Circulating miRNAs Related to Epithelial–Mesenchymal Transitions (EMT) as the New Molecular Markers in Endometriosis
Abstract
1. Introduction
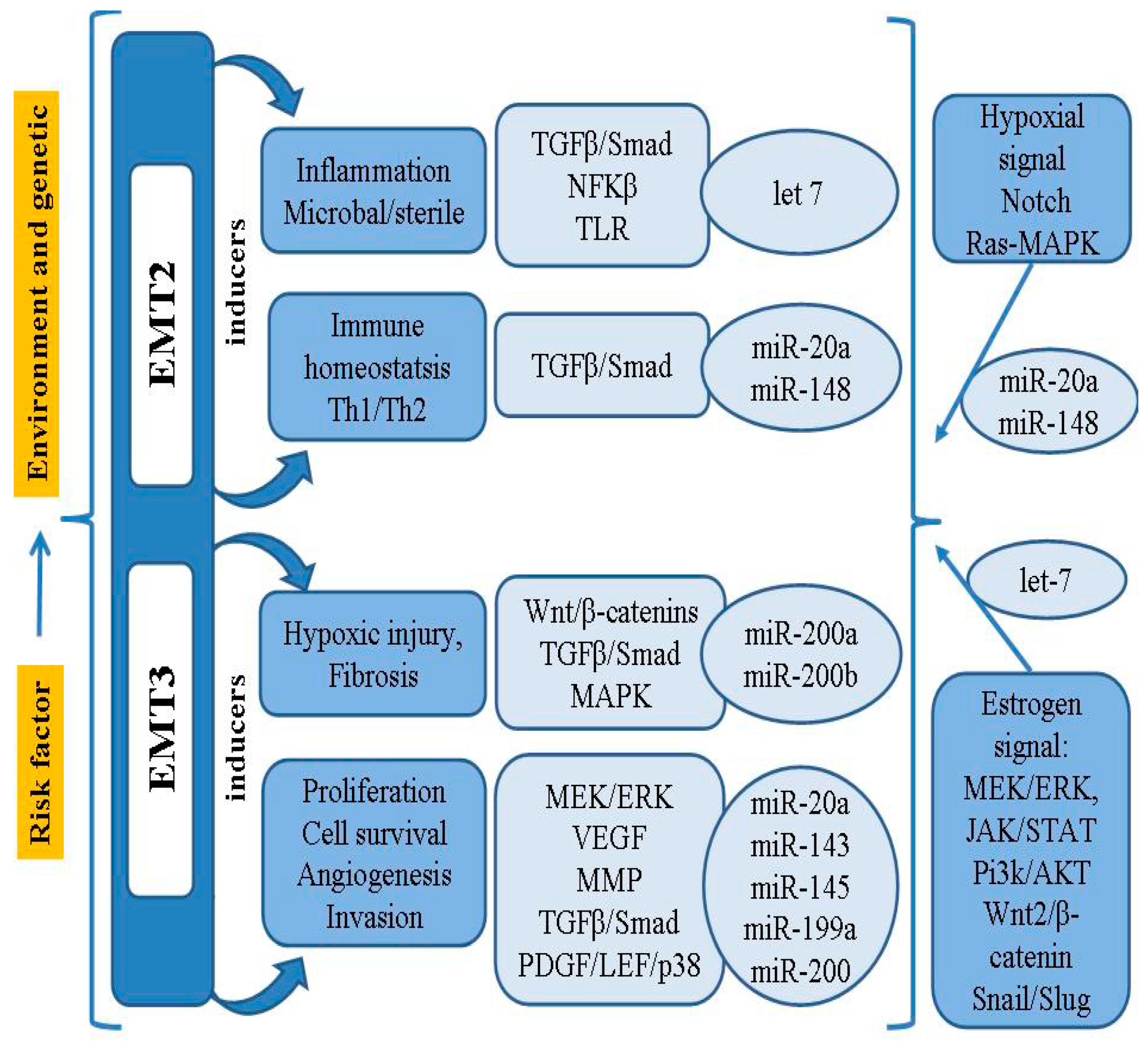
2. Key EMT Regulators Related to Endometriosis
3. Role of miRNAs in the Pathophysiology of Endometriosis
4. Candidates of Circulating miRNAs Related to EMT in Endometriosis
4.1. miR-200
4.2. miR-20a
4.3. miR-199a
4.4. miR-143 and miR-145
4.5. Let-7
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moga, M.A.; Bălan, A.; Dimienescu, O.G.; Burtea, V.; Dragomir, R.M.; Anastasiu, C.V. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer-An Overview. J. Clin. Med. 2019, 8, 735. [Google Scholar] [CrossRef]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet Lond. Engl. 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef]
- Johnson, N.P.; Hummelshoj, L.; World Endometriosis Society Montpellier Consortium. Consensus on current management of endometriosis. Hum. Reprod. Oxf. Engl. 2013, 28, 1552–1568. [Google Scholar] [CrossRef] [PubMed]
- Kavoussi, S.K.; Lim, C.S.; Skinner, B.D.; Lebovic, D.I.; As-Sanie, S. New paradigms in the diagnosis and management of endometriosis. Curr. Opin. Obstet. Gynecol. 2016, 28, 267–276. [Google Scholar] [CrossRef]
- Kajihara, H.; Yamada, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Sado, T.; Oi, H.; Kobayashi, H. New insights into the pathophysiology of endometriosis: From chronic inflammation to danger signal. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2011, 27, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Götte, M.; Viganò, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef]
- Sarria-Santamera, A.; Orazumbekova, B.; Terzic, M.; Issanov, A.; Chaowen, C.; Asúnsolo-Del-Barco, A. Systematic Review and Meta-Analysis of Incidence and Prevalence of Endometriosis. Healthcare 2020, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Bougie, O.; Yap, M.I.; Sikora, L.; Flaxman, T.; Singh, S. Influence of race/ethnicity on prevalence and presentation of endometriosis: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1104–1115. [Google Scholar] [CrossRef]
- Gerlinger, C.; Faustmann, T.; Hassall, J.J.; Seitz, C. Treatment of endometriosis in different ethnic populations: A meta-analysis of two clinical trials. BMC Womens Health 2012, 12, 9. [Google Scholar] [CrossRef]
- Asghari, S.; Valizadeh, A.; Aghebati-Maleki, L.; Nouri, M.; Yousefi, M. Endometriosis: Perspective, lights, and shadows of etiology. Biomed. Pharmacother. 2018, 106, 163–174. [Google Scholar] [CrossRef]
- Borghese, B.; Zondervan, K.T.; Abrao, M.S.; Chapron, C.; Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 2017, 91, 254–264. [Google Scholar] [CrossRef]
- Albertsen, H.M.; Ward, K. Genes Linked to Endometriosis by GWAS Are Integral to Cytoskeleton Regulation and Suggests That Mesothelial Barrier Homeostasis Is a Factor in the Pathogenesis of Endometriosis. Reprod. Sci. 2017, 24, 803–811. [Google Scholar] [CrossRef]
- Yovich, J.L.; Rowlands, P.K.; Lingham, S.; Sillender, M.; Srinivasan, S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod. Biomed. Online 2020, 40, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Sekhon, L.; Krajcir, N.; Cocuzza, M.; Falcone, T. Serum and peritoneal abnormalities in endometriosis: Potential use as diagnostic markers. Minerva Ginecol. 2006, 58, 527–551. [Google Scholar]
- Laganà, A.S.; Salmeri, F.M.; Ban Frangež, H.; Ghezzi, F.; Vrtačnik-Bokal, E.; Granese, R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 441–444. [Google Scholar] [CrossRef]
- Eggers, J.C.; Martino, V.; Reinbold, R.; Schäfer, S.D.; Kiesel, L.; Starzinski-Powitz, A.; Schüring, A.N.; Kemper, B.; Greve, B.; Götte, M. microRNA miR-200b affects proliferation, invasiveness and stemness of endometriotic cells by targeting ZEB1, ZEB2 and KLF4. Reprod. Biomed. Online 2016, 32, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, N.; Taylor, H.S. Endometriosis and Stem Cell Trafficking. Reprod. Sci. 2016, 23, 1616–1619. [Google Scholar] [CrossRef]
- Laganà, A.S.; Salmeri, F.M.; Vitale, S.G.; Triolo, O.; Götte, M. Stem Cell Trafficking During Endometriosis: May Epigenetics Play a Pivotal Role? Reprod. Sci. 2018, 25, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J.; Jülicher, A.; Hotz, B.; Mechsner, S.; Hotz, H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch. Gynecol. Obstet. 2014, 289, 871–881. [Google Scholar] [CrossRef]
- Mashayekhi, P.; Noruzinia, M.; Zeinali, S.; Khodaverdi, S. Endometriotic Mesenchymal Stem Cells Epigenetic Pathogenesis: Deregulation of miR-200b, miR-145, and let7b in A Functional Imbalanced Epigenetic Disease. Cell J. 2019, 21, 179–185. [Google Scholar]
- Anger, D.L.; Zhang, B.; Boutross-Tadross, O.; Foster, W.G. Tyrosine receptor kinase B (TrkB) protein expression in the human endometrium. Endocrine 2007, 31, 167–173. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-M.; Yang, W.-X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Herington, J.L.; Bruner-Tran, K.L.; Lucas, J.A.; Osteen, K.G. Immune interactions in endometriosis. Expert Rev. Clin. Immunol. 2011, 7, 611–626. [Google Scholar] [CrossRef]
- Podgaec, S.; Dias Junior, J.A.; Chapron, C.; Oliveira, R.M.D.; Baracat, E.C.; Abrão, M.S. Th1 and Th2 ummune responses related to pelvic endometriosis. Rev. Assoc. Medica Bras. 1992 2010, 56, 92–98. [Google Scholar]
- Grund, E.M.; Kagan, D.; Tran, C.A.; Zeitvogel, A.; Starzinski-Powitz, A.; Nataraja, S.; Palmer, S.S. Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells. Mol. Pharmacol. 2008, 73, 1394–1404. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- González-Ramos, R.; Van Langendonckt, A.; Defrère, S.; Lousse, J.-C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Leconte, M.; Nicco, C.; Ngô, C.; Chéreau, C.; Chouzenoux, S.; Marut, W.; Guibourdenche, J.; Arkwright, S.; Weill, B.; Chapron, C.; et al. The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am. J. Pathol. 2011, 179, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Tang, N.; Wang, J.; Zhang, Y.; Chang, J.; Liu, Z.; Liu, H. TGR5 agonist INT-777 mitigates inflammatory response in human endometriotic stromal cells: A therapeutic implication for endometriosis. Int. Immunopharmacol. 2019, 71, 93–99. [Google Scholar] [CrossRef]
- Ngô, C.; Chéreau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, X.; Guo, S.-W. Platelets and Regulatory T Cells May Induce a Type 2 Immunity That Is Conducive to the Progression and Fibrogenesis of Endometriosis. Front. Immunol. 2020, 11, 610963. [Google Scholar] [CrossRef]
- Omwandho, C.O.A.; Konrad, L.; Halis, G.; Oehmke, F.; Tinneberg, H.-R. Role of TGF-betas in normal human endometrium and endometriosis. Hum. Reprod. Oxf. Engl. 2010, 25, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Itoga, T.; Matsumoto, T.; Takeuchi, H.; Yamasaki, S.; Sasahara, N.; Hoshi, T.; Kinoshita, K. Fibrosis and smooth muscle metaplasia in rectovaginal endometriosis. Pathol. Int. 2003, 53, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Liu, X.; Xu, H.; Guo, S.-W. Mesothelial Cells Participate in Endometriosis Fibrogenesis through Platelet-Induced Mesothelial-Mesenchymal Transition. J. Clin. Endocrinol. Metab. 2020, 105, e4124–e4147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Duan, J.; Liu, X.; Guo, S.-W. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol. Cell. Endocrinol. 2016, 428, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Duan, J.; Olson, M.; Fazleabas, A.; Guo, S.-W. Cellular Changes Consistent with Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 2016, 23, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dai, Y.; Zhu, H.; Jiang, Y.; Zhang, S. Endometriotic mesenchymal stem cells significantly promote fibrogenesis in ovarian endometrioma through the Wnt/β-catenin pathway by paracrine production of TGF-β1 and Wnt1. Hum. Reprod. Oxf. Engl. 2016, 31, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Involvement of the Wnt/β-Catenin Signaling Pathway in the Cellular and Molecular Mechanisms of Fibrosis in Endometriosis. PLoS ONE 2013, 8, e76808. [Google Scholar] [CrossRef]
- Douchi, D.; Ohtsuka, H.; Ariake, K.; Masuda, K.; Kawasaki, S.; Kawaguchi, K.; Fukase, K.; Oikawa, M.; Motoi, F.; Naitoh, T.; et al. Silencing of LRRFIP1 reverses the epithelial-mesenchymal transition via inhibition of the Wnt/β-catenin signaling pathway. Cancer Lett. 2015, 365, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ghahhari, N.M.; Babashah, S. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur. J. Cancer Oxf. Engl. 1990 2015, 51, 1638–1649. [Google Scholar] [CrossRef]
- Loverro, G.; Maiorano, E.; Napoli, A.; Selvaggi, L.; Marra, E.; Perlino, E. Transforming growth factor-beta 1 and insulin-like growth factor-1 expression in ovarian endometriotic cysts: A preliminary study. Int. J. Mol. Med. 2001, 7, 423–429. [Google Scholar]
- Young, V.J.; Ahmad, S.F.; Brown, J.K.; Duncan, W.C.; Horne, A.W. Peritoneal VEGF-A expression is regulated by TGF-β1 through an ID1 pathway in women with endometriosis. Sci. Rep. 2015, 5, 16859. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.W.; Zhang, R.; Tan, Z.; Chung, J.P.W.; Zhang, T.; Wang, C.C. Pharmaceuticals targeting signaling pathways of endometriosis as potential new medical treatment: A review. Med. Res. Rev. 2021, 41, 2489–2564. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, T.; Man, G.C.W.; May, K.E.; Becker, C.M.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wong, A.W.Y.; et al. Vascular endothelial growth factor C is increased in endometrium and promotes endothelial functions, vascular permeability and angiogenesis and growth of endometriosis. Angiogenesis 2013, 16, 541–551. [Google Scholar] [CrossRef]
- Sharkey, A.M.; Day, K.; McPherson, A.; Malik, S.; Licence, D.; Smith, S.K.; Charnock-Jones, D.S. Vascular endothelial growth factor expression in human endometrium is regulated by hypoxia. J. Clin. Endocrinol. Metab. 2000, 85, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.N.; Yu, J.; Torres, P.B.; Schickedanz, A.C.; Park, J.K.; Mueller, M.D.; Sidell, N. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod. Sci. 2009, 16, 140–146. [Google Scholar] [CrossRef]
- Lin, S.-C.; Lee, H.-C.; Hsu, C.-T.; Huang, Y.-H.; Li, W.-N.; Hsu, P.-L.; Wu, M.-H.; Tsai, S.-J. Targeting Anthrax Toxin Receptor 2 Ameliorates Endometriosis Progression. Theranostics 2019, 9, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Dai, Y.; Xu, W.; Shi, L.; Jin, X.; Li, C.; Zhou, F.; Pan, Y.; Zhang, Y.; Lin, X.; et al. Hypoxia Promotes Ectopic Adhesion Ability of Endometrial Stromal Cells via TGF-β1/Smad Signaling in Endometriosis. Endocrinology 2018, 159, 1630–1641. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-N.; Wu, M.-H.; Tsai, S.-J. Hypoxia and reproductive health: The role of hypoxia in the development and progression of endometriosis. Reprod. Camb. Engl. 2021, 161, F19–F31. [Google Scholar]
- Bruner-Tran, K.L.; Eisenberg, E.; Yeaman, G.R.; Anderson, T.A.; McBean, J.; Osteen, K.G. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J. Clin. Endocrinol. Metab. 2002, 87, 4782–4791. [Google Scholar] [CrossRef]
- Jana, S.; Chatterjee, K.; Ray, A.K.; DasMahapatra, P.; Swarnakar, S. Regulation of Matrix Metalloproteinase-2 Activity by COX-2-PGE2-pAKT Axis Promotes Angiogenesis in Endometriosis. PLoS ONE 2016, 11, e0163540. [Google Scholar] [CrossRef] [PubMed]
- Pino, M.; Galleguillos, C.; Torres, M.; Sovino, H.; Fuentes, A.; Boric, M.A.; Johnson, M.C. Association between MMP1 and MMP9 activities and ICAM1 cleavage induced by tumor necrosis factor in stromal cell cultures from eutopic endometria of women with endometriosis. Reprod. Camb. Engl. 2009, 138, 837–847. [Google Scholar] [CrossRef][Green Version]
- Yu, M.-M.; Zhou, Q.-M. 3,6-dihydroxyflavone suppresses the epithelial-mesenchymal transition, migration and invasion in endometrial stromal cells by inhibiting the Notch signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4009–4017. [Google Scholar]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. Oxf. Engl. 2005, 20, 2698–2704. [Google Scholar] [CrossRef]
- Scarselli, G.; Rizzello, F.; Cammilli, F.; Ginocchini, L.; Coccia, M.E. Diagnosis and treatment of endometriosis. A review. Minerva Ginecol. 2005, 57, 55–78. [Google Scholar]
- Chamié, L.P.; Blasbalg, R.; Pereira, R.M.A.; Warmbrand, G.; Serafini, P.C. Findings of pelvic endometriosis at transvaginal US, MR imaging, and laparoscopy. Radiogr. Rev. Publ. Radiol. Soc. N. Am. Inc. 2011, 31, E77–E100. [Google Scholar] [CrossRef]
- Hull, M.L.; Nisenblat, V. Tissue and circulating microRNA influence reproductive function in endometrial disease. Reprod. Biomed. Online 2013, 27, 515–529. [Google Scholar] [CrossRef]
- Braza-Boïls, A.; Marí-Alexandre, J.; Gilabert, J.; Sánchez-Izquierdo, D.; España, F.; Estellés, A.; Gilabert-Estellés, J. MicroRNA expression profile in endometriosis: Its relation to angiogenesis and fibrinolytic factors. Hum. Reprod. Oxf. Engl. 2014, 29, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Marí-Alexandre, J.; García-Oms, J.; Barceló-Molina, M.; Gilabert-Aguilar, J.; Estellés, A.; Braza-Boíls, A.; Gilabert-Estellés, J. MicroRNAs and angiogenesis in endometriosis. Thromb. Res. 2015, 135 (Suppl. 1), S38–S40. [Google Scholar] [CrossRef]
- Nyholt, D.R.; Low, S.-K.; Anderson, C.A.; Painter, J.N.; Uno, S.; Morris, A.P.; MacGregor, S.; Gordon, S.D.; Henders, A.K.; Martin, N.G.; et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat. Genet. 2012, 44, 1355–1359. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Nyholt, D.R.; Morris, A.P.; Missmer, S.A.; Montgomery, G.W.; Zondervan, K.T. Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum. Reprod. Update 2014, 20, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Haider, B.A.; Baras, A.S.; McCall, M.N.; Hertel, J.A.; Cornish, T.C.; Halushka, M.K. A critical evaluation of microRNA biomarkers in non-neoplastic disease. PLoS ONE 2014, 9, e89565. [Google Scholar] [CrossRef]
- Khalaj, K.; Miller, J.E.; Lingegowda, H.; Fazleabas, A.T.; Young, S.L.; Lessey, B.A.; Koti, M.; Tayade, C. Extracellular vesicles from endometriosis patients are characterized by a unique miRNA-lncRNA signature. JCI Insight 2019, 4, e128846. [Google Scholar] [CrossRef] [PubMed]
- Michell, D.L.; Vickers, K.C. Lipoprotein carriers of microRNAs. Biochim. Biophys. Acta 2016, 1861, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Petracco, R.; Dias, A.C.D.O.; Taylor, H.; Petracco, Á.; Badalotti, M.; Michelon, J.D.R.; Marinowic, D.R.; Hentschke, M.; Azevedo, P.N.D.; Zanirati, G.; et al. Evaluation of miR-135a/b expression in endometriosis lesions. Biomed. Rep. 2019, 11, 181–187. [Google Scholar] [CrossRef]
- Cho, S.; Mutlu, L.; Grechukhina, O.; Taylor, H.S. Circulating microRNAs as potential biomarkers for endometriosis. Fertil. Steril. 2015, 103, 1252–1260.e1. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-Z.; Yang, Y.; Lang, J.; Sun, P.; Leng, J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum. Reprod. Oxf. Engl. 2013, 28, 322–330. [Google Scholar] [CrossRef]
- Rekker, K.; Saare, M.; Roost, A.M.; Kaart, T.; Sõritsa, D.; Karro, H.; Sõritsa, A.; Simón, C.; Salumets, A.; Peters, M. Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil. Steril. 2015, 104, 938–946.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-T.; Zhao, Y.-N.; Han, B.-W.; Hong, S.-J.; Chen, Y.-Q. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J. Clin. Endocrinol. Metab. 2013, 98, 281–289. [Google Scholar] [CrossRef]
- Saare, M.; Rekker, K.; Laisk-Podar, T.; Rahmioglu, N.; Zondervan, K.; Salumets, A.; Götte, M.; Peters, M. Challenges in endometriosis miRNA studies—From tissue heterogeneity to disease specific miRNAs. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2282–2292. [Google Scholar] [CrossRef]
- Agrawal, S.; Tapmeier, T.; Rahmioglu, N.; Kirtley, S.; Zondervan, K.; Becker, C. The miRNA Mirage: How Close Are We to Finding a Non-Invasive Diagnostic Biomarker in Endometriosis? A Systematic Review. Int. J. Mol. Sci. 2018, 19, 599. [Google Scholar] [CrossRef]
- Papari, E.; Noruzinia, M.; Kashani, L.; Foster, W.G. Identification of candidate microRNA markers of endometriosis with the use of next-generation sequencing and quantitative real-time polymerase chain reaction. Fertil. Steril. 2020, 113, 1232–1241. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Taheri, M. Role of Non-coding RNAs in the Pathogenesis of Endometriosis. Front. Oncol. 2020, 10, 1370. [Google Scholar] [CrossRef]
- Moustafa, S.; Burn, M.; Mamillapalli, R.; Nematian, S.; Flores, V.; Taylor, H.S. Accurate diagnosis of endometriosis using serum microRNAs. Am. J. Obstet. Gynecol. 2020, 223, e1–e557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Yuan, M.; Li, D.; Sun, C.; Wang, G. Serum Exosomal MicroRNAs as Potential Circulating Biomarkers for Endometriosis. Dis. Markers 2020, 2020, 2456340. [Google Scholar] [CrossRef]
- Bashti, O.; Noruzinia, M.; Garshasbi, M.; Abtahi, M. miR-31 and miR-145 as Potential Non-Invasive Regulatory Biomarkers in Patients with Endometriosis. Cell J. 2018, 20, 84–89. [Google Scholar] [PubMed]
- Pateisky, P.; Pils, D.; Szabo, L.; Kuessel, L.; Husslein, H.; Schmitz, A.; Wenzl, R.; Yotova, I. hsa-miRNA-154-5p expression in plasma of endometriosis patients is a potential diagnostic marker for the disease. Reprod. Biomed. Online 2018, 37, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.-H.; Yuan, M.; Li, D.; Wang, G.-Y. Exosomal miR-22-3p derived from peritoneal macrophages enhances proliferation, migration, and invasion of ectopic endometrial stromal cells through regulation of the SIRT1/NF-κB signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 571–580. [Google Scholar] [PubMed]
- Kästingschäfer, C.S.; Schäfer, S.D.; Kiesel, L.; Götte, M. miR-142-3p is a novel regulator of cell viability and proinflammatory signalling in endometrial stroma cells. Reprod. Biomed. Online 2015, 30, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Jin, D.; Zhang, Y. Serum miR-17, IL-4, and IL-6 levels for diagnosis of endometriosis. Medicine (Baltimore) 2018, 97, e10853. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Zhao, Z.; Gao, B.; Meng, L.; Feng, X. miR-146b level and variants is associated with endometriosis related macrophages phenotype and plays a pivotal role in the endometriotic pain symptom. Taiwan J. Obstet. Gynecol. 2019, 58, 401–408. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.; Bersinger, N.A.; Wotzkow, C.; Mueller, M.D. Endometriosis-associated nerve fibers, peritoneal fluid cytokine concentrations, and pain in endometriotic lesions from different locations. Fertil. Steril. 2012, 97, 373–380. [Google Scholar] [CrossRef]
- Nematian, S.E.; Mamillapalli, R.; Kadakia, T.S.; Majidi Zolbin, M.; Moustafa, S.; Taylor, H.S. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. J. Clin. Endocrinol. Metab. 2018, 103, 64–74. [Google Scholar] [CrossRef]
- Kats, R.; Al-Akoum, M.; Guay, S.; Metz, C.; Akoum, A. Cycle-dependent expression of macrophage migration inhibitory factor in the human endometrium. Hum. Reprod. Oxf. Engl. 2005, 20, 3518–3525. [Google Scholar] [CrossRef]
- Akoum, A.; Kong, J.; Metz, C.; Beaumont, M.C. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil. Steril. 2002, 77, 989–994. [Google Scholar] [CrossRef]
- Morin, M.; Bellehumeur, C.; Therriault, M.-J.; Metz, C.; Maheux, R.; Akoum, A. Elevated levels of macrophage migration inhibitory factor in the peripheral blood of women with endometriosis. Fertil. Steril. 2005, 83, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kats, R.; Metz, C.N.; Akoum, A. Macrophage migration inhibitory factor is markedly expressed in active and early-stage endometriotic lesions. J. Clin. Endocrinol. Metab. 2002, 87, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Kats, R.; Collette, T.; Metz, C.N.; Akoum, A. Marked elevation of macrophage migration inhibitory factor in the peritoneal fluid of women with endometriosis. Fertil. Steril. 2002, 78, 69–76. [Google Scholar] [CrossRef]
- Graham, A.; Falcone, T.; Nothnick, W.B. The expression of microRNA-451 in human endometriotic lesions is inversely related to that of macrophage migration inhibitory factor (MIF) and regulates MIF expression and modulation of epithelial cell survival. Hum. Reprod. Oxf. Engl. 2015, 30, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qin, S.; Lei, A.; Li, X.; Gao, Q.; Dong, J.; Xiao, Q.; Zhou, J. Expansion of monocytic myeloid-derived suppressor cells in endometriosis patients: A pilot study. Int. Immunopharmacol. 2017, 47, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Marí-Alexandre, J.; Barceló-Molina, M.; Belmonte-López, E.; García-Oms, J.; Estellés, A.; Braza-Boïls, A.; Gilabert-Estellés, J. Micro-RNA profile and proteins in peritoneal fluid from women with endometriosis: Their relationship with sterility. Fertil. Steril. 2018, 109, 675–684.e2. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, K.; Xu, Y.; Guo, P.; Hong, B.; Cao, Y.; Wei, Z.; Xue, R.; Wang, C.; Jiang, H. Alteration of Myeloid-Derived Suppressor Cells, Chronic Inflammatory Cytokines, and Exosomal miRNA Contribute to the Peritoneal Immune Disorder of Patients With Endometriosis. Reprod. Sci. 2019, 26, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.-B.; Li, Z.-L.; Luo, D.-H.; Huang, B.-J.; Chen, Y.-S.; Zhang, X.-S.; Cui, J.; Zeng, Y.-X.; Li, J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 2014, 5, 5439–5452. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Z.; Wang, J.; Li, S. MiR-130b promotes obesity associated adipose tissue inflammation and insulin resistance in diabetes mice through alleviating M2 macrophage polarization via repression of PPAR-γ. Immunol. Lett. 2016, 180, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Hill, A.S.; Beste, M.T.; Kumar, M.P.; Chiswick, E.; Fedorcsak, P.; Isaacson, K.B.; Lauffenburger, D.A.; Griffith, L.G.; Qvigstad, E. Peritoneal fluid cytokines related to endometriosis in patients evaluated for infertility. Fertil. Steril. 2017, 107, 1191–1199.e2. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Monsavais, D.; Pavone, M.E.; Dyson, M.; Xue, Q.; Attar, E.; Tokunaga, H.; Su, E.J. Role of estrogen receptor-β in endometriosis. Semin. Reprod. Med. 2012, 30, 39–45. [Google Scholar] [CrossRef]
- Humphries, B.; Yang, C. The microRNA-200 family: Small molecules with novel roles in cancer development, progression and therapy. Oncotarget 2015, 6, 6472–6498. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson Teague, E.M.C.; Van der Hoek, K.H.; Van der Hoek, M.B.; Perry, N.; Wagaarachchi, P.; Robertson, S.A.; Print, C.G.; Hull, L.M. MicroRNA-regulated pathways associated with endometriosis. Mol. Endocrinol. Baltim. Md. 2009, 23, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, S.M.; Creighton, C.J.; Han, D.Y.; Zariff, A.; Anderson, M.L.; Gunaratne, P.H.; Matzuk, M.M. Functional microRNA involved in endometriosis. Mol. Endocrinol. Baltim. Md. 2011, 25, 821–832. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. Oxf. Engl. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Park, S.-M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, Y.; Zhao, Y.; Xu, C.; Zhang, A.; Zhang, Q.; Wang, D.; He, J.; Hua, W.; Duan, P. miR-200c suppresses endometriosis by targeting MALAT1 in vitro and in vivo. Stem Cell Res. Ther. 2017, 8, 251. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Z.; Xiong, W.; Li, N.; Liu, H.; He, H.; Li, Q.; Liu, Y.; Zhang, L. Estradiol promotes EMT in endometriosis via MALAT1/miR200s sponge function. Reprod. Camb. Engl. 2019, 157, 179–188. [Google Scholar] [CrossRef]
- Filigheddu, N.; Gregnanin, I.; Porporato, P.E.; Surico, D.; Perego, B.; Galli, L.; Patrignani, C.; Graziani, A.; Surico, N. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J. Biomed. Biotechnol. 2010, 2010, 369549. [Google Scholar] [CrossRef]
- Shi, X.-Y.; Gu, L.; Chen, J.; Guo, X.-R.; Shi, Y.-L. Downregulation of miR-183 inhibits apoptosis and enhances the invasive potential of endometrial stromal cells in endometriosis. Int. J. Mol. Med. 2014, 33, 59–67. [Google Scholar] [CrossRef]
- Yang, R.Q.; Teng, H.; Xu, X.H.; Liu, S.Y.; Wang, Y.H.; Guo, F.J.; Liu, X.J. Microarray analysis of microRNA deregulation and angiogenesis-related proteins in endometriosis. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Zhang, T.; Deng, J.; Xia, X.; Fang, X. microRNA-141 inhibits TGF-β1-induced epithelial-to-mesenchymal transition through inhibition of the TGF-β1/SMAD2 signalling pathway in endometriosis. Arch. Gynecol. Obstet. 2020, 301, 707–714. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Tang, L.; Wang, X.; Zhang, T.; Xia, X.; Fang, X. Downregulated circular RNA hsa_circ_0067301 regulates epithelial-mesenchymal transition in endometriosis via the miR-141/Notch signaling pathway. Biochem. Biophys. Res. Commun. 2019, 514, 71–77. [Google Scholar] [CrossRef]
- Ramón, L.A.; Braza-Boïls, A.; Gilabert-Estellés, J.; Gilabert, J.; España, F.; Chirivella, M.; Estellés, A. microRNAs expression in endometriosis and their relation to angiogenic factors. Hum. Reprod. Oxf. Engl. 2011, 26, 1082–1090. [Google Scholar] [CrossRef]
- Wu, J.; Cui, S.H.; Li, H.Z.; Li, Q.H.; Yuan, R.; Zhang, Y.P.; Zhao, T.W. Ultrasound diagnosis in gynecological acute abdomen. J. Biol. Regul. Homeost. Agents 2016, 30, 211–217. [Google Scholar]
- Lin, S.-C.; Wang, C.-C.; Wu, M.-H.; Yang, S.-H.; Li, Y.-H.; Tsai, S.-J. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J. Clin. Endocrinol. Metab. 2012, 97, E1515–E1523. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tang, Q.; Wu, W.; Xia, Y.; Chen, D.; Wang, X. miR-20a contributes to endometriosis by regulating NTN4 expression. Mol. Biol. Rep. 2014, 41, 5793–5797. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Yanagisawa, K.; Tanaka, M.; Cao, K.; Matsuyama, Y.; Goto, H.; Takahashi, T. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008, 68, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Li, B.; Yang, Z.; Fang, H.; Zhang, G.-M.; Feng, Z.-H.; Huang, B. Regulation of HIF-1alpha and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS ONE 2009, 4, e7629. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Okada, H.; Cho, H.; Tsuji, S.; Nishigaki, A.; Yasuda, K.; Kanzaki, H. Hypoxic stress simultaneously stimulates vascular endothelial growth factor via hypoxia-inducible factor-1α and inhibits stromal cell-derived factor-1 in human endometrial stromal cells. Hum. Reprod. Oxf. Engl. 2012, 27, 523–530. [Google Scholar] [CrossRef]
- Wing, L.-Y.C.; Chuang, P.-C.; Wu, M.-H.; Chen, H.-M.; Tsai, S.-J. Expression and mitogenic effect of fibroblast growth factor-9 in human endometriotic implant is regulated by aberrant production of estrogen. J. Clin. Endocrinol. Metab. 2003, 88, 5547–5554. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Hsieh, T.-H.; Tsai, C.-F.; Tsai, H.-P.; Chen, H.-S.; Chang, Y.; Chuang, H.-Y.; Lee, J.-N.; Hsu, Y.-L.; Tsai, E.-M. miRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J. Pathol. 2014, 232, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gu, L.; Di, W. MiR-199a attenuates endometrial stromal cell invasiveness through suppression of the IKKβ/NF-κB pathway and reduced interleukin-8 expression. Mol. Hum. Reprod. 2012, 18, 136–145. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, C.; Fan, L.; Wang, J.; Li, T.; Liu, Z.; Sheng, J.; Qian, R.; Duan, A.; Lu, D. MiR-199a-5p Targets ZEB1 to Inhibit the Epithelial-Mesenchymal Transition of Ovarian Ectopic Endometrial Stromal Cells Via PI3K/Akt/mTOR Signal Pathway In Vitro and In Vivo. Reprod. Sci. 2020, 27, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Deeb, W.S.; El Amir, A.; Zaki, S.S.; El Sawah, H.; Al Mohamady, M.; Metwally, A.A.; Katta, M.A. Diagnostic accuracy of serum miR-122 and miR-199a in women with endometriosis. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2018, 141, 14–19. [Google Scholar] [CrossRef]
- Dai, L.; Lou, W.; Zhu, J.; Zhou, X.; Di, W. MiR-199a inhibits the angiogenic potential of endometrial stromal cells under hypoxia by targeting HIF-1α/VEGF pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 4735–4744. [Google Scholar] [PubMed]
- Cosar, E.; Mamillapalli, R.; Ersoy, G.S.; Cho, S.; Seifer, B.; Taylor, H.S. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil. Steril. 2016, 106, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Xue, X.; Zhao, Y.; Chen, J.; Xu, C.-Y.; Duan, P. The differential expression of microRNA-143,145 in endometriosis. Iran. J. Reprod. Med. 2014, 12, 555–560. [Google Scholar] [PubMed]
- Nimi-Hoveidi, E.; Kohan, L.; Hashemi, S.S. Association of miR-143 rs41291957 and rs4705342 genetic variants with endometriosis risk in infertile women. KAUMS J. FEYZ 2016, 20, 441–446. [Google Scholar]
- Adammek, M.; Greve, B.; Kässens, N.; Schneider, C.; Brüggemann, K.; Schüring, A.N.; Starzinski-Powitz, A.; Kiesel, L.; Götte, M. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil. Steril. 2013, 99, 1346–1355.e5. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, J.; Xu, C.; Tang, S.-C.; Ren, H. The insights of Let-7 miRNAs in oncogenesis and stem cell potency. J. Cell. Mol. Med. 2016, 20, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Mutlu, L.; Zhou, Y.; Taylor, H.S. Aromatase inhibitor regulates let-7 expression and let-7f-induced cell migration in endometrial cells from women with endometriosis. Fertil. Steril. 2016, 106, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Grechukhina, O.; Petracco, R.; Popkhadze, S.; Massasa, E.; Paranjape, T.; Chan, E.; Flores, I.; Weidhaas, J.B.; Taylor, H.S. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol. Med. 2012, 4, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Sahin, C.; Mamillapalli, R.; Yi, K.W.; Taylor, H.S. microRNA Let-7b: A Novel treatment for endometriosis. J. Cell. Mol. Med. 2018, 22, 5346–5353. [Google Scholar] [CrossRef]
- Woo, J.-H.; Yang, Y.-I.; Ahn, J.-H.; Choi, Y.S.; Choi, J.-H. Interleukin 6 secretion from alternatively activated macrophages promotes the migration of endometriotic epithelial cells. Biol. Reprod. 2017, 97, 660–670. [Google Scholar] [CrossRef][Green Version]
- Chen, P.-Y.; Qin, L.; Barnes, C.; Charisse, K.; Yi, T.; Zhang, X.; Ali, R.; Medina, P.P.; Yu, J.; Slack, F.J.; et al. FGF regulates TGF-β signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012, 2, 1684–1696. [Google Scholar] [CrossRef]
- Dai, X.; Jiang, Y.; Tan, C. Let-7 Sensitizes KRAS Mutant Tumor Cells to Chemotherapy. PLoS ONE 2015, 10, e0126653. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubrzycka, A.; Migdalska-Sęk, M.; Jędrzejczyk, S.; Brzeziańska-Lasota, E. Circulating miRNAs Related to Epithelial–Mesenchymal Transitions (EMT) as the New Molecular Markers in Endometriosis. Curr. Issues Mol. Biol. 2021, 43, 900-916. https://doi.org/10.3390/cimb43020064
Zubrzycka A, Migdalska-Sęk M, Jędrzejczyk S, Brzeziańska-Lasota E. Circulating miRNAs Related to Epithelial–Mesenchymal Transitions (EMT) as the New Molecular Markers in Endometriosis. Current Issues in Molecular Biology. 2021; 43(2):900-916. https://doi.org/10.3390/cimb43020064
Chicago/Turabian StyleZubrzycka, Anna, Monika Migdalska-Sęk, Sławomir Jędrzejczyk, and Ewa Brzeziańska-Lasota. 2021. "Circulating miRNAs Related to Epithelial–Mesenchymal Transitions (EMT) as the New Molecular Markers in Endometriosis" Current Issues in Molecular Biology 43, no. 2: 900-916. https://doi.org/10.3390/cimb43020064
APA StyleZubrzycka, A., Migdalska-Sęk, M., Jędrzejczyk, S., & Brzeziańska-Lasota, E. (2021). Circulating miRNAs Related to Epithelial–Mesenchymal Transitions (EMT) as the New Molecular Markers in Endometriosis. Current Issues in Molecular Biology, 43(2), 900-916. https://doi.org/10.3390/cimb43020064





