Metagenomic Shotgun Sequencing Analysis of Canalicular Concretions in Lacrimal Canaliculitis Cases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Recruitment
2.2. Sampling of Canalicular Concretions
2.3. DNA Extraction and Shotgun Metagenomic Sequencing Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Metagenomic Shotgun Sequencing Analysis
3.3. Taxonomy of Canalicular Concretions and Identification of Bacterial Phyla and Shannon Index
3.4. Taxonomy of Canalicular Concretions and Identification of Bacterial Genera
3.5. The Treatment Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CNS | coagulase-negative staphylococci |
| SD | standard deviation |
| spp. | species |
| PCR | polymerase chain reaction |
| NGS | next-generation sequencing |
| DNA | deoxyribonucleic acid |
| RNA | ribonucleic acid |
References
- Freedman, J.R.; Markert, M.S.; Cohen, A.J. Primary and secondary lacrimal canaliculitis: A review of literature. Surv. Ophthalmol. 2011, 56, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Gautam, N.; Agarwal, A.; Kaur, M. Primary lacrimal canaliculitis-A clinical entity often misdiagnosed. J. Curr. Ophthalmol. 2018, 30, 87–90. [Google Scholar] [CrossRef]
- Lin, S.C.; Kao, S.C.; Tsai, C.C.; Cheng, C.Y.; Kau, H.C.; Hsu, W.M.; Lee, S.M. Clinical characteristics and factors associated the outcome of lacrimal canaliculitis. Acta Ophthalmol. 2011, 89, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Lin, B.; Pan, Q.; Zheng, M.; Qin, X.; Wang, Y.; Zhang, Z. Clinical features and surgical outcomes of primary canaliculitis with concretions. Medicine 2017, 96, e6188. [Google Scholar] [CrossRef] [PubMed]
- Gogandy, M.; Al-Sheikh, O.; Chaudhry, I. Clinical features and bacteriology of lacrimal canaliculitis in patients presenting to a tertiary eye care center in the Middle East. Saudi J. Ophthalmol. 2014, 28, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.; Hollingworth, K.; Kumar, V.; Sandramouli, S. Canaliculitis: The incidence of long-term epiphora following canaliculotomy. Orbit 2004, 23, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.R.; Wadwekar, B.; Prajna, L. Primary canaliculitis: The incidence, clinical features, outcome and long-term epiphora after snip-punctoplasty and curettage. Saudi J. Ophthalmol. 2015, 29, 274–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vécsei, V.P.; Huber-Spitzy, V.; Arocker-Mettinger, E.; Steinkogler, F.J. Canaliculitis: Difficulties in diagnosis, differential diagnosis and comparison between conservative and surgical treatment. Ophthalmologica 1994, 208, 314–317. [Google Scholar] [CrossRef]
- Briscoe, D.; Edelstein, E.; Zacharopoulos, I.; Keness, Y.; Kilman, A.; Zur, F.; Assia, E.I. Actinomyces canaliculitis: Diagnosis of a masquerading disease. Graefes. Arch. Clin. Exp. Ophthalmol. 2004, 242, 682–686. [Google Scholar] [CrossRef]
- Hussain, I.; Bonshek, R.E.; Loudon, K.; Armstrong, M.; Tullo, A.B. Canalicular infection caused by Actinomyces. Eye 1993, 7, 542–544. [Google Scholar] [CrossRef]
- Li, Z.; Breitwieser, F.P.; Lu, J.; Jun, A.S.; Asnaghi, L.; Salzberg, S.L.; Eberhart, C.G. Identifying Corneal Infections in Formalin-Fixed Specimens Using Next Generation Sequencing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 280–288. [Google Scholar] [CrossRef]
- Shigeyasu, C.; Yamada, M.; Aoki, K.; Ishii, Y.; Tateda, K.; Yaguchi, T.; Hori, Y. Metagenomic analysis for detecting Fusarium solani in a case of fungal keratitis. J. Infect. Chemother. 2018, 24, 664–668. [Google Scholar] [CrossRef]
- Seitzman, G.D.; Thulasi, P.; Hinterwirth, A.; Chen, C.; Shantha, J.; Doan, T. Capnocytophaga Keratitis: Clinical Presentation and Use of Metagenomic Deep Sequencing for Diagnosis. Cornea 2019, 38, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Borroni, D.; Romano, V.; Kaye, S.B.; Somerville, T.; Napoli, L.; Fasolo, A.; Ferrari, S. Metagenomics in ophthalmology: Current findings and future prospectives. BMJ Open Ophthalmol. 2019, 4, e000248. [Google Scholar] [CrossRef] [PubMed]
- Gallon, P.; Parekh, M.; Ferrari, S.; Fasolo, A.; Ponzin, D.; Borroni, D. Metagenomics in ophthalmology: Hypothesis or real prospective? Biotechnol. Rep. 2019, 23, e00355. [Google Scholar] [CrossRef]
- Doan, T.; Wilson, M.R.; Crawford, E.D.; Chow, E.D.; Khan, L.M.; Knopp, K.A.; DeRisi, J.L. Illuminating uveitis: Metagenomic deep sequencing identifies common and rare pathogens. Genome Med. 2016, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Norman, J.M.; Handley, S.A.; Virgin, H.W. Kingdom-agnostic metagenomics and the importance of complete characterization of enteric microbial communities. Gastroenterology 2014, 146, 1459–1469. [Google Scholar] [CrossRef]
- Deurenberg, R.H.; Bathoorn, E.; Chlebowicz, M.A.; Couto, N.; Ferdous, M.; García-Cobos, S.; Rossen, J.W. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017, 243, 16–24. [Google Scholar] [CrossRef]
- Doan, T.; Acharya, N.R.; Pinsky, B.A.; Sahoo, M.K.; Chow, E.D.; Banaei, N.; DeRisi, J.L. Metagenomic DNA Sequencing for the Diagnosis of Intraocular Infections. Ophthalmology 2017, 124, 1247–1248. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Lei, R.; Ding, S.W.; Zhu, S. Skewer: A fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 2014, 15, 182. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huson, D.H.; Mitra, S.; Ruscheweyh, H.J.; Weber, N.; Schuster, S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011, 21, 1552–1560. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yang, B.; Li, W. Defining the normal core microbiome of conjunctival microbial communities. Clin. Microbiol. Infect. 2016, 22, 643.e7–643.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Q.; Brulc, J.M.; Iovieno, A.; Bates, B.; Garoutte, A.; Miller, D.; Shestopalov, V.I. Diversity of bacteria at healthy human conjunctiva. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5408–5413. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, J.; Nielsen, S.; Diez-Vives, C.; Coroneo, M.; Thomas, T.; Willcox, M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci. Rep. 2017, 7, 9880. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.M.; Luo, T.; Lee, K.H.; Guerreiro, D.; Botero, T.M.; McDonald, N.J.; Rickard, A.H. Deciphering Endodontic Microbial Communities by Next-generation Sequencing. J. Endod. 2018, 44, 1080–1087. [Google Scholar] [CrossRef]
- Salmon, J.F.; Kanski, J.J. Kanski’s Clinical Ophthalmology: A Systematic Approach, 9th ed.; Elsevier Limited: Edinburgh, UK, 2020; p. 100. [Google Scholar]
- Eguchi, H.; Hotta, F.; Kuwahara, T.; Imaohji, H.; Miyazaki, C.; Hirose, M.; Shimomura, Y. Diagnostic Approach to Ocular Infections Using Various Techniques From Conventional Culture to Next-Generation Sequencing Analysis. Cornea 2017, 36, S46–S52. [Google Scholar] [CrossRef] [PubMed]
- Perumal, B.; Carlson, J.A.; Meyer, D.R. A Pathological Analysis of Canaliculitis Concretions: More Than Just Actinomyces. Scientifica 2016. [Google Scholar] [CrossRef] [Green Version]
- Pulverer, G.; Schütt-Gerowitt, H.; Schaal, K.P. Human cervicofacial actinomycoses: Microbiological data for 1997 cases. Clin. Infect. Dis. 2003, 37, 490–497. [Google Scholar] [CrossRef]
- Dentino, A.; Lee, S.; Mailhot, J.; Hefti, A.F. Principles of periodontology. Periodontol 2000, 61, 16–53. [Google Scholar] [CrossRef] [Green Version]
- Moschioni, M.; Pansegrau, W.; Barocchi, M.A. Adhesion determinants of the Streptococcus species. Microb. Biotechnol. 2010, 3, 370–388. [Google Scholar] [CrossRef] [Green Version]
- Ormerod, L.D.; Puklin, J.E.; Giles, C.L. Chronic Propionibacterium acnes endophthalmitis as a cause of intermediate uveitis. Ocul. Immunol. Inflamm. 1997, 5, 67–68. [Google Scholar] [CrossRef]
- Abrahams, I.W. Propionibacterium acnes endophthalmitis: An unusual manner of presentation. J. Cataract. Refract. Surg. 1989, 15, 698–701. [Google Scholar] [CrossRef]
- Sousa, V.; Nibali, L.; Spratt, D.; Dopico, J.; Mardas, N.; Petrie, A.; Donos, N. Peri-implant and periodontal microbiome diversity in aggressive periodontitis patients: A pilot study. Clin. Oral Implant. Res. 2017, 28, 558–570. [Google Scholar] [CrossRef]
- Daniluk, T.; Tokajuk, G.; Cylwik-Rokicka, D.; Rozkiewicz, D.; Zaremba, M.L.; Stokowska, W. Aerobic and anaerobic bacteria in subgingival and supragingival plaques of adult patients with periodontal disease. Adv. Med. Sci. 2006, 51, 81–85. [Google Scholar] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valm, A.M.; Mark Welch, J.L.; Rieken, C.W.; Welch, J.L.M.; Rieken, C.W.; Hasegawa, Y.; Sogin, M.L.; Oldenbourg, R.; Borisy, G.G. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 4152–4157. [Google Scholar] [CrossRef] [Green Version]
- Suresh Unniachan, A.; Krishnavilasom Jayakumari, N.; Sethuraman, S. Association between Candida species and periodontal disease: A systematic review. Curr. Med. Mycol. 2020, 6, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Doh, S.H.; Kim, E.C.; Chung, S.Y.; Kim, M.S.; Chung, S.K.; Shin, M.C.; Hwang, H.S. Optical Coherence Tomography Imaging of Human Lacrimal Glands: An In Vivo Study. Ophthalmology 2015, 122, 2364–2366. [Google Scholar]
- Napoli, P.E.; Nioi, M.; d’Aloja, E.; Fossarello, M. The Bull′s Eye Pattern of the Tear Film in Humans during Visual Fixation on En-Face Optical Coherence Tomography. Sci. Rep. 2019, 9, 1413. [Google Scholar] [CrossRef]
- Napoli, P.E.; Nioi, M.; Mangoni, L.; Gentile, P.; Braghiroli, M.; d′Aloja, E.; Fossarello, M. Fourier-Domain OCT Imaging of the Ocular Surface and Tear Film Dynamics: A Review of the State of the Art and an Integrative Model of the Tear Behavior During the Inter-Blink Period and Visual Fixation. J. Clin. Med. 2020, 9, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larvin, H.; Kang, J.; Aggarwal, V.R.; Pavitt, S.; Wu, J. Risk of incident cardiovascular disease in people with periodontal disease: A systematic review and meta-analysis. Clin. Exp. Dent. Res. 2021, 7, 109–122. [Google Scholar] [CrossRef] [PubMed]
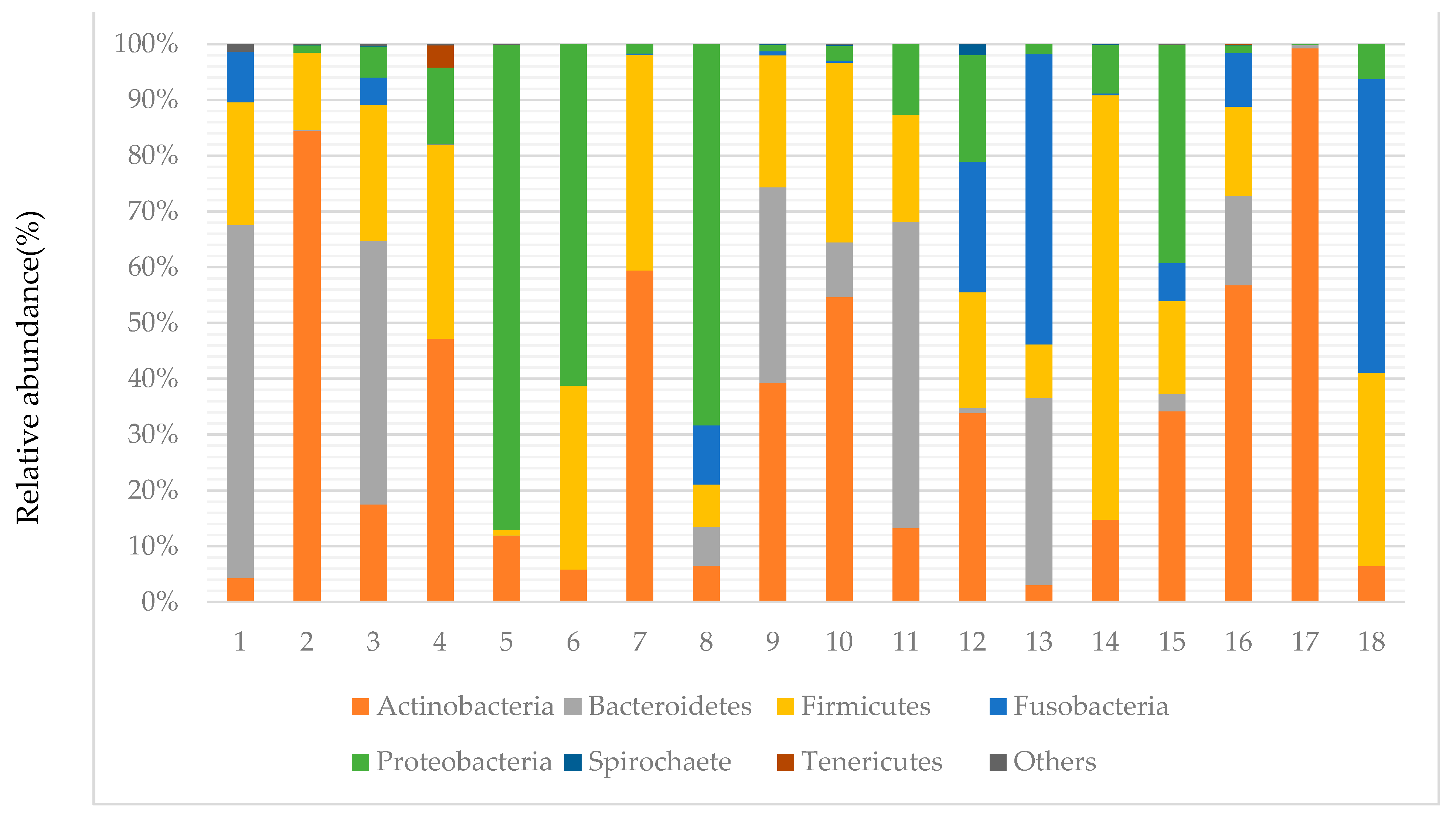
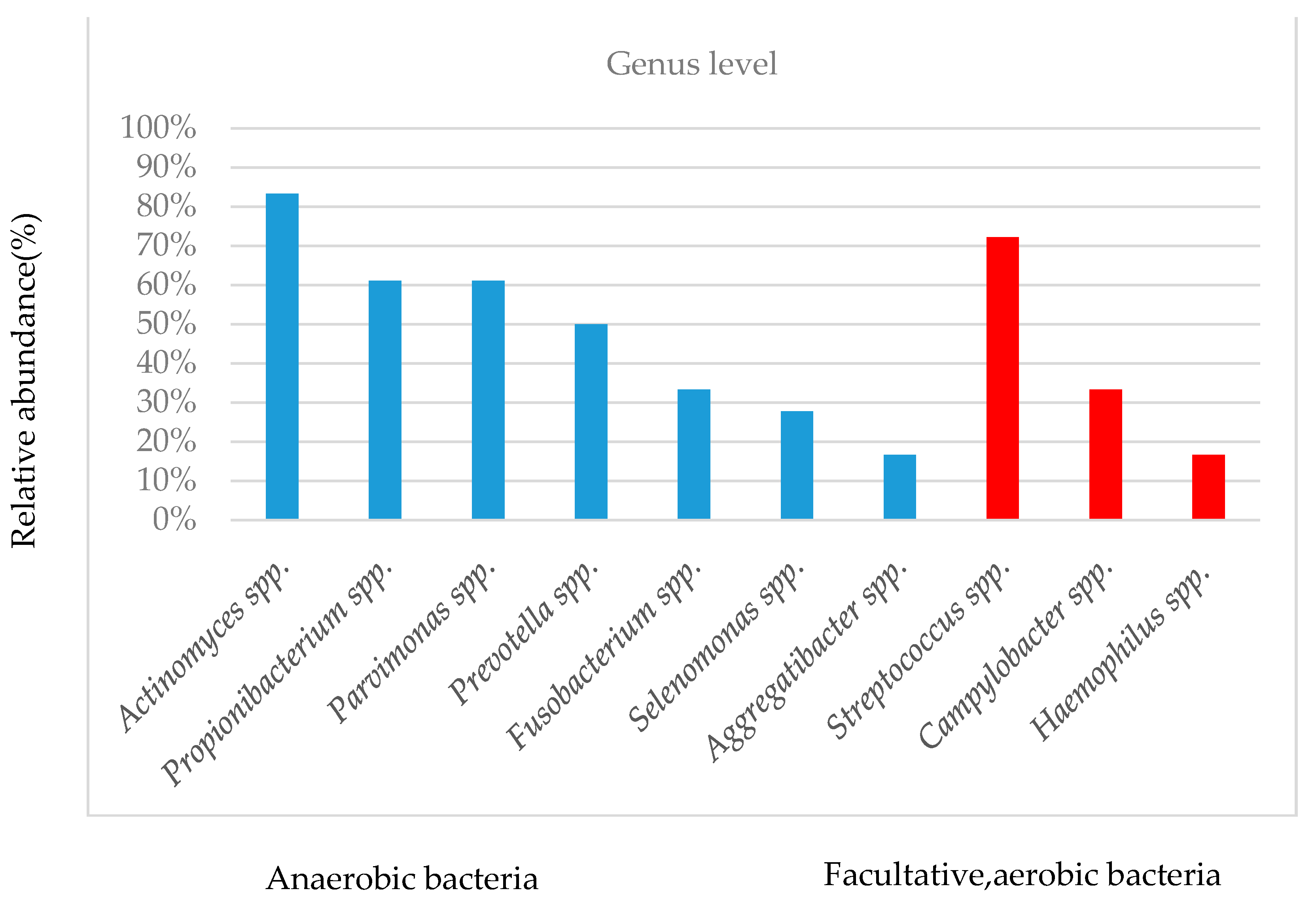
| Sample | |
|---|---|
| Mean age (years ± SD), n = 18 | 77.1 ± 6.1 |
| No. women (%), n = 18 | 14 (82.4%) |
| Side of involvement Upper right; lower right; upper left; lower left, eyes (%) | 6 eyes; 1 eye; 5 eyes; 6 eyes |
| Sample collection method using lacrimal passage endoscope or curettage, eyes (%) n = 18 | 12 eyes; 6 eyes |
| Before sample collection, antibacterial eye drops were used, eyes (%) n = 18 | 18/18 |
| Before sample collection, antibacterial internal drugs were used, eyes (%), n = 16 (unknown in 2 eyes) | 6/16 |
| Sample | Microbiology Laboratory Culture |
|---|---|
| 1 | Prevotella melaninogenica, Fusobacterium nucleatum, Streptococcus milleri |
| 2 | Actinomyces spp., Streptococcus constellatus |
| 3 | No culture test |
| 4 | Actinomyces odontolyticus, Eikenella corrodens |
| 5 | Actinomyces spp. Haemophilus parainfluenzae |
| 6 | Streptococcus spp. |
| 7 | Peptostereptococcus spp., α-streptococcus |
| 8 | Negative culture for anaerobic bacteria |
| 9 | Negative culture for anaerobic bacteria |
| 10 | CNS, Coynebacteirum spp. |
| 11 | No culture test |
| 12 | Propionibacterium spp. |
| 13 | No culture test |
| 14 | Streptococcus milleri group Peptostreptococcus spp. |
| 15 | Haemophilus influenzae |
| 16 | Haemophilus influenzae |
| 17 | Actinomyces israelii |
| 18 | Streptococcus milleri group, CNS |
| Sample | Total Genome | Bacteria | % | Virus | % | Fungi | % | Eukaryota | % |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 968,005 | 698,597 | 72.2 | 0 | 0 | 10,061 | 1.04 | 1603 | 0.17 |
| 2 | 118,762 | 26,548 | 22.4 | 0 | 0 | 13 | 0.01 | 1938 | 1.63 |
| 3 | 974,992 | 181,299 | 18.6 | 0 | 0 | 120 | 0.01 | 1803 | 0.18 |
| 4 | 166,885 | 58,750 | 35.2 | 0 | 0 | 21 | 0.01 | 959 | 0.57 |
| 5 | 77,720 | 48,107 | 61.9 | 0 | 0 | 10 | 0.01 | 1758 | 2.26 |
| 6 | 260,161 | 222,246 | 85.4 | 0 | 0 | 0 | 0 | 2340 | 0.90 |
| 7 | 103,639 | 55,698 | 53.7 | 0 | 0 | 15 | 0.01 | 1546 | 1.49 |
| 8 | 180,213 | 129,314 | 71.8 | 0 | 0 | 0 | 0 | 91 | 0.05 |
| 9 | 1,087,307 | 451,326 | 41.5 | 0 | 0 | 143 | 0.01 | 760 | 0.07 |
| 10 | 209,085 | 67,158 | 32.1 | 0 | 0 | 41 | 0.02 | 1496 | 0.72 |
| 11 | 1,148,458 | 501,681 | 43.7 | 0 | 0 | 110 | 0.01 | 693 | 0.06 |
| 12 | 191,096 | 118,846 | 62.2 | 0 | 0 | 15 | 0.01 | 1553 | 0.81 |
| 13 | 78,334 | 65,926 | 84.2 | 0 | 0 | 0 | 0 | 1352 | 1.73 |
| 14 | 273,766 | 140,082 | 51.2 | 0 | 0 | 34 | 0.01 | 3524 | 1.29 |
| 15 | 413,328 | 153,523 | 37.1 | 33 | 0.01 | 63 | 0.02 | 2317 | 0.56 |
| 16 | 336,017 | 126,406 | 37.6 | 0 | 0 | 35 | 0.01 | 1564 | 0.47 |
| 17 | 27,552 | 21,000 | 76.2 | 2 | 0.01 | 2 | 0.01 | 2773 | 10.06 |
| 18 | 638,880 | 588,396 | 92.1 | 0 | 0 | 0 | 0 | 1243 | 0.19 |
| Sample | Number of Detected Bacterial Genera | Number of Detected Bacterial Genera, 1% or More (Ratio of Anaerobic Bacteria to Facultative, Aerobic Bacteria) |
|---|---|---|
| 1 | 13 | 5 (4:1) |
| 2 | 139 | 4 (3:1) |
| 3 | 380 | 12 (10:2) |
| 4 | 233 | 7 (3:4) |
| 5 | 99 | 3 (2:1) |
| 6 | 16 | 4(2:2) |
| 7 | 103 | 5 (4:1) |
| 8 | 54 | 7(6:1) |
| 9 | 109 | 8 (7:1) |
| 10 | 204 | 8 (7:1) |
| 11 | 82 | 4 (2:2) |
| 12 | 68 | 7 (5:2) |
| 13 | 45 | 5 (4:1) |
| 14 | 95 | 7 (5:2) |
| 15 | 157 | 7 (5:2) |
| 16 | 119 | 4 (4:0) |
| 17 | 21 | 2 (2:0) |
| 18 | 15 | 4(2:2) |
| Average ± SD | 108.4 ± 90.0 | 5.7 ± 2.3 |
| Sample | Treatment Results |
|---|---|
| 1 | No recurrence |
| 2 | No recurrence |
| 3 | No recurrence |
| 4 | No recurrence |
| 5 | No recurrence |
| 6 | No recurrence |
| 7 | Recurrence |
| 8 | No recurrence |
| 9 | No recurrence |
| 10 | No recurrence |
| 11 | No recurrence |
| 12 | No recurrence |
| 13 | No recurrence |
| 14 | No recurrence |
| 15 | No recurrence |
| 16 | No recurrence |
| 17 | No recurrence |
| 18 | No recurrence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okajima, Y.; Suzuki, T.; Miyazaki, C.; Goto, S.; Ishikawa, S.; Suzuki, Y.; Aoki, K.; Ishii, Y.; Tateda, K.; Hori, Y. Metagenomic Shotgun Sequencing Analysis of Canalicular Concretions in Lacrimal Canaliculitis Cases. Curr. Issues Mol. Biol. 2021, 43, 676-686. https://doi.org/10.3390/cimb43020049
Okajima Y, Suzuki T, Miyazaki C, Goto S, Ishikawa S, Suzuki Y, Aoki K, Ishii Y, Tateda K, Hori Y. Metagenomic Shotgun Sequencing Analysis of Canalicular Concretions in Lacrimal Canaliculitis Cases. Current Issues in Molecular Biology. 2021; 43(2):676-686. https://doi.org/10.3390/cimb43020049
Chicago/Turabian StyleOkajima, Yukinobu, Takashi Suzuki, Chika Miyazaki, Satoshi Goto, Sho Ishikawa, Yuka Suzuki, Kotaro Aoki, Yoshikazu Ishii, Kazuhiro Tateda, and Yuichi Hori. 2021. "Metagenomic Shotgun Sequencing Analysis of Canalicular Concretions in Lacrimal Canaliculitis Cases" Current Issues in Molecular Biology 43, no. 2: 676-686. https://doi.org/10.3390/cimb43020049
APA StyleOkajima, Y., Suzuki, T., Miyazaki, C., Goto, S., Ishikawa, S., Suzuki, Y., Aoki, K., Ishii, Y., Tateda, K., & Hori, Y. (2021). Metagenomic Shotgun Sequencing Analysis of Canalicular Concretions in Lacrimal Canaliculitis Cases. Current Issues in Molecular Biology, 43(2), 676-686. https://doi.org/10.3390/cimb43020049






