Phytochemical Screening, Nutritional Value, Anti-Diabetic, Anti-Cancer, and Anti-Bacterial Assessment of Aqueous Extract from Abelmoschus esculentus Pods
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical, Reagents, and Cell Line
2.2. Collection and Drying of Okra Pods
2.3. Preparation of Extract
2.4. Phytochemical Estimations of Okra Pods Extract
2.4.1. Assessment of Moisture, Ash, Crude Protein, Fiber, and Fat Content
2.4.2. Mineral Determination
2.4.3. Determination of Total Phenolic Content
2.4.4. Determination of Chlorophyll and Carotenoids
2.4.5. Diphenyl-1-Picrylhydrazyl Assay
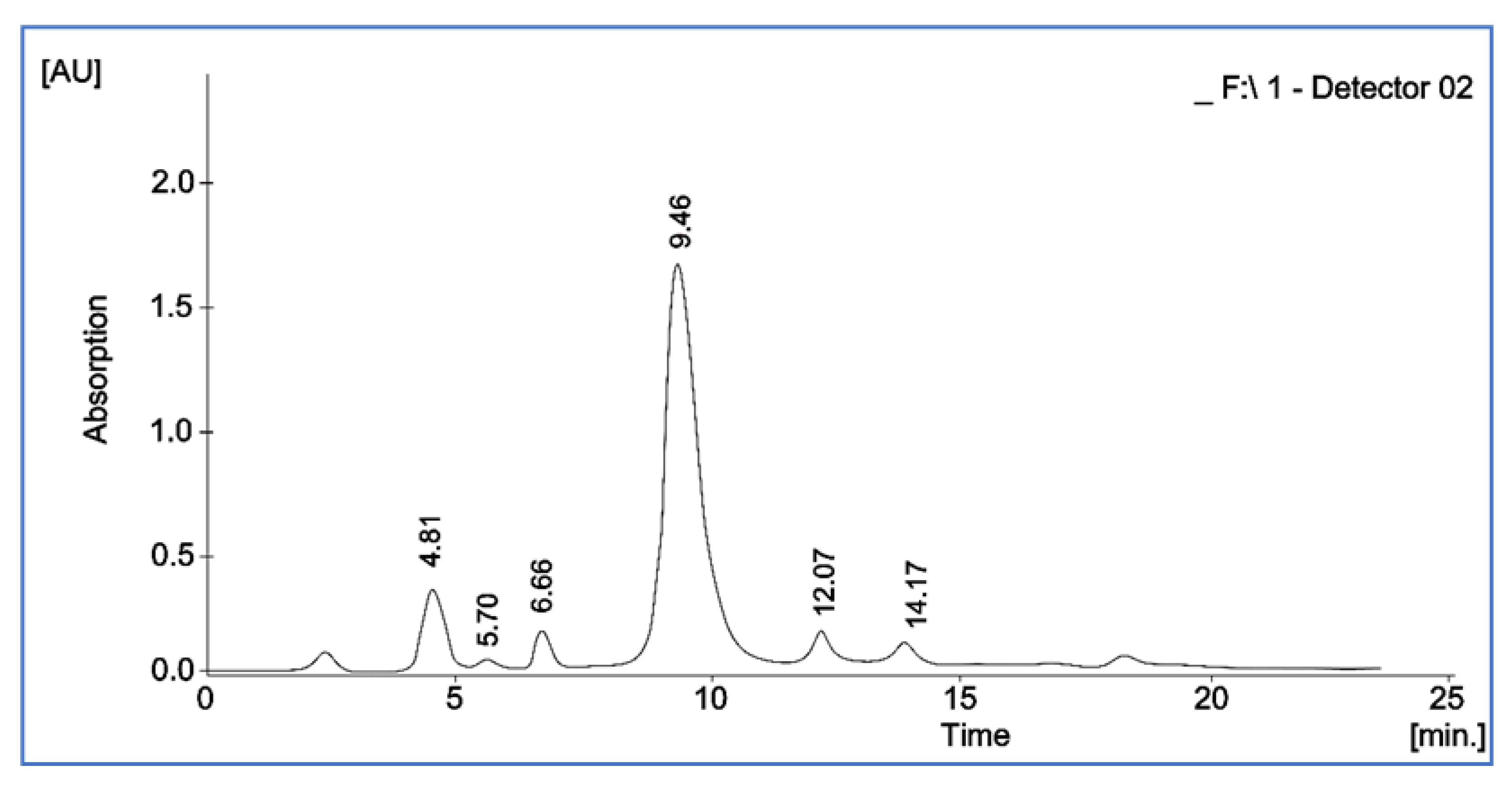
2.4.6. Estimation of Vitamin E (Tocopherol) by High-Performance Liquid Chromatography
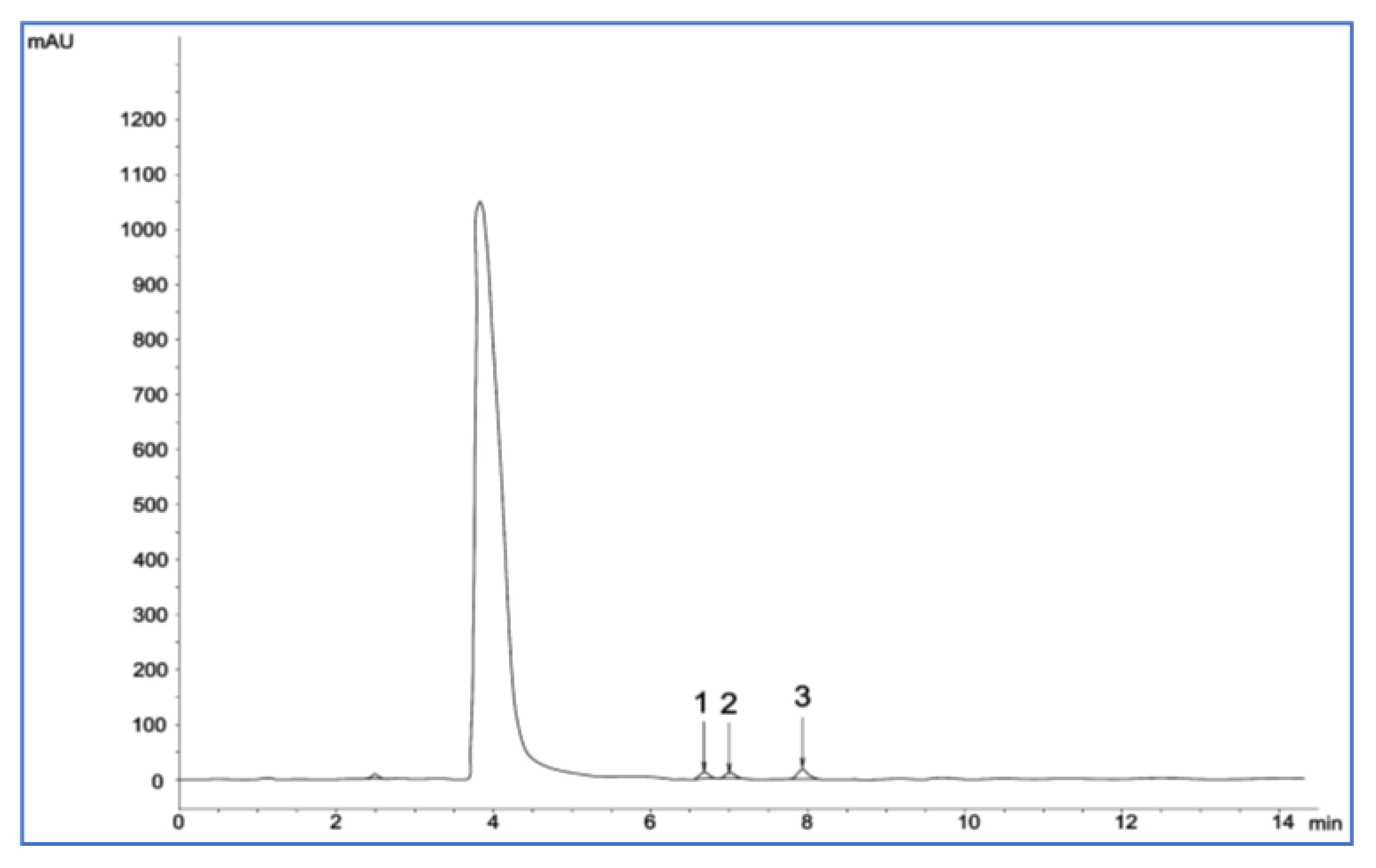
2.4.7. Analysis of Sugars by High-Performance Liquid Chromatography
2.4.8. Gas Chromatography–Mass Spectrometry Analysis of Okra Pod Extract
2.5. In Vitro Antidiabetic Activity of Aqueous Extract of Okra Pods
2.5.1. Amylase Inhibition Assay
2.5.2. Glucose Diffusion Inhibition Assay
2.6. Anticancer Activity of Okra Pod Extract against HepG2 Cells
2.6.1. Determination of Cell Viability by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl
Tetrazolium Bromide Assay
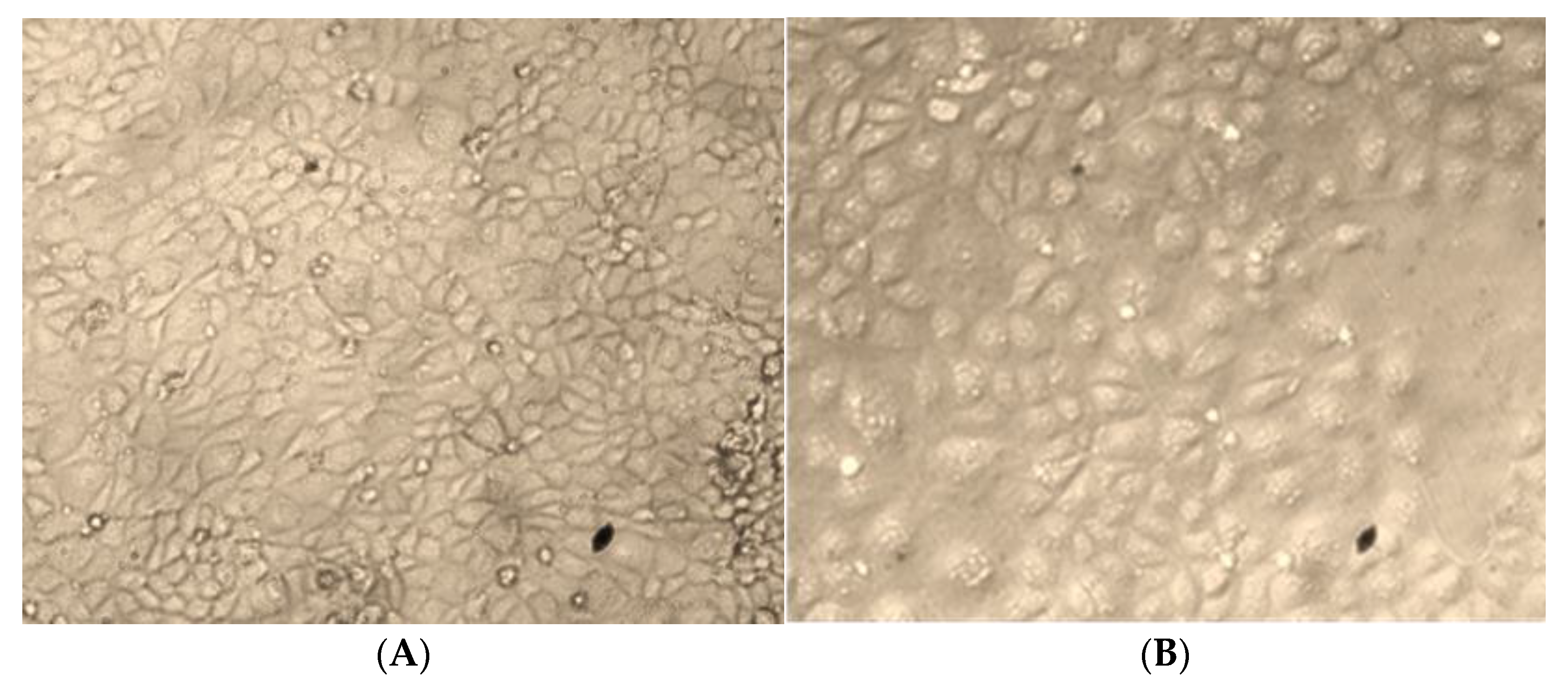
2.6.2. Morphological Analysis by Phase-Contrast Microscopy
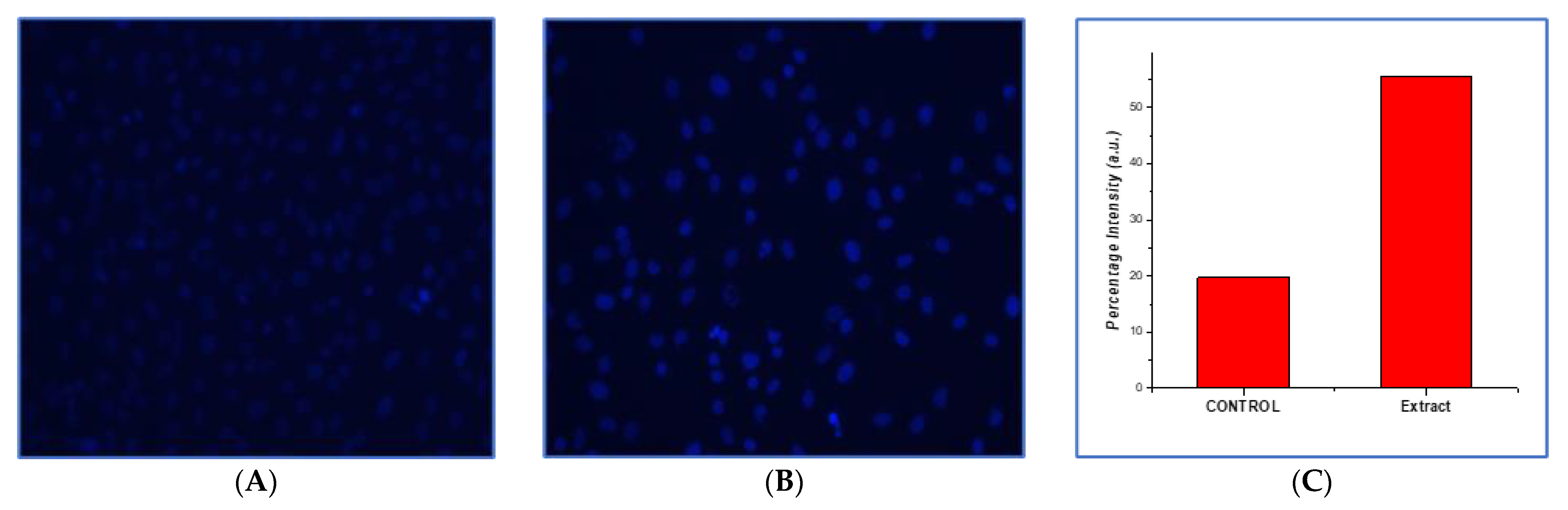
2.6.3. Nuclear Condensation Detection by 4,6-Diamidino-2-phenylindole Staining
2.6.4. Quantification of Intracellular Reactive Oxygen Species
2.7. Antimicrobial Activity of Okra Pod Extract
2.8. Statistical Analysis
3. Results
3.1. Proximate Nutritional Composition
3.2. Proximate Mineral Composition
3.3. Determination of Phenolic Compounds
3.4. Estimation of Chlorophyll and Carotenoid Content
3.5. Antioxidant Potential of Okra
3.6. Estimation of Vitamin E (Tocopherol) Content
3.7. Determination of Sugar Content
3.8. GC–MS Analysis of Okra Pods
3.9. α-Amylase Inhibition and Glucose Diffusion Inhibition Activity
3.10. In Vitro Anticancer Activity of Okra Extract
3.10.1. Morphological Changes in HepG2 Cells
3.10.2. Analysis of Morphological and Nuclear Structural Changes
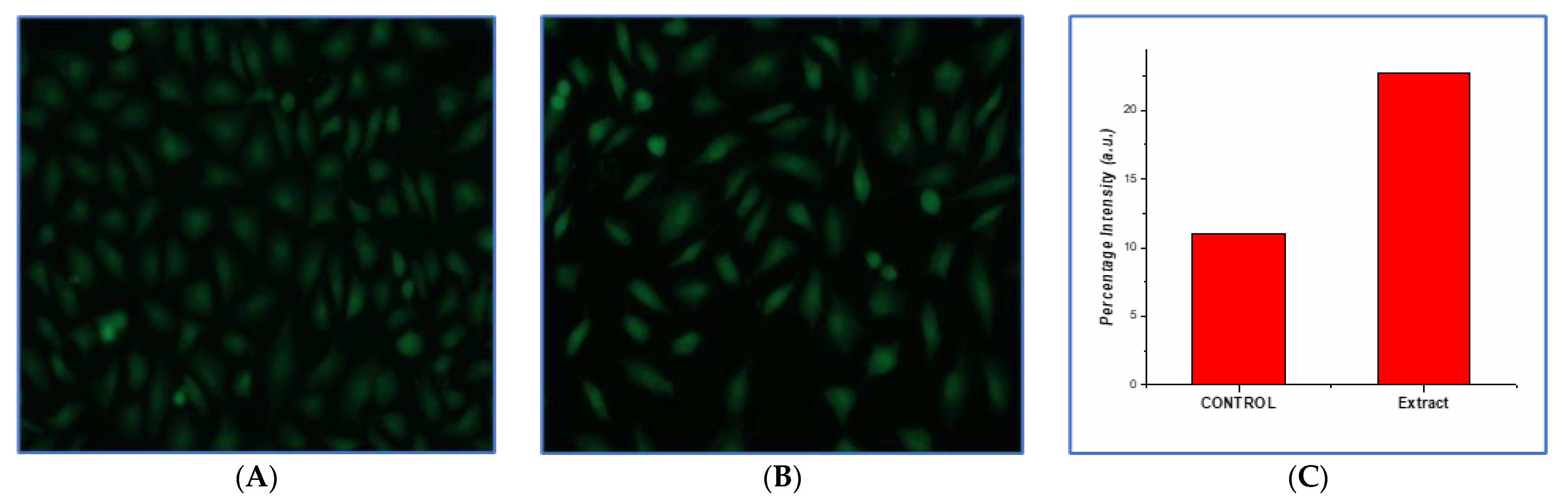
3.10.3. Production of Reactive Oxygen Species
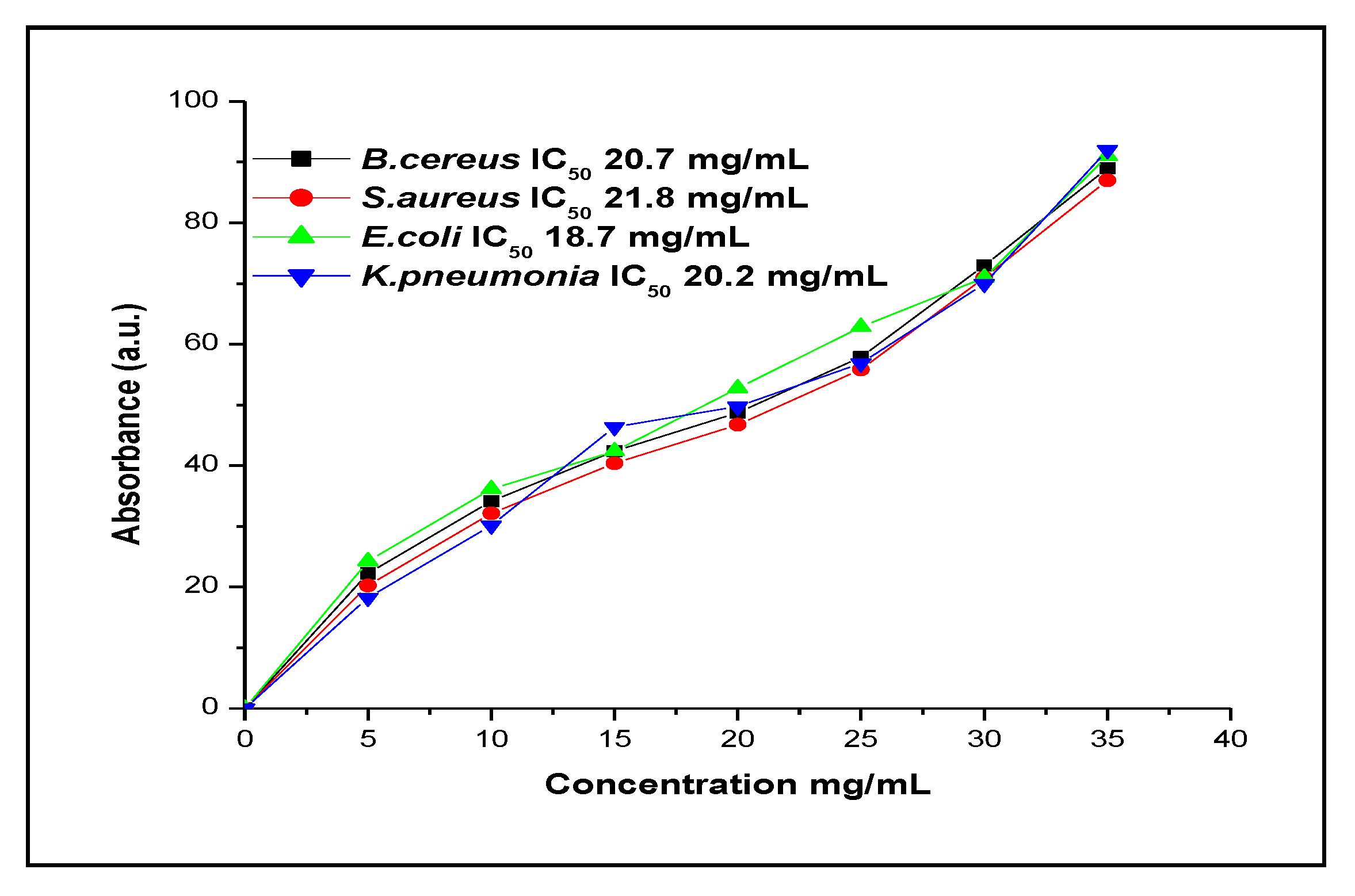
3.11. Antimicrobial Activity of Okra Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshahrani, M.Y.; Rafi, Z.; Alabdallah, N.M.; Shoaib, A.; Ahmad, I.; Asiri, M.; Zaman, G.S.; Wahab, S.; Saeed, M.; Khan, S. A Comparative Antibacterial, Antioxidant, and Antineoplastic Potential of Rauwolfia serpentina (L.) Leaf Extract with Its Biologically Synthesized Gold Nanoparticles (R-AuNPs). Plants 2021, 10, 2278. [Google Scholar] [CrossRef]
- Kumar, S.; Dagnoko, S.; Haougui, A.; Ratnadass, A.; Pasternak, N.; Kouame, C. Okra (Abelmoschus spp.) in West and Central Africa: Potential and progress on its improvement. Afr. J. Agric. Res. 2010, 5, 3590–3598. [Google Scholar]
- Al-Shawi, A.A.; Hameed, M.F.; Hussein, K.A.; Thawini, H.K. Review on the “Biological Applications of Okra Polysaccharides and Prospective Research”. Future J. Pharm. Sci. 2021, 7, 102. [Google Scholar] [CrossRef]
- Bhat, U.R.; Tharanathan, R.N. Functional properties of okra (Hibiscus esculentus) mucilage. Starch-Stärke 1987, 39, 165–167. [Google Scholar] [CrossRef]
- Somasundaram, G.; Rajan, J. Effectual Role of Abelmoschus esculentus (Okra) extract on morphology, microbial and photocatalytic activities of CdO Tetrahedral Clogs. J. Inorg. Organomet. Polym. Mater. 2018, 28, 152–167. [Google Scholar] [CrossRef]
- Prommakool, A.; Sajjaanantakul, T.; Janjarasskul, T.; Krochta, J.M. Whey protein-okra polysaccharide fraction blend edible films: Tensile properties, water vapor permeability and oxygen permeability. J. Sci. Food Agric. 2011, 91, 362–369. [Google Scholar] [CrossRef]
- Lee, C.S.; Chong, M.F.; Robinson, J.; Binner, E. Optimisation of extraction and sludge dewatering efficiencies of bio-flocculants extracted from Abelmoschus esculentus (okra). J. Environ. Manag. 2015, 157, 320–325. [Google Scholar] [CrossRef]
- Sabitha, V.; Panneerselvam, K.; Ramachandran, S. In vitro α-glucosidase and α-amylase enzyme inhibitory effects in aqueous extracts of Abelmoscus esculentus (L.) Moench. Asian Pac. J. Trop. Biomed. 2012, 2, S162–S164. [Google Scholar] [CrossRef]
- Sabitha, V.; Ramachandran, S.; Naveen, K.R.; Panneerselvam, K. Antidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic rats. J. Pharm. Bioallied. Sci. 2011, 3, 397–402. [Google Scholar]
- Durazzo, A.; Lucarini, M.; Novellino, E.; Souto, E.B.; Daliu, P.; Santini, A. Abelmoschus esculentus (L.): Bioactive Components’ Beneficial Properties-Focused on Antidiabetic Role-For Sustainable Health Applications. Molecules 2018, 24, 38. [Google Scholar] [CrossRef]
- Whistler, R.L.; Conrad, H. 2-O-(D-Galactopyranosyluronic Acid)-L-rhamnose from Okra Mucilage1. J. Am. Chem. Soc. 1954, 76, 3544–3546. [Google Scholar] [CrossRef]
- Ameena, K.; Dilip, C.; Saraswathi, R.; Krishnan, P.; Sankar, C.; Simi, S. Isolation of the mucilages from Hibiscus rosasinensis linn. and Okra (Abelmoschus esculentus linn.) and studies of the binding effects of the mucilages. Asian Pac. J. Trop. Med. 2010, 3, 539–543. [Google Scholar] [CrossRef]
- Ogungbenle, H.; Omosola, S. The comparative assessment of nutritive values of dry Nigerian okra (Abelmoschus esculentus) fruit and oil. Int. J. Food Sci. Nutr. Eng. 2015, 5, 8–14. [Google Scholar]
- Al-Kanani, E.A.S.; Al-Hilifi, S.A.H.; Al-Kareem, A.H. The nutritional composition and vitamin E of three Iraqi okra (Abelmoschus esculentus L.) seeds oil. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Kaur, G.; Singh, D.; Brar, V. Bioadhesive okra polymer based buccal patches as platform for controlled drug delivery. Int. J. Biol. Macromol. 2014, 70, 408–419. [Google Scholar] [CrossRef]
- Freitas, T.; Oliveira, V.; de Souza, M.; Geraldino, H.; Almeida, V.; Fávaro, S.; Garcia, J. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crops Prod. 2015, 76, 538–544. [Google Scholar] [CrossRef]
- Adamma, E.P.; Sani, S.M.; Israel, O.K.; Yusuf, Z.S. Proximate and anti-nutritional constituents of Abelmoschus esculentus grown in Fadaman Kubanni, Zaria, Kaduna State, Nigeria. J. Sci. Res. Rep. 2014, 3, 2015–2027. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bessada, S.M.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb. f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Mounir, S.; Ghandour, A.; Téllez-Pérez, C.; Aly, A.A.; Mujumdar, A.S.; Allaf, K. Phytochemicals, chlorophyll pigments, antioxidant activity, relative expansion ratio, and microstructure of dried okra pods: Swell-drying by instant controlled pressure drop versus conventional shade drying. Dry. Technol. 2020, 39, 2145–2159. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Mansoor, S.; Khan, I.; Fatima, J.; Saeed, M.; Mustafa, H. Anti-bacterial, anti-oxidant and cytotoxicity of aqueous and organic extracts of Ricinus communis. Afr. J. Microbiol. Res. 2016, 10, 260–270. [Google Scholar]
- Lengsfeld, C.; Titgemeyer, F.; Faller, G.; Hensel, A. Glycosylated compounds from okra inhibit adhesion of Helicobacter pylori to human gastric mucosa. J. Agric. Food Chem. 2004, 52, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Iram, S.; Zahera, M.; Khan, S.; Khan, I.; Syed, A.; Ansary, A.A.; Ameen, F.; Shair, O.H.; Khan, M.S. Gold nanoconjugates reinforce the potency of conjugated cisplatin and doxorubicin. Colloids Surf. B Biointerfaces 2017, 160, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Pakos, E.E.; Nearchou, A.D.; Grimer, R.J.; Koumoullis, H.D.; Abudu, A.; Bramer, J.A.; Jeys, L.M.; Franchi, A.; Scoccianti, G.; Campanacci, D.; et al. Prognostic factors and outcomes for osteosarcoma: An international collaboration. Eur. J. Cancer 2009, 45, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Haque, E.; Hameed, R.; Maier, P.N.; Irfan, S.; Kamil, M.; Nazir, A.; Mir, S.S. Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy. Life Sci. 2020, 256, 118000. [Google Scholar] [CrossRef]
- Wahyuningsih, S.P.A.; Savira, N.I.I.; Anggraini, D.W.; Winarni, D.; Suhargo, L.; Kusuma, B.W.A.; Nindyasari, F.; Setianingsih, N.; Mwendolwa, A.A. Antioxidant and Nephroprotective Effects of Okra Pods Extract (Abelmoschus esculentus L.) against Lead Acetate-Induced Toxicity in Mice. Scientifica 2020, 2020, 4237205. [Google Scholar] [CrossRef]
- Romdhane, M.H.; Al-Mohammadi, A.R.; Mahgoub, S.; Abdel-Shafi, S.; Askar, E.; Ghaly, M.F.; Taha, M.A.; El-Gazzar, N. Chemical Composition, Nutritional Value, and Biological Evaluation of Tunisian Okra Pods (Abelmoschus esculentus L. Moench). Molecules 2020, 25, 4739. [Google Scholar] [CrossRef]
- Ilodibia, C.V.; Achebe, U.A.; Chiafor, C. Nutrient characteristics assessment of two variants of okra (Abelmoschus esculentus L. Moench.) found in Anambra State, Nigeria. Arch. Agric. Environ. Sci. 2017, 2, 298–300. [Google Scholar] [CrossRef]
- Miranda, M.; Vega-Gálvez, A.; López, J.; Parada, G.; Sanders, M.; Aranda, M.; Uribe, E.; Di Scala, K. Impact of air-drying temperature on nutritional properties, total phenolic content and antioxidant capacity of quinoa seeds (Chenopodium quinoa Willd.). Ind. Crops Prod. 2010, 32, 258–263. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Fernández-Ruiz, V.; Matallana-González, M.C.; Morales, P. Dietary fiber sources and human benefits: The case study of cereal and pseudocereals. Adv. Food Nutr. Res. 2019, 90, 83–134. [Google Scholar]
- Omoniyi, S.A.; Muhammad, A.M.; Ayuba, R. Nutrient composition and anti-nutritional properties of okra (Abelmoschus esculentus) calyx flour. Nutr. Food Sci. 2021, 51, 30–40. [Google Scholar] [CrossRef]
- Hussain, M.S.; Fareed, S.; Saba Ansari, M.; Rahman, A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioallied. Sci. 2012, 4, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Fujita, K.I.; Ishikura, T.; Jono, Y.; Yamaguchi, Y.; Ogita, A.; Kubo, I.; Tanaka, T. Anethole potentiates dodecanol’s fungicidal activity by reducing PDR5 expression in budding yeast. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Jwa, S.K. Inhibitory effects of beta-caryophyllene on Streptococcus mutans biofilm. Arch. Oral Biol. 2018, 88, 42–46. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.; Totti, B.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K.; et al. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab. Syndr. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Kalhoro, M.S.; Kalhoro, D.H.; Adebowale, T.; Mazhar, M.U.; Rehman, Z.U.; et al. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef]
- Saeed, M.; Shoaib, A.; Tasleem, M.; Alabdallah, N.; Alam, J.; Asmar, Z.; Jamal, Q.; Bardakci, F.; Alqahtani, S.; Ansari, I.; et al. Assessment of Antidiabetic Activity of the Shikonin by Allosteric Inhibition of Protein-Tyrosine Phosphatase 1B (PTP1B) Using State of Art: An In Silico and In Vitro Tactics. Molecules 2021, 26, 3996. [Google Scholar] [CrossRef]
- de Carvalho, C.C.; Cruz, P.A.; da Fonseca, M.M.R.; Xavier-Filho, L. Antibacterial properties of the extract of Abelmoschus esculentus. Biotechnol. Bioprocess Eng. 2011, 16, 971–977. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Barros, L.; Ciric, A.; Sokovic, M.; Ferreira, I.C. The chemical composition, nutritional value and antimicrobial properties of Abelmoschus esculentus seeds. Food Funct. 2017, 8, 4733–4743. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Alshammari, E.; Adnan, M.; Alcantara, J.C.C.; Awadelkareem, A.M.; Eltoum, N.E.; Mehmood, K.; Panda, B.P.; Ashraf, S.A. Okra (Abelmoschus Esculentus) as a Potential Dietary Medicine with Nutraceutical Importance for Sustainable Health Applications. Molecules 2021, 26, 696. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Shoaib, A.; Kandimalla, R.; Javed, S.; Almatroudi, A.; Gupta, R.; Aqil, F. Microbe-based therapies for colorectal cancer: Advantages and limitations. Semin. Cancer Biol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, S.; Wang, M.; Chen, X.; Tian, L.; Wang, L.; Yang, W.; Chen, L.; He, F.; Yin, W. Flavonoid-rich extracts from okra flowers exert antitumor activity in colorectal cancer through induction of mitochondrial dysfunction-associated apoptosis, senescence and autophagy. Food Funct. 2020, 11, 10448–10466. [Google Scholar] [CrossRef]
- Ping, M.H. Hyperin Controls the Development and Therapy of Gastric Cancer via Regulating Wnt/beta-Catenin Signaling. Cancer Manag. Res. 2020, 12, 11773–11782. [Google Scholar] [CrossRef]
- Chaemsawang, W.; Prasongchean, W.; Papadopoulos, K.I.; Ritthidej, G.; Sukrong, S.; Wattanaarsakit, P. The Effect of Okra (Abelmoschus esculentus (L.) Moench) Seed Extract on Human Cancer Cell Lines Delivered in Its Native Form and Loaded in Polymeric Micelles. Int. J. Biomater. 2019, 2019, 9404383. [Google Scholar] [CrossRef]
- de Oliveira Figueiroa, E.; Da Cunha, C.R.A.; Albuquerque, P.B.; De Paula, R.A.; Aranda-Souza, M.A.; Alves, M.S.; Zagmignan, A.; Carneiro-Da-Cunha, M.G.; Da Silva, L.C.N.; Correia, M.T.D.S. Lectin-Carbohydrate Interactions: Implications for the Development of New Anticancer Agents. Curr. Med. Chem. 2017, 24, 3667–3680. [Google Scholar] [CrossRef] [PubMed]



| S.No. | Nutrient Composition | Content (g/100 g) |
|---|---|---|
| 1 | Moisture | 84.67–87.65 |
| 2 | Total ash | 1.514–1.197 |
| 3 | Crude protein | 2.367–3.41 |
| 4 | Total fat | 0.066–0.1 |
| 5 | Total carbohydrate | 7.857–8.261 |
| 6 | Dietary fiber | 6.781–8.314 |
| 7 | Energy (kcal/100 g) | 51.258 |
| S.No. | Mineral Composition | Content (mg/100 g) |
|---|---|---|
| 1 | Iron | 1.5–1.7 |
| 2 | Copper | 0.48–0.5 |
| 3 | Manganese | 0.12–0.15 |
| 4 | Zinc | 1.8–2.2 |
| 5 | Magnesium | 100–104 |
| 6 | Calcium | 260–270 |
| 7 | Sodium | 120–130 |
| 8 | Potassium | 330–350 |
| S.No. | Soluble Sugars | Content mg/100 g |
|---|---|---|
| 1 | Fructose | 33–35 |
| 2 | Glucose | 28–32 |
| 3 | Sucrose | 105–115 |
| 4 | Total sugars | 166–182 |
| Compounds | Abundance/ Values | Chemical Formula | Molecular Weight (g/mol) |
|---|---|---|---|
| 4-Hydroxy-d-methyl-2-pentanone | 834; 3.45 | ||
| α-Pinene | 939; 1.85 | C10H16 | 136.23 |
| (E)-2-Heptanal | 959; 0.67 | C7H12O | 112.17 |
| Benzaldehyde | 961; 0.47 | C7H6O | 106.12 |
| 1-Octn-3-ol | 978; 2.95 | C8H16O | 128.21 |
| β-Pinene | 980; 0.85 | C10H16 | 136.23 |
| 6-Methyl-5-hepten-2-one | 984; 2.32 | C8H14O | 126.20 |
| Limonene | 1030; 6.65 | C10H16 | 136.24 |
| (E)-3-Octen-1-ol | 1059; 3.94 | C8H16O | 128.21 |
| (E)-2-Octen-1-ol | 1068; 4.22 | C8H16O | 128.21 |
| 1-Octanol | 1072; 3.85 | C8H18O | 130.23 |
| n-Undecane | 1098; 0.55 | C11H24 | 156.31 |
| Linalool | 1098; 1.4 | C10H18O | 154.25 |
| Nonanal | 1102; 3.25 | C9H18O | 142.23 |
| cis-ρ-Menth-2-en-1-ol | 1120; 1.45 | C10H18O | 154.24 |
| Camphor | 1142; 3.23 | C10H16O | 152.23 |
| Menthol | 1172; 1.3 | C10H20O | 156.27 |
| α-Terpineol | 1184; 0.75 | C10H18O | 154.25 |
| cis-Dihydrocarvone | 1193; 0.42 | C10H16O | 152.23 |
| n-Dodecane | 1197; 2.35 | C12H26 | 170.33 |
| Decanal | 1204; 4.45 | C10H20O | 156.2 |
| β-Cyclocitral | 1219; 1.55 | C10H16O | 152.23 |
| Exo-fenchyl acetate | 1228; 2.05 | C12H20O2 | 196.29 |
| Carvone | 1241; 4.35 | C10H14O | 150.22 |
| (E)-Anethole | 1283; 6.7 | C10H12O | 148.20 |
| n-Tridecane | 1297; 1.85 | C13H28 | 184.37 |
| α-Cubebene | 1349; 1.55 | C15H24 | 204.35 |
| Eugenol | 1356; 4.05 | C10H12O2 | 164.2 |
| n-Tetradecane | 1395; 1.95 | C14H30 | 198.39 |
| β-Caryophyllene | 1017; 5.2 | C15H24 | 204.36 |
| 2,5-Dimethoxy-ρ-cymene | 1419; 1.43 | C12H18O2 | 194.27 |
| Δ8,9-Dehydro-4-hydroxythymol dimethyl ether | 1442; 4.15 | C12H16O2 | 192.26 |
| Δ8,9-Dehydro-4-hydroxythymol | 1457; 2.48 | C10H12O2 | 164.20 |
| γ-Muurolene | 1476; 1.6 | C15H24 | 204.35 |
| 3,4-Dehydro-β-ionone | 1781; 2.35 | C13H22O | 194.31 |
| Class of Compounds | Percentage | ||
| Monoterpene hydrocarbons | 8.50 | ||
| Oxygenated monoterpenes | 21.10 | ||
| Sesquiterpene hydrocarbons | 10.50 | ||
| Phenylpropanoids | 10.30 | ||
| Apocarotenes | 3.70 | ||
| Non-terpene derivatives | 37.53 | ||
| Total identified (%) | 91.63 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Rafi, Z.; Baker, A.; Shoaib, A.; Alkhathami, A.G.; Asiri, M.; Alshahrani, M.Y.; Ahmad, I.; Alraey, Y.; Hakamy, A.; et al. Phytochemical Screening, Nutritional Value, Anti-Diabetic, Anti-Cancer, and Anti-Bacterial Assessment of Aqueous Extract from Abelmoschus esculentus Pods. Processes 2022, 10, 183. https://doi.org/10.3390/pr10020183
Khan S, Rafi Z, Baker A, Shoaib A, Alkhathami AG, Asiri M, Alshahrani MY, Ahmad I, Alraey Y, Hakamy A, et al. Phytochemical Screening, Nutritional Value, Anti-Diabetic, Anti-Cancer, and Anti-Bacterial Assessment of Aqueous Extract from Abelmoschus esculentus Pods. Processes. 2022; 10(2):183. https://doi.org/10.3390/pr10020183
Chicago/Turabian StyleKhan, Salman, Zeeshan Rafi, Abu Baker, Ambreen Shoaib, Ali G. Alkhathami, Mohammed Asiri, Mohammad Y. Alshahrani, Irfan Ahmad, Yasser Alraey, Ali Hakamy, and et al. 2022. "Phytochemical Screening, Nutritional Value, Anti-Diabetic, Anti-Cancer, and Anti-Bacterial Assessment of Aqueous Extract from Abelmoschus esculentus Pods" Processes 10, no. 2: 183. https://doi.org/10.3390/pr10020183
APA StyleKhan, S., Rafi, Z., Baker, A., Shoaib, A., Alkhathami, A. G., Asiri, M., Alshahrani, M. Y., Ahmad, I., Alraey, Y., Hakamy, A., Saeed, M., & Mansoor, S. (2022). Phytochemical Screening, Nutritional Value, Anti-Diabetic, Anti-Cancer, and Anti-Bacterial Assessment of Aqueous Extract from Abelmoschus esculentus Pods. Processes, 10(2), 183. https://doi.org/10.3390/pr10020183







