Regenerative Potential of Blood-Derived Products in 3D Osteoarthritic Chondrocyte Culture System
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Preparation of Blood Products
2.2. OA Chondrocytes Isolation
2.3. OA Chondrocytes Pellet Culture
2.4. Metabolic Activity Assay (XTT Assay)
2.5. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analysis
3. Results
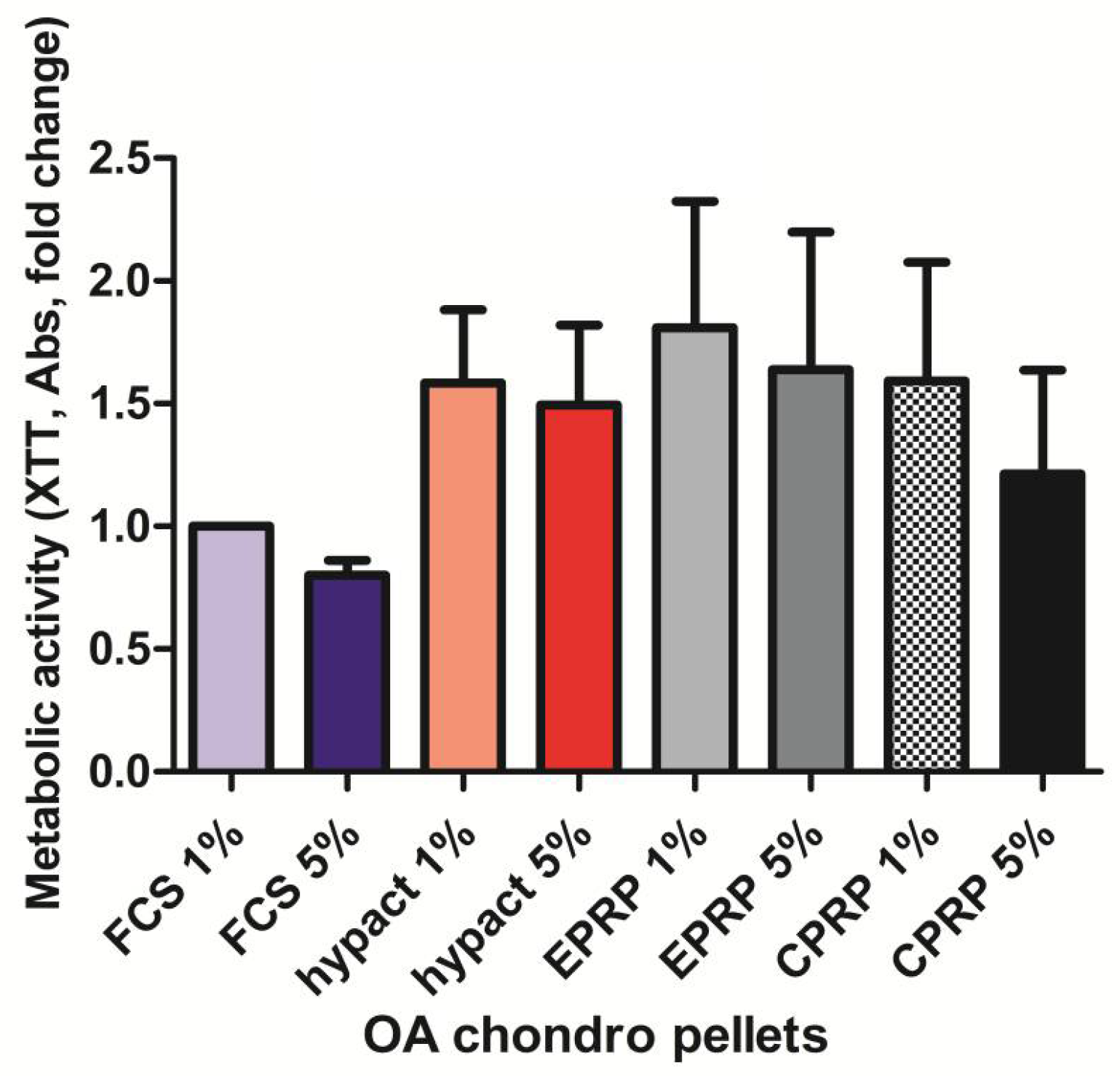
3.1. There Is No Significant Difference in Metabolic Activity between the OA Chondrocytes from Pellet Culture with Different Blood Products
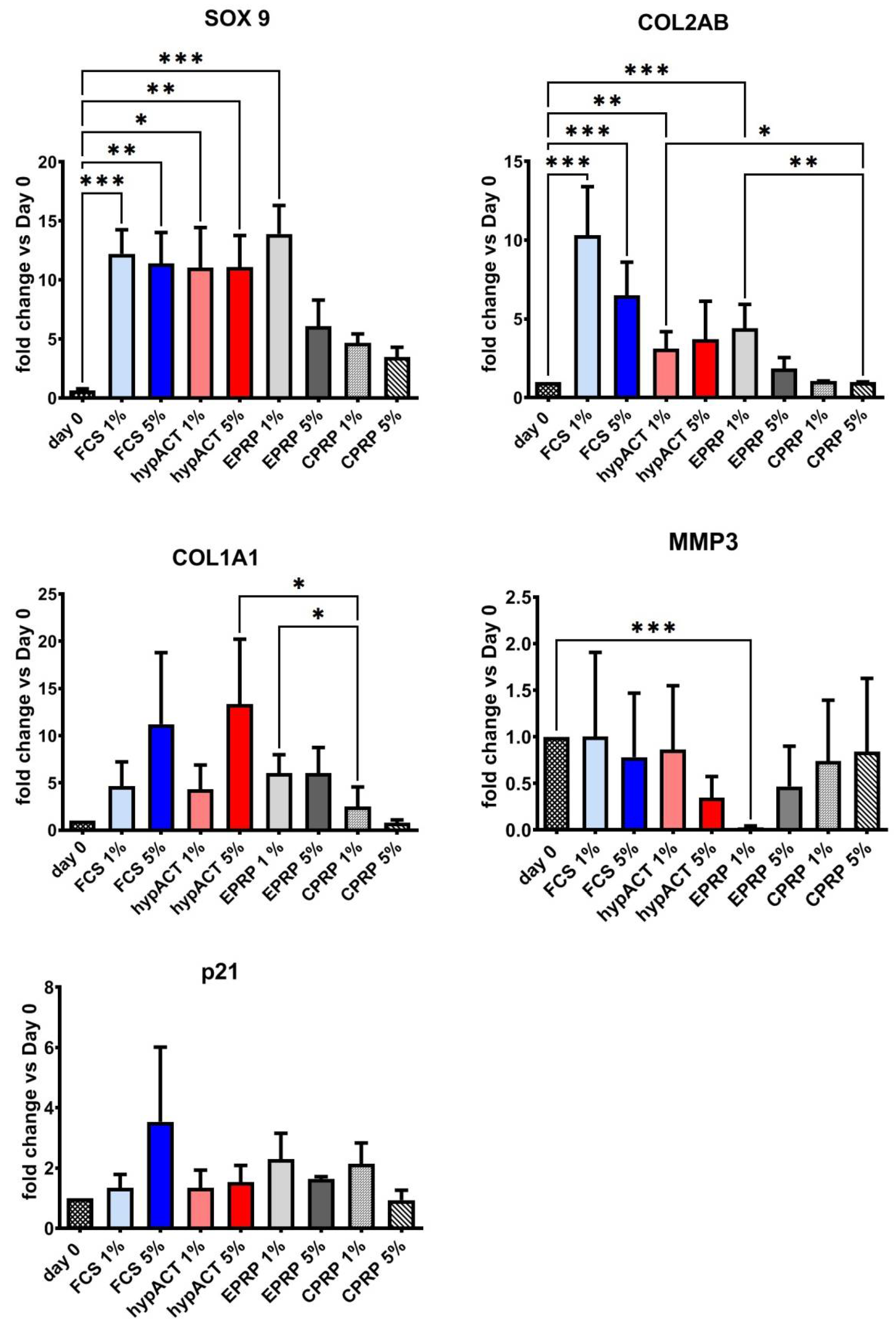
3.2. Blood Products Influence the Chondrogenic Genes Expression
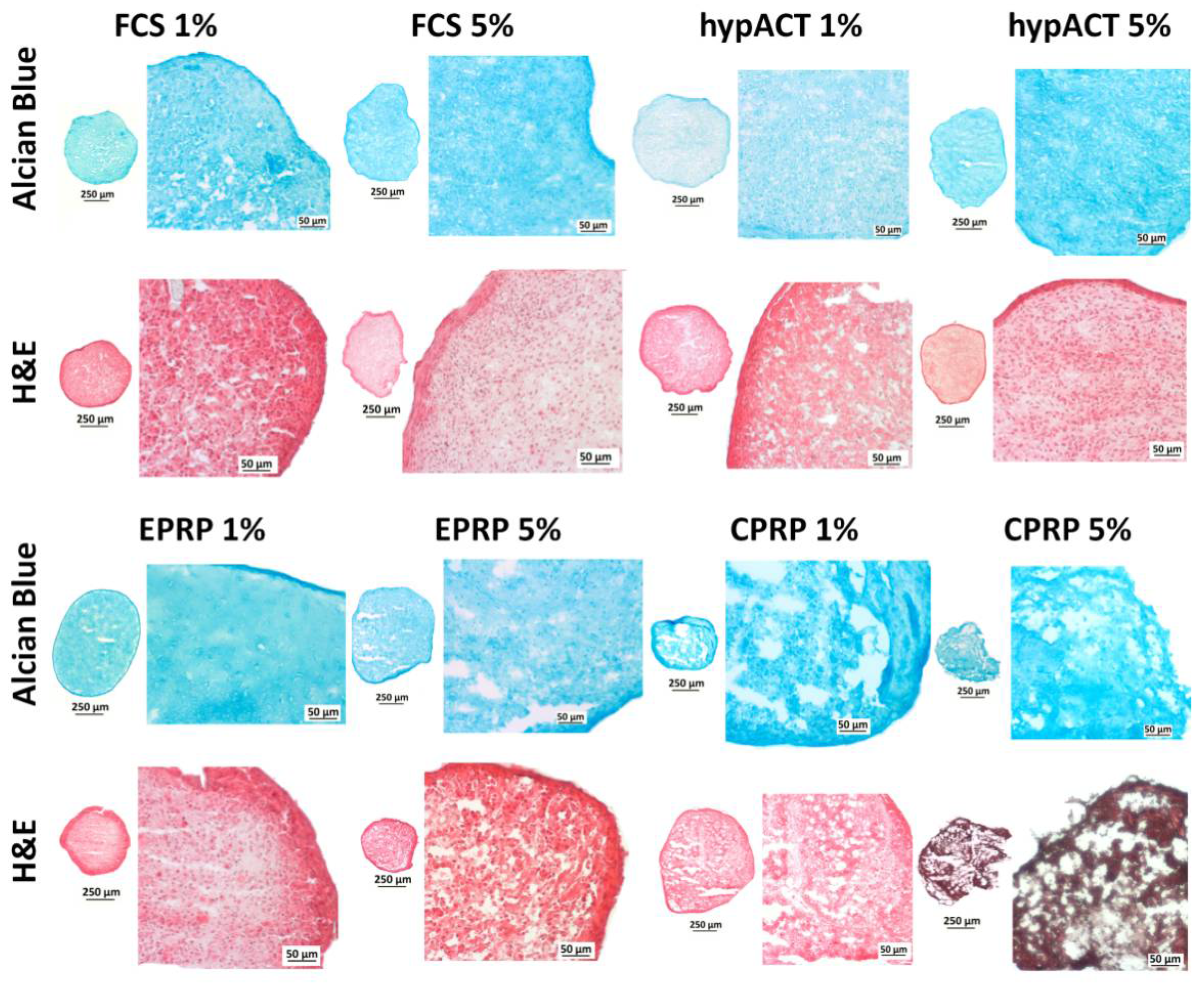
3.3. Blood Products Influence ECM Morphology and the Tissue Quality of Chondrocyte Pellets
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Felson, D.; Naimark, A.; Anderson, J.; Kazis, L.; Castelli, W.; Meenan, R.F. The prevalence of knee osteoarthritis in the elderly. the framingham osteoarthritis study. Arthritis Rheum. 1987, 30, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacitharan, P.K.; Vincent, T.L. Cellular ageing mechanisms in osteoarthritis. Mamm. Genome 2016, 27, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, F.; Bennardo, L.; Del Duca, E.; Patruno, C.; Fortunato, L.; Giudice, A.; Nisticò, S.P. Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 2020, 33, e13334. [Google Scholar] [CrossRef] [PubMed]
- Amrichová, J.; Špaková, T.; Rosocha, J.; Harvanová, D.; Bačenková, D.; Lacko, M.; Horňák, S. Effect of PRP and PPP on proliferation and migration of human chondrocytes and synoviocytes in vitro. Open Life Sci. 2014, 9, 139–148. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Wu, H.; Hogan, M.V.; Wang, J.H.-C. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells—Implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res. Ther. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kierulf, P.; Sandset, P.M.; Klingenberg, O.; Joø, G.B.; Godal, H.C.; Skjønsberg, O.H.; Jensen, T. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb. Haemost. 2007, 97, 822–829. [Google Scholar] [CrossRef]
- Kardos, D.; Simon, M.; Vácz, G.; Hinsenkamp, A.; Holczer, T.; Cseh, D.; Sárközi, A.; Szenthe, K.; Bánáti, F.; Szathmary, S.; et al. The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method. Int. J. Mol. Sci. 2019, 20, 721. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; Major, B.; Vácz, G.; Kuten, O.; Hornyak, I.; Hinsenkamp, A.; Kardos, D.; Bagó, M.; Cseh, D.; Sarkozi, A.; et al. The Effects of Hyperacute Serum on the Elements of the Human Subchondral Bone Marrow Niche. Stem Cells Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, M.; Kuten, O.; Stotter, C.; Kramer, K.; De Luna, A.; Muellner, T.; Lacza, Z.; Nehrer, S. The Effect of Blood-Derived Products on the Chondrogenic and Osteogenic Differentiation Potential of Adipose-Derived Mesenchymal Stem Cells Originated from Three Different Locations. Stem Cells Int. 2019, 2019, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Niculescu-Morzsa, E.; Jeyakumar, V.; Kern, D.; Späth, S.S.; Nehrer, S. Chondroprotective effect of high-molecular-weight hyaluronic acid on osteoarthritic chondrocytes in a co-cultivation inflammation model with M1 macrophages. J. Inflamm. 2016, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripmeester, E.G.J.; Timur, U.T.; Caron, M.M.J.; Welting, T.J.M. Recent insights into the contribution of the changing hypertrophic chondrocyte phenotype in the development and progression of osteoarthritis. Front. Bioeng. Biotechnol. 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Van der Kraan, P.M.; Van den Berg, W.B. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Marcu, K.B.; Goldring, M.B.; Otero, M. Phenotypic instability of chondrocytes in osteoarthritis: On a path to hypertrophy. Ann. N. Y. Acad. Sci. 2019, 1442, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, A.A.; Beier, F. Cartilage biology in osteoarthritis—lessons from developmental biology. Nat. Rev. Rheumatol. 2011, 7, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Samvelyan, H.J.; Hughes, D.; Stevens, C.; Staines, K.A. Models of Osteoarthritis: Relevance and New Insights. Calcif. Tissue Int. 2020, 1, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Muraglia, A.; Nguyen, V.T.; Nardini, M.; Mogni, M.; Coviello, D.; Dozin, B.; Strada, P.; Baldelli, I.; Formica, M.; Cancedda, R.; et al. Culture Medium Supplements Derived from Human Platelet and Plasma: Cell Commitment and Proliferation Support. Front. Bioeng. Biotechnol. 2017, 5, 66. [Google Scholar] [CrossRef] [Green Version]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Platelet-Rich Plasma Supports Proliferation and Redifferentiation of Chondrocytes during In Vitro Expansion. Front. Bioeng. Biotechnol. 2017, 5, 75. [Google Scholar] [CrossRef]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z.; Nehrer, S. Redifferentiation of Articular Chondrocytes by Hyperacute Serum and Platelet Rich Plasma in Collagen Type I Hydrogels. Int. J. Mol. Sci. 2019, 20, 316. [Google Scholar] [CrossRef] [Green Version]
- Kardos, D.; Marschall, B.; Simon, M.; Hornyák, I.; Hinsenkamp, A.; Kuten, O.; Gyevnár, Z.; Erdélyi, G.; Bárdos, T.; Paukovits, T.M.; et al. Investigation of Cytokine Changes in Osteoarthritic Knee Joint Tissues in Response to Hyperacute Serum Treatment. Cells 2019, 8, 824. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Rigueur, D.; Lyons, K.M. TGFβ signaling in cartilage development and maintenance. Birth Defects Res. Part C Embryo Today Rev. 2014, 102, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Tekari, A.; Luginbuehl, R.; Hofstetter, W.; Egli, R. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Yamaguchi, Y.; Ambe, K.; Horie, M.; Saito, A.; Nagase, T.; Nakashima, K.; Ohki, H.; Kawai, T.; Abiko, Y.; et al. Fibroblast VEGF-receptor 1 expression as molecular target in periodontitis. J. Clin. Periodontol. 2015, 43, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, M.; Yamaguchi, Y.; Matsumoto, N.; Micke, P.; Takenouchi, Y.; Nishida, T.; Kato, M.; Komiyama, K.; Abiko, Y.; Ito, K.; et al. TGF-β Signaling in Gingival Fibroblast-Epithelial Interaction. J. Dent. Res. 2010, 89, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, Inflammation, and Pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, 12–26. [Google Scholar]
- Giusti, I.; D’Ascenzo, S.; Mancò, A.; Di Stefano, G.; Di Francesco, M.; Rughetti, A.; Mas, A.D.; Properzi, G.; Calvisi, V.; Dolo, V. Platelet Concentration in Platelet-Rich Plasma Affects Tenocyte BehaviorIn Vitro. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Graziani, F.; Ivanovski, S.; Cei, S.; Ducci, F.; Tonetti, M.; Gabriele, M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral Implant. Res. 2006, 17, 212–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuten, O.; Simon, M.; Hornyák, I.; De Luna-Preitschopf, A.; Nehrer, S.; Lacza, Z. The Effects of Hyperacute Serum on Adipogenesis and Cell Proliferation of Mesenchymal Stromal Cells. Tissue Eng. Part A 2018, 24, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- De Luna-Preitschopf, A.; Zwickl, H.; Nehrer, S.; Hengstschläger, M.; Mikula, M. Rapamycin Maintains the Chondrocytic Phenotype and Interferes with Inflammatory Cytokine Induced Processes. Int. J. Mol. Sci. 2017, 18, 1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, J.; Gebhard, P.M.; Aigner, T. SOX Gene Expression in Human Osteoarthritic Cartilage. Pathobiol. 2008, 75, 195–199. [Google Scholar] [CrossRef]
- Rousseau, J.; Garnero, P. Biological markers in osteoarthritis. Bone 2012, 51, 265–277. [Google Scholar] [CrossRef]
- Lafont, J.E.; Talma, S.; Hopfgarten, C.; Murphy, C.L. Hypoxia Promotes the Differentiated Human Articular Chondrocyte Phenotype through SOX9-dependent and -independent Pathways. J. Biol. Chem. 2008, 283, 4778–4786. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Ma, B.; Liang, Y.; Chen, J.; Zhu, W.; Li, M.; Wang, D. Cytokine networking of chondrocyte dedifferentiation in vitro and its implications for cell-based cartilage therapy. Am. J. Transl. Res. 2015, 7, 194–208. [Google Scholar]
- Wang, X.; Zhang, Y.; Choukroun, J.; Ghanaati, S.; Miron, R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets 2018, 29, 48–55. [Google Scholar] [CrossRef]
- Mellor, L.F.; Mohiti-Asli, M.; Williams, J.; Kannan, A.; Dent, M.R.; Guilak, F.; Loboa, E.G. Extracellular Calcium Modulates Chondrogenic and Osteogenic Differentiation of Human Adipose-Derived Stem Cells: A Novel Approach for Osteochondral Tissue Engineering Using a Single Stem Cell Source. Tissue Eng. Part A 2015, 21, 2323–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Markers |
|---|
| GAPDH FORWARD: 5′-CTCTGCTCCTCCTGTTCGAC-3′ REVERSE: 5′-ACGACCAAATCCGTTGACTC-3′ |
| COL1A1 FORWARD: 5′-GGGATTCCCTGGACCTAAAG-3′ REVERSE: 5′-GGAACACCTCGCTCTCCAG-3′ |
| COL2AB FORWARD: 5′-GCACCTGCAGAGACCTGA-3′ REVERSE: 5′-GGGTCAATCCAGTAGTCTCCAC-3′ |
| SOX9 FORWARD: 5′-TACCCGCACTTGCACAAC-3′ REVERSE: 5′-TCTCGCTCTCGTTCAGAAGTC-3′ |
| MMP3 FORWARD: 5′-CAAAACATATTTCTTTGTAGAGGACAA-3′ REVERSE: 5′-TTCAGCTATTTGCTTGGGAAA-3′ |
| P21 FORWARD: 5′-TCACTGTCTTGTACCCTTGTGC-3′ REVERSE: 5′-GGCGTTTGGAGTGGTAGAAA-3′ |
| Gene | Dunn’s Multiple Comparisons Test | Summary | Adjusted p Value |
|---|---|---|---|
| SOX9 | day 0 vs. FCS 1% | *** | 0.0007 |
| day 0 vs. FCS 5% | ** | 0.0046 | |
| day 0 vs. hypACT 1% | * | 0.0169 | |
| day 0 vs. hypACT 5% | ** | 0.0043 | |
| day 0 vs. EPRP 1% | *** | 0.0002 | |
| COL2AB | day 0 vs. FCS 1% | *** | <0.001 |
| day 0 vs. FCS 5% | *** | <0.001 | |
| day 0 vs. hypACT 1% | ** | 0.0040 | |
| day 0 vs. EPRP 1% | *** | <0.001 | |
| FCS 1% vs. CPRP 1% | * | 0.0100 | |
| FCS 1% vs. CPRP 5% | *** | <0.001 | |
| FCS 5% vs. CPRP 1% | * | 0.0300 | |
| FCS 5% vs. CPRP 5% | *** | <0.001 | |
| hypACT 1% vs. CPRP 5% | * | 0.0100 | |
| EPRP 1% vs. CPRP 5% | ** | 0.0030 | |
| COL1A1 | hypACT 5% vs. CPRP 1% | * | 0.0200 |
| EPRP 1 % vs. CPRP 1% | * | 0.0200 | |
| MMP3 | day 0 vs. EPRP 1% | *** | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuten-Pella, O.; De Luna, A.; Kramer, K.; Neubauer, M.; Nehrer, S.; Lacza, Z. Regenerative Potential of Blood-Derived Products in 3D Osteoarthritic Chondrocyte Culture System. Curr. Issues Mol. Biol. 2021, 43, 665-675. https://doi.org/10.3390/cimb43020048
Kuten-Pella O, De Luna A, Kramer K, Neubauer M, Nehrer S, Lacza Z. Regenerative Potential of Blood-Derived Products in 3D Osteoarthritic Chondrocyte Culture System. Current Issues in Molecular Biology. 2021; 43(2):665-675. https://doi.org/10.3390/cimb43020048
Chicago/Turabian StyleKuten-Pella, Olga, Andrea De Luna, Karina Kramer, Markus Neubauer, Stefan Nehrer, and Zsombor Lacza. 2021. "Regenerative Potential of Blood-Derived Products in 3D Osteoarthritic Chondrocyte Culture System" Current Issues in Molecular Biology 43, no. 2: 665-675. https://doi.org/10.3390/cimb43020048
APA StyleKuten-Pella, O., De Luna, A., Kramer, K., Neubauer, M., Nehrer, S., & Lacza, Z. (2021). Regenerative Potential of Blood-Derived Products in 3D Osteoarthritic Chondrocyte Culture System. Current Issues in Molecular Biology, 43(2), 665-675. https://doi.org/10.3390/cimb43020048







