140.36 ± 4.03 b | 19.36 ± 2.15 c | 5.02 ± 1.02 b | 2.98 ± 0.87 c |
| STZ plus selenium tetrachloride group | 129.36 ± 2.75 c | 20.03 ± 2.11 c | 4.01 ± 0.87 c,d | 3.74 ± 0.87 b |
| STZ plus VOSO4 and selenium tetrachloride group | 97.25 ± 4.26 d,e | 23.65 ± 2.02 b | 3.54 ± 0.96 d | 4.00 ± 0.96 a |
| Groups | Parameters | |||
|---|---|---|---|---|
| Pancreatic Catalase (U/g) | Pancreatic SOD (U/g) | Pancreatic MDA (U/g) | Pancreatic GPx (U/g) | |
| Control group | 1.88 ± 0.21 a | 22.05 ± 1.15 a,b | 3.05 ± 0.48 e | 34.05 ± 1.85 a |
| STZ group | 0.26 ± 0.10 d | 5.22 ± 1.35 d | 81.15 ± 0.96 a | 7.56 ± 1.18 e |
| STZ plus VOSO4 group | 1.42 ± 0.36 c | 19.91 ± 1.58 c | 20.42 ± 1.02 b | 23.15 ± 1.15 d |
| STZ plus selenium tetrachloride group | 1.63 ± 0.48 b | 20.52 ± 2.16 b | 12.26 ± 1.45 c | 26.41 ± 1.28 c |
| STZ plus VOSO4 and selenium tetrachloride group | 1.74 ± 0.22 a | 21.19 ± 2.25 b | 8.78 ± 1.25 d | 31.58 ±1.58 b,c |
| Groups | Parameters | |
|---|---|---|
| MPO (nmol/min/mL) | XO (U/g) | |
| Control group | 15.16 ± 1.36 e | 16.25 ± 1.25 e |
| STZ group | 25.16 ± 1.15 a | 38.55 ± 1.16 a |
| STZ plus VOSO4 group | 18.24 ± 1.25 b | 20.15 ± 1.25 b |
| STZ plus selenium tetrachloride group | 17.55 ± 1.19 c | 19.65 ± 1.36 c |
| STZ plus VOSO4 and selenium tetrachloride group | 16.48 ± 2.16 d,e | 17.16 ± 1.39 d,e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Salmi, F.A.; Hamza, R.Z. RETRACTED: Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats. Curr. Issues Mol. Biol. 2022, 44, 94-104. https://doi.org/10.3390/cimb44010007
Al-Salmi FA, Hamza RZ. RETRACTED: Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats. Current Issues in Molecular Biology. 2022; 44(1):94-104. https://doi.org/10.3390/cimb44010007
Chicago/Turabian StyleAl-Salmi, Fawziah A., and Reham Z. Hamza. 2022. "RETRACTED: Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats" Current Issues in Molecular Biology 44, no. 1: 94-104. https://doi.org/10.3390/cimb44010007
APA StyleAl-Salmi, F. A., & Hamza, R. Z. (2022). RETRACTED: Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats. Current Issues in Molecular Biology, 44(1), 94-104. https://doi.org/10.3390/cimb44010007






