Unveiling a Hidden Biomarker of Inflammation and Tumor Progression: The 65 kDa Isoform of MMP-9 New Horizons for Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
- Lung cancer at an advanced stage, treated with Opdivo.
- Lung cancer at an advanced stage, treated with Opdivo.
- Breast cancer at an early stage treated with paclitaxel adjuvant.
- Advanced breast cancer with liver metastases treated with docetaxel (taxotere), in combination with pertuzumab (Perjeta) and trastuzumab (Herceptin).
2.2. Detection of Gelatinases by 2-D Gelatin Zymography (2-DZ)
2.3. Western Blot Analysis
- -
- Anti-MMP-9 (Ab-8) mAb (IA5) at a concentration of 2.66 µg/mL (Calbiochem, Milano, Italy). This antibody recognizes both the 92 kDa latent and the 86 kDa active forms.
- -
- Anti-NGAL Ab (5G5) at a concentration of 2.0 µg/mL (EuroClone, Pero, MI, Italy). It recognizes the human NGAL.
- -
- Anti-MMP-9 (4A3) Ab at a concentration of 2.0 µg/mL (Novus Biologicals, Segrate, MI, Italy). It recognizes both the 82 kDa and the 65 kD active forms,
- -
- Anti-MMP-2 Ab (Ab-4) mAb (75-7F7) at a concentration of 1.0 µg/mL (Calbiochem, Milano, Italy). It recognizes the both 72 kDa latent and the 66 kDa active forms.
2.4. Gelatinase Purification
3. Results
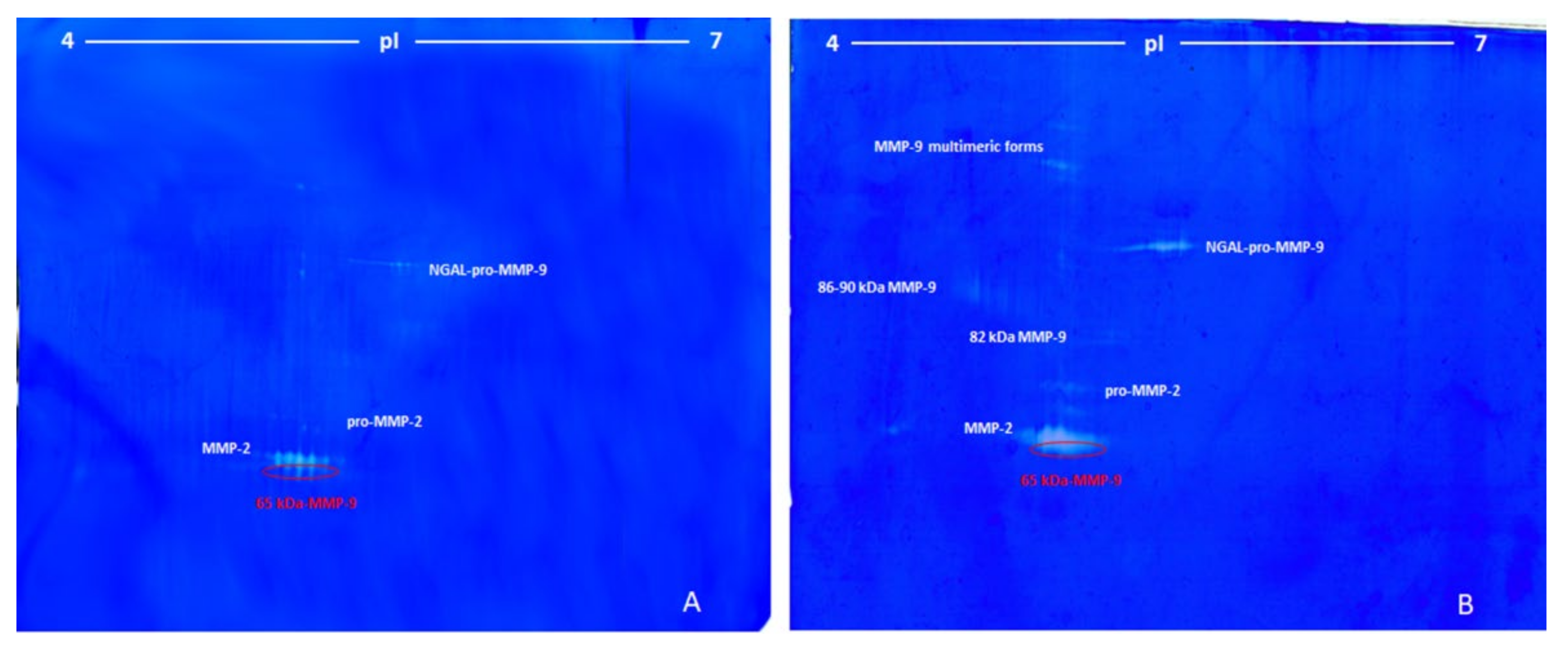
3.1. Identification of MMP-9 on Zymograms
- 1)
- The pro-MMP-9—a broad spot around Mw 90–92 kDa and pI 4.2–5.0;
- 2), 3)
- The activated MMP-9—two groups of spots of about 86 and 82 kDa and pI range of 4.2–5.0 and 4.2–4.6, respectively;
- 4)
- The 65–67 kDa MMP-9 active form—a group of spots with pI range 4.6–4.9, corresponding to the marker suggested in this work.
3.2. Lung Cancer
- (1)
- The first group was made of some unresolved spots around 120 kDa and pI between 5.50 and 5.72 (ascribed to the complex NGAL-pro-MMP-9).
- (2)
- The second group (three spots) was located at around 72 kDa and pI 5.25–5.40 (pro-MMP-2).
- (3)
- The third group, characterized by the highest proteolytic activity, consisted of seven spots of 68 kDa at pI between 5.11–5.45 (MMP-2).
- (4)
- The fourth and last group was made of three spots at 65 kDa and pI between 5.20 and 5.35 (65-kDa MMP-9 active form).
3.3. Breast Cancer
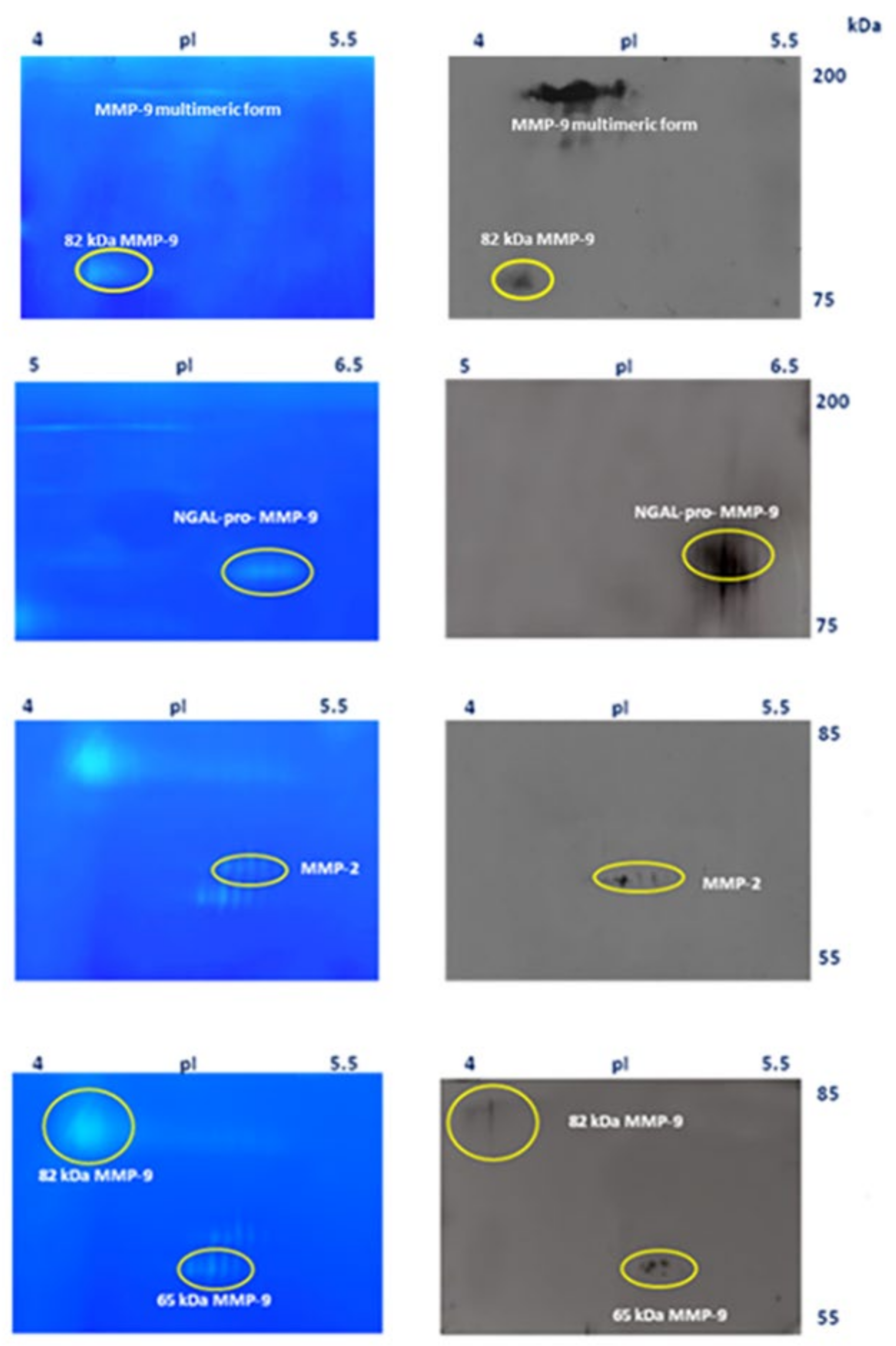
3.4. Identification of MMP-9 and MMP-2 by Western Blot Analysis
4. Discussion
4.1. On the Origin of the 65 kDa MMP-9 and the Inhibition of Its Formation
4.2. The Urokinase System
4.3. Peptidyl Arginase Deiminases (PAD) and MMP-9 Citrullination
4.4. Cathepsin K
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nat. Cell Biol. 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ravindranathan, D.; Master, V.; Bilen, M. Inflammatory Markers in Cancer Immunotherapy. Biology 2021, 10, 325. [Google Scholar] [CrossRef]
- Barraclough, R.; Vasieva, O.; Bell, R.B.A.O.V.R. Gene Expression Meta-Analysis of Potential Metastatic Breast Cancer Markers. Curr. Mol. Med. 2017, 17, 200–210. [Google Scholar] [CrossRef][Green Version]
- Pouliquen, D.L.; Boissard, A.; Coqueret, O.; Guette, C. Biomarkers of tumor invasiveness in proteomics (Review). Int. J. Oncol. 2020, 57, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Leber, M.F.; Efferth, T. Molecular principles of cancer invasion and metastasis (review). Int. J. Oncol. 2009, 34, 881–895. [Google Scholar] [PubMed]
- Barillari, G. The Impact of Matrix Metalloproteinase-9 on the Sequential Steps of the Metastatic Process. Int. J. Mol. Sci. 2020, 21, 4526. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Psaila, B.; Kaplan, R.N.; Port, E.R.; Lyden, D. Priming the ‘Soil’ for Breast Cancer Metastasis: The Pre-Metastatic Niche. Breast Dis. 2007, 26, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Shay, G.; Lynch, C.C.; Fingleton, B. Moving targets: Emerging roles for MMPs in cancer progression and metastasis. Matrix Biol. 2015, 44–46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix Metalloproteinases As Novel Biomarker s and Potential Therapeutic Targets in Human Cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef]
- Radisky, E.S.; Raeeszadeh-Sarmazdeh, M.; Radisky, D.C. Therapeutic Potential of Matrix Metalloproteinase Inhibition in Breast Cancer. J. Cell. Biochem. 2017, 118, 3531–3548. [Google Scholar] [CrossRef] [PubMed]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metallo-proteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Cauwe, B.; Opdenakker, G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 351–423. [Google Scholar] [CrossRef]
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2010, 41, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Boon, L.; Ugarte-Berzal, E.; Martens, E.; Fiten, P.; Vandooren, J.; Janssens, R.; Blanter, M.; Yu, K.; Boon, M.; Struyf, S.; et al. Citrullination as a novel posttranslational modification of matrix metalloproteinases. Matrix Biol. 2021, 95, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Madzharova, E.; Kastl, P.; Sabino, F.; Auf dem Keller, U. Post-translational modification-dependent activity of matrix met-alloproteinases. Int. J. Mol. Sci. 2019, 20, 3077. [Google Scholar] [CrossRef]
- Rossano, R.; Larocca, M.; Riviello, L.; Coniglio, M.G.; Vandooren, J.; Liuzzi, G.M.; Opdenakker, G.; Riccio, P. Heterogeneity of serum gelatinases MMP-2 and MMP-9 isoforms and charge variants. J. Cell Mol. Med. 2014, 18, 242–252. [Google Scholar] [CrossRef]
- Christensen, J.; Shastri, V.P. Matrix-metalloproteinase-9 is cleaved and activated by cathepsin K. BMC Res. Notes 2015, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Enghild, J.; Nagase, H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J. Biol. Chem. 1992, 267, 3581–3584. [Google Scholar] [CrossRef]
- Inuzuka, K.; Ogata, Y.; Nagase, H.; Shirouzu, K. Significance of Coexpression of Urokinase-type Plasminogen Activator, and Matrix Metalloproteinase 3 (Stromelysin) and 9 (Gelatinase B) in Colorectal Carcinoma. J. Surg. Res. 2000, 93, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Gonoji, Y.; Naka, K.; Tomita, K.; Nakanishi, I.; Iwata, K.; Yamashita, K.; Hayakawa, T. Matrix metalloproteinase 9 (92-kDa gelatinase/type IV collagenase) from HT 1080 human fibrosarcoma cells. Purification and activation of the precursor and enzymic properties. J. Biol. Chem. 1992, 267, 21712–21719. [Google Scholar] [CrossRef]
- Lovett, D.H.; Mahimkar, R.; Raffai, R.L.; Cape, L.; Maklashina, E.; Cecchini, G.; Karliner, J.S. A Novel Intracellular Isoform of Matrix Metalloproteinase-2 Induced by Oxidative Stress Activates Innate Immunity. PLoS ONE 2012, 7, e34177. [Google Scholar] [CrossRef]
- Bellini, T.; Trentini, A.; Manfrinato, M.C.; Tamborino, C.; Volta, C.A.; Di Foggia, V.; Fainardi, E.; Dallocchio, F.; Castellazzi, M. Matrix metalloproteinase-9 activity detected in body fluids is the result of two different enzyme forms. J. Biochem. 2012, 151, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Trentini, A.; Manfrinato, M.C.; Castellazzi, M.; Tamborino, C.; Roversi, G.; Volta, C.A.; Baldi, E.; Tola, M.R.; Granieri, E.; Dallocchio, F.; et al. Emilia-Romagna network for Multiple Sclerosis (ERMES) study group. TIMP-1 re-sistant matrix metalloproteinase-9 is the predominant serum active isoform associated with MRI activity in patients with multiple sclerosis. Mult. Scler. 2015, 21, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- La Rocca, G.; Pucci-Minafra, I.; Marrazzo, A.; Taormina, P.; Minafra, S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. Br. J. Cancer 2004, 90, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Yousef, E.M.; Tahir, M.R.; St-Pierre, Y.; Gaboury, L.A. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer 2014, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Abu Rabi, Z.; Todorović-Raković, N.; Vujasinović, T.; Milovanović, J.; Nikolić-Vukosavljević, D. Markers of progression and invasion in short term follow up of untreated breast cancer patients. Cancer Biomark. 2015, 15, 745–754. [Google Scholar] [CrossRef]
- Avolio, C.; Ruggieri, M.; Giuliani, F.; Liuzzi, G.M.; Leante, R.; Riccio, P.; Livrea, P.; Trojano, M. Serum MMP-2 and MMP-9 are elevated in different multiple sclerosis subtypes. J. Neuroimmunol. 2003, 136, 46–53. [Google Scholar] [CrossRef]
- Alam, M.; Shuaib, A. Complexity in differentiating the expression of truncated or matured forms of MMP-2 and MMP-9 through zymography in rat brain tissues after acute ischaemic stroke. J. Neurosci. Methods 2013, 216, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R. Nutrition Facts in Multiple Sclerosis. ASN Neuro 2015, 7, 1759091414568185. [Google Scholar] [CrossRef] [PubMed]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; de Jesus, A.A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 2021, 6. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Han, B.; Siegel, E.; Cui, Y.; Giuliano, A.; Cui, X. Breast cancer lung metastasis: Molecular biology and therapeutic im-plications. Cancer Biol. Ther. 2018, 19, 858–868. [Google Scholar] [CrossRef]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, X.; Zhu, G.; Ye, H. Circulating uPA as a potential prognostic biomarker for resectable esophageal squamous cell carcinoma. Medicine 2019, 98, e14717. [Google Scholar] [CrossRef]
- Rath, M.; Pauling, L. Plasmin-induced proteolysis and the role of apoprotein (a), lysine and synthetic analogs. Orthomol. Med. 1992, 7, 17–23. [Google Scholar]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Down-regulation of urokinase plasminogen activator and matrix metalloproteinases and up-regulation of their inhibitors by a novel nutrient mixture in human prostate cancer cell lines PC-3 and DU-145. Oncol. Rep. 2011, 26, 1407–1413. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Latronico, T.; Branà, M.T.; Gramegna, P.; Coniglio, M.G.; Rossano, R.; Larocca, M.; Riccio, P. Struc-ture-dependent inhibition of gelatinases by dietary antioxidants in rat astrocytes and sera of multiple sclerosis patients. Neurochem. Res. 2011, 36, 518–527. [Google Scholar] [CrossRef]
- Amara, F.; Berbenni, M.; Fragni, M.; Leoni, G.; Viggiani, S.; Ippolito, V.M.; Larocca, M.; Rossano, R.; Alberghina, L.; Riccio, P.; et al. Neuroprotection by cocktails of dietary antioxidants under conditions of nerve growth factor depriva-tion. Oxidative Med. Cell. Longev. 2015, 2015, 217258. [Google Scholar] [CrossRef]
- Yuzhalin, A.E. Citrullination in Cancer. Cancer Res. 2019, 79, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R.; Larocca, M.; Trotta, V.; Mennella, I.; Vitaglione, P.; Ettorre, M.; Graverini, A.; De Santis, A.; Di Monte, E.; et al. Anti-inflammatory nutritional intervention in patients with relapsing-remitting and primary-progressive multiple sclerosis: A pilot study. Exp. Biol. Med. 2016, 241, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Duong, L.T.; Wesolowski, G.A.; Leung, P.; Oballa, R.; Pickarski, M. Efficacy of a Cathepsin K Inhibitor in a Preclinical Model for Prevention and Treatment of Breast Cancer Bone Metastasis. Mol. Cancer Ther. 2014, 13, 2898–2909. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossano, R.; Larocca, M.; Macellaro, M.; Bilancia, D.; Riccio, P. Unveiling a Hidden Biomarker of Inflammation and Tumor Progression: The 65 kDa Isoform of MMP-9 New Horizons for Therapy. Curr. Issues Mol. Biol. 2022, 44, 105-116. https://doi.org/10.3390/cimb44010008
Rossano R, Larocca M, Macellaro M, Bilancia D, Riccio P. Unveiling a Hidden Biomarker of Inflammation and Tumor Progression: The 65 kDa Isoform of MMP-9 New Horizons for Therapy. Current Issues in Molecular Biology. 2022; 44(1):105-116. https://doi.org/10.3390/cimb44010008
Chicago/Turabian StyleRossano, Rocco, Marilena Larocca, Margherita Macellaro, Domenico Bilancia, and Paolo Riccio. 2022. "Unveiling a Hidden Biomarker of Inflammation and Tumor Progression: The 65 kDa Isoform of MMP-9 New Horizons for Therapy" Current Issues in Molecular Biology 44, no. 1: 105-116. https://doi.org/10.3390/cimb44010008
APA StyleRossano, R., Larocca, M., Macellaro, M., Bilancia, D., & Riccio, P. (2022). Unveiling a Hidden Biomarker of Inflammation and Tumor Progression: The 65 kDa Isoform of MMP-9 New Horizons for Therapy. Current Issues in Molecular Biology, 44(1), 105-116. https://doi.org/10.3390/cimb44010008





