Development of Thermal Barrier Coating Systems from Al Microparticles—Part II: Characterisation of Mechanical and Thermal Transport Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microstructural Characterisation
2.2. Scratch Tests and Micro-Indentation
2.3. Thermal Insulation Assessment
3. Results
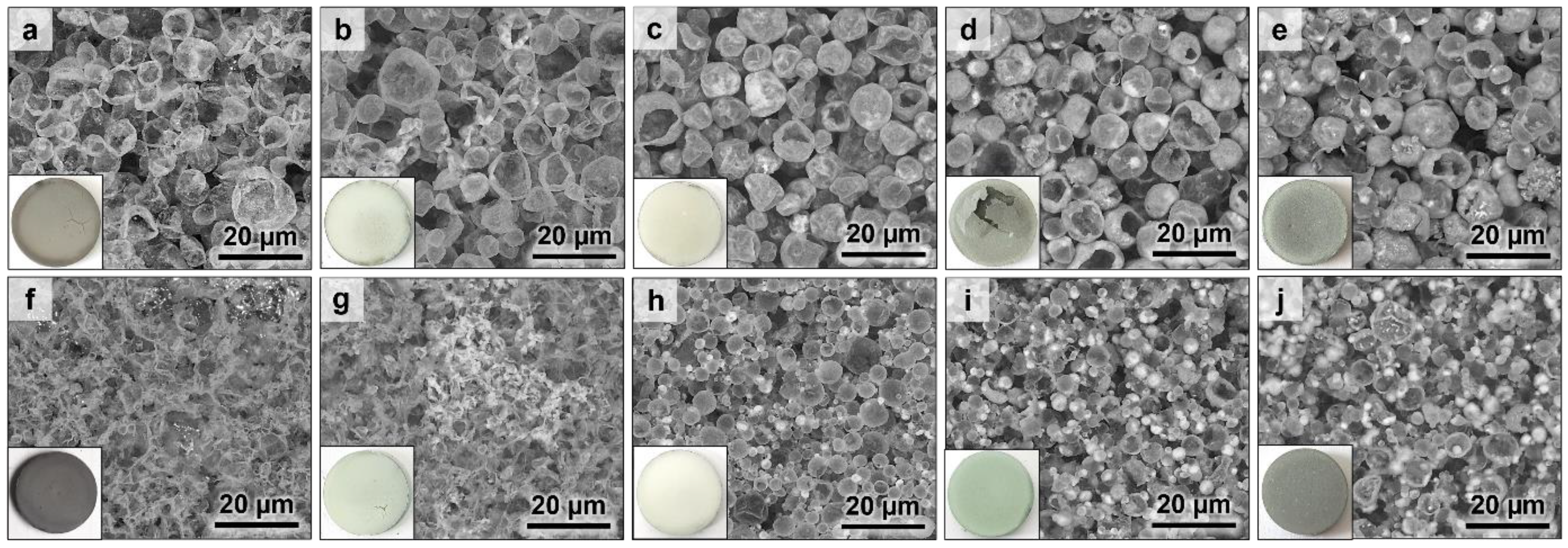
3.1. Microstructure of the Thermal Barrier Systems
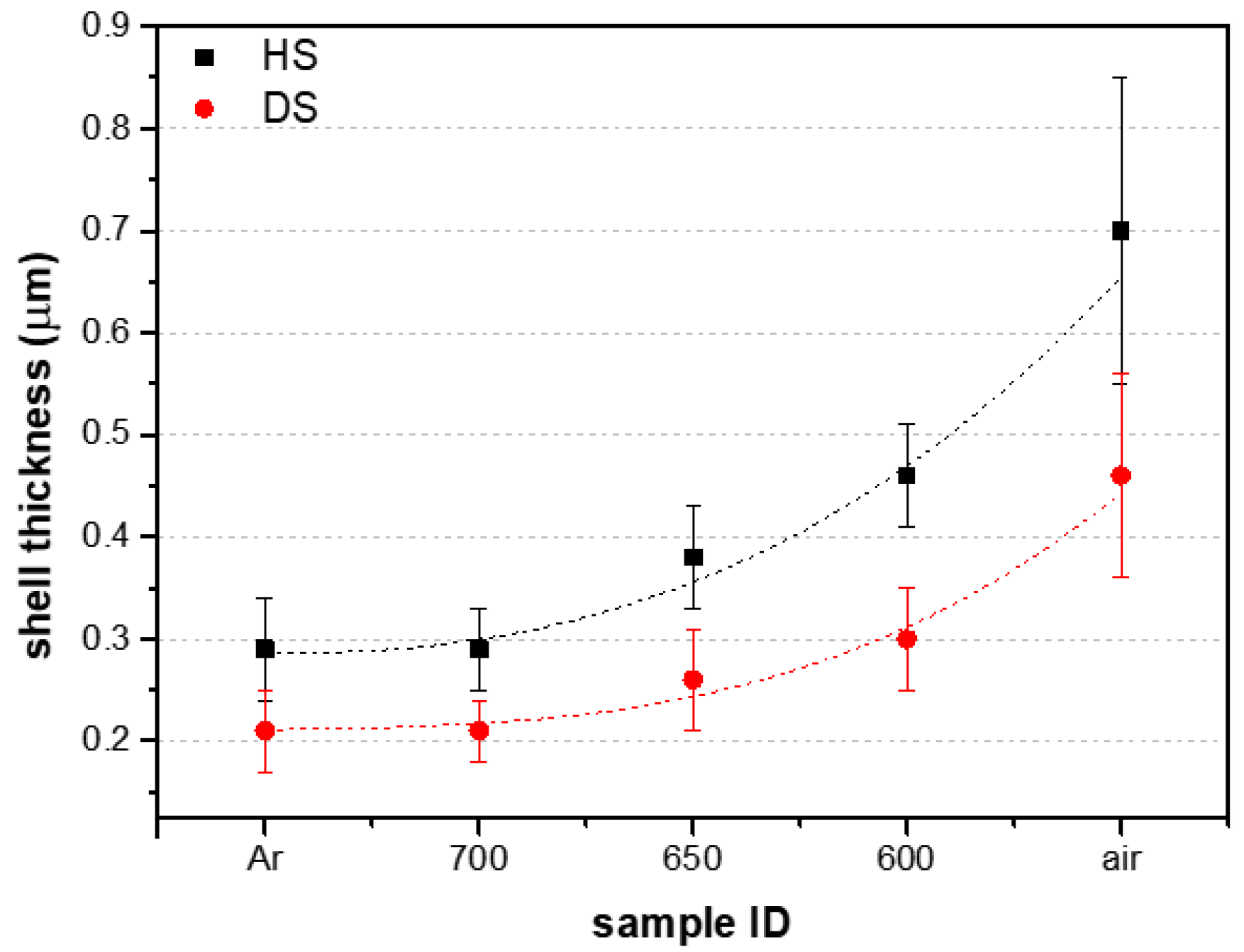
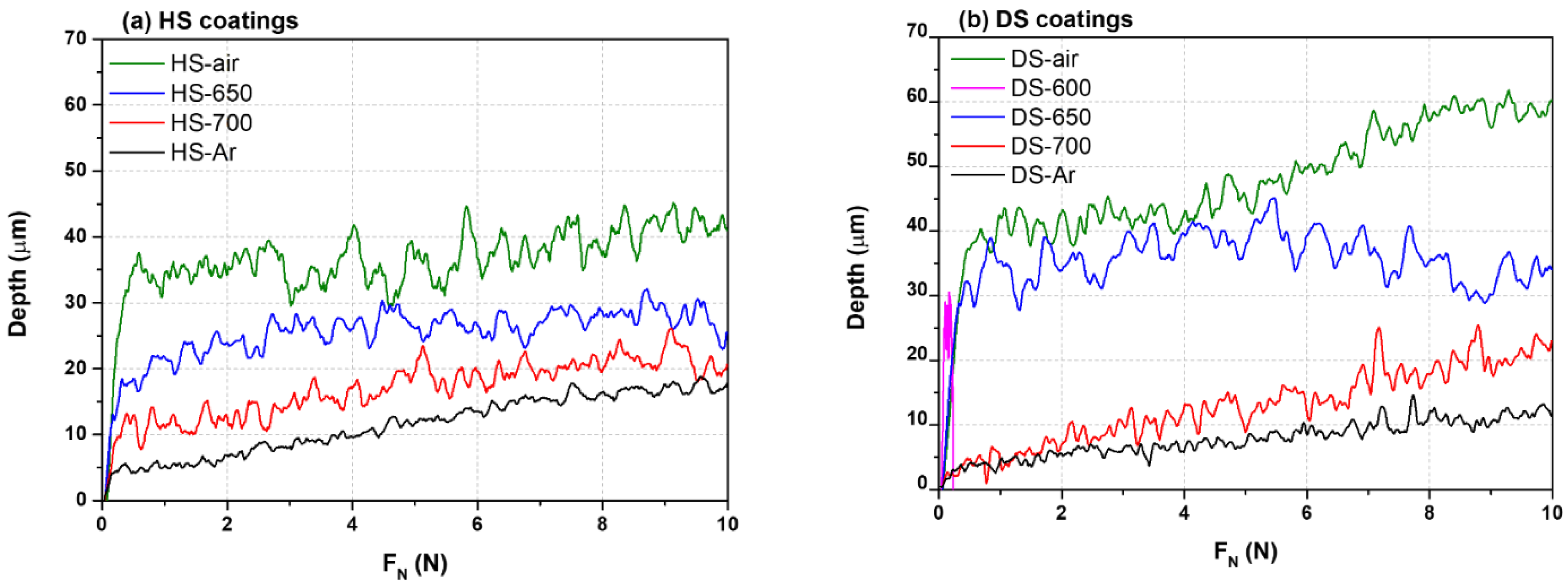
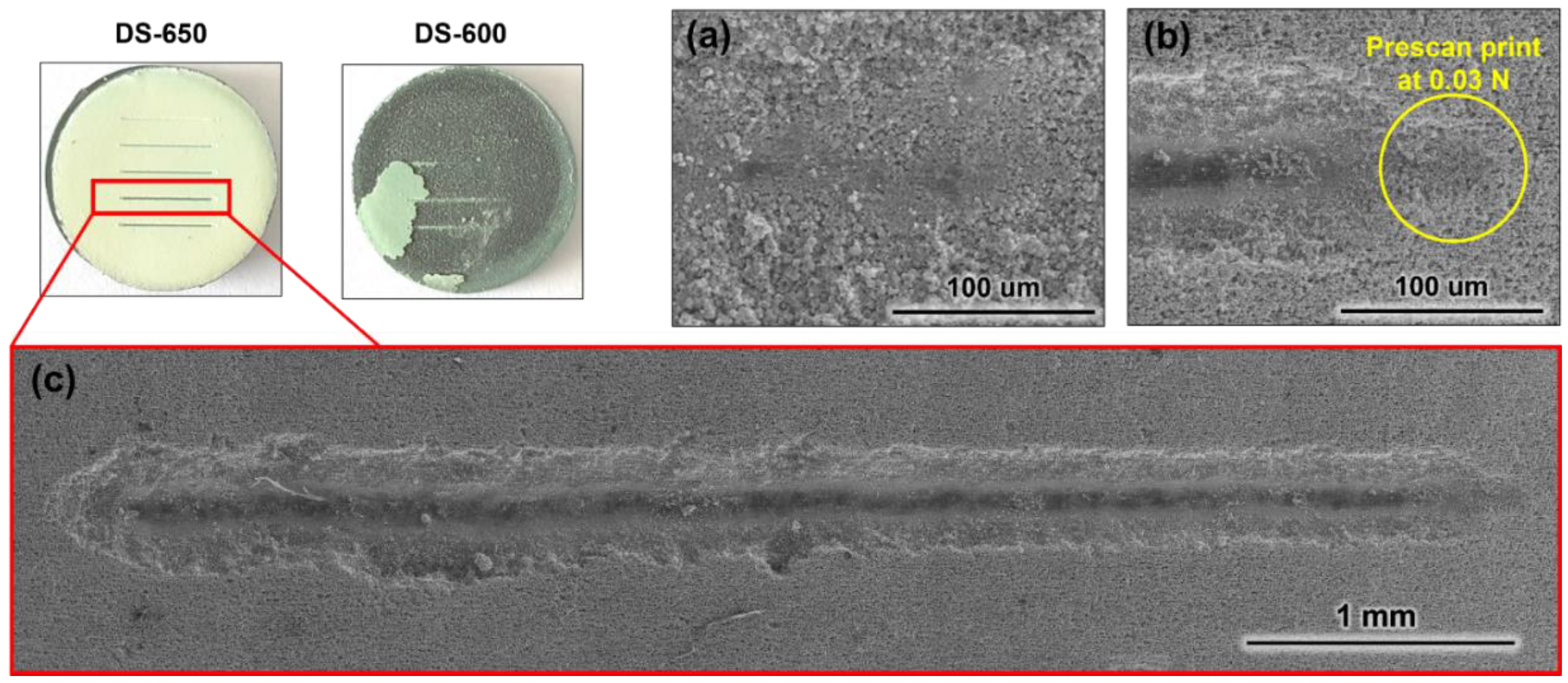
3.2. Mechanical Tests
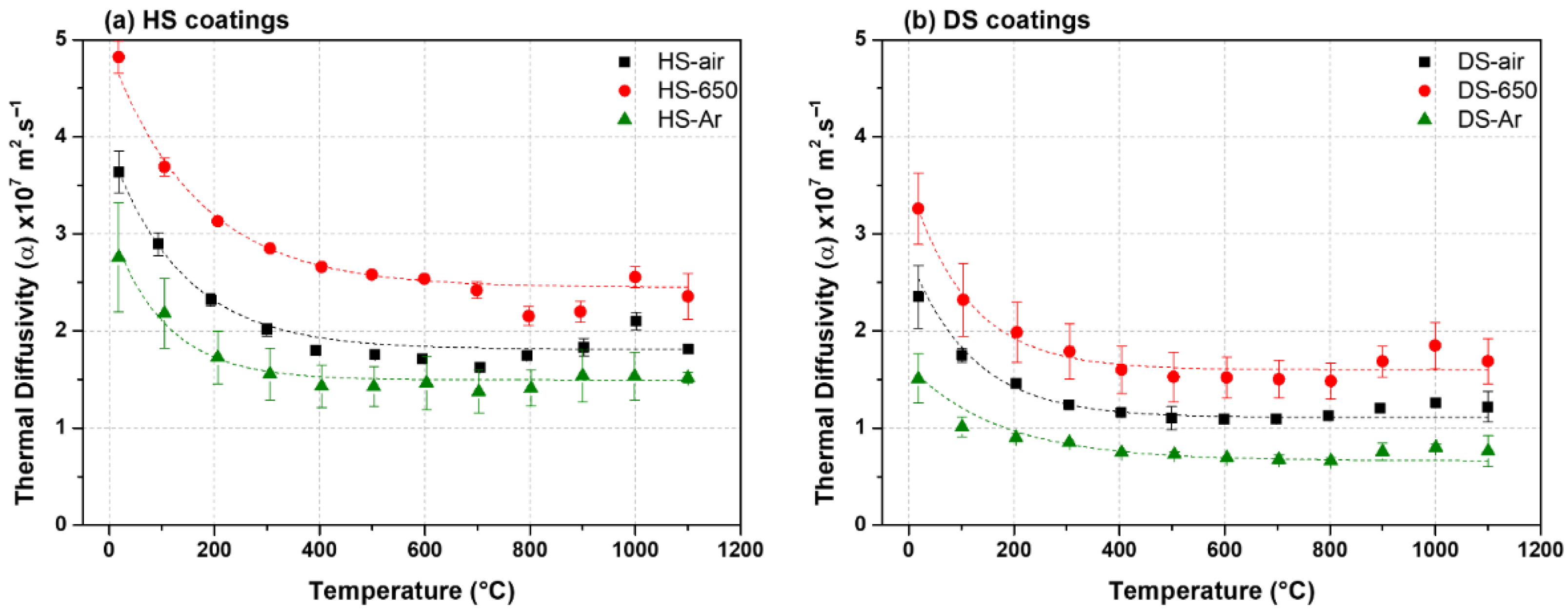
3.3. Thermal Diffusivity
4. Discussion
5. Conclusions
- All the coatings showed great thermal insulation properties on a par with the ones of the state-of-the-art YSZ TBCs made by plasma spraying or EB-PVD. Considering the foam coating and disregarding the potential thermal insulation of an interfacial thermally grown oxide, better thermal insulation is achieved with thinner shells.
- The greater mechanical resistance of the foam coating is obtained with thicker shells of the hollow alumina spheres. This is achieved by increasing the exposure to air during the annealing step.
- The scratch tests also allowed the comparison of the cohesion of the different coatings and only the DS-600 and HS-600 coatings spalled. All the other coatings possess good adhesive properties in comparison with the strength of cohesion of the alumina foam itself.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padture, N.P. Advanced Structural Ceramics in Aerospace Propulsion. Nat. Mater. 2016, 15, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Mollard, M.; Rannou, B.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. Comparative Degradation of Nickel Aluminized by Slurry and by Pack Cementation under Isothermal Conditions. Corros. Sci. 2013, 66, 118–124. [Google Scholar] [CrossRef]
- Montero, X.; Galetz, M.; Schütze, M. A Single Step Process to Form In-Situ an Alumina Foam/Aluminide TBC System for Alloys in Extreme Environments at High Temperatures. Surf. Coat. Technol. 2011, 206, 1586–1594. [Google Scholar] [CrossRef]
- Pedraza, F.; Mollard, M.; Rannou, B.; Balmain, J.; Bouchaud, B.; Bonnet, G. Potential Thermal Barrier Coating Systems from Al Microparticles. Mechanisms of Coating Formation on Pure Nickel. Mater. Chem. Phys. 2012, 134, 700–705. [Google Scholar] [CrossRef]
- Pedraza, F.; Rannou, B.; Boissonnet, G.; Bouchaud, B.; Maache-Rezzoug, Z. Rheological Behaviour, Synthesis and Performance of Smart Thermal Barrier Coating Systems Based on Hollow Alumina. J. Mater. Sci. Chem. Eng. 2015, 3, 17–22. [Google Scholar] [CrossRef][Green Version]
- Pedraza, F.; Boissonnet, G.; Fernandez, B.; Bouchaud, B.; Podor, R. Synthesis and Related Properties of Low k TBCs with Hollow Alumina Microspheres. Presented at the International Conference on Metallurgical Coatings and Thin Films (ICMTF), San Diego, CA, USA, April 2016. [Google Scholar]
- Kolarik, V.; Roussel, R.; Juez Lorenzo, M.; Fietzek, H. Factors Influencing the Formation of a Diffusion Zone and the Adherence of the Top Coat of High Temperature Coatings from Micro-Sized Spherical Aluminium Particles. Mater. High Temp. 2012, 29, 89–94. [Google Scholar] [CrossRef]
- Galetz, M.C.; Montero, X.; Mollard, M.; Günthner, M.; Pedraza, F.; Schütze, M. The Role of Combustion Synthesis in the Formation of Slurry Aluminization. Intermetallics 2014, 44, 8–17. [Google Scholar] [CrossRef]
- Brossard, M.; Bouchaud, B.; Pedraza, F. Influence of Water Vapour on the Oxidation Behaviour of a Conventional Aluminide and a New Thermal Barrier Coating System Sintered from a Slurry: Water Vapour Oxidation of Aluminide Coatings. Mater. Corros. 2014, 65, 161–168. [Google Scholar] [CrossRef]
- Pedraza, F.; Podor, R. Influence of Annealing Conditions on the Formation of Hollow Al2O3 Microspheres Studied by in Situ ESEM. Mater. Charact. 2016, 113, 198–206. [Google Scholar] [CrossRef]
- Boissonnet, G.; Grégoire, B.; Bonnet, G.; Pedraza, F. Development of Thermal Barrier Coating Systems from Al Microparticles. Part I: Influence of Processing Conditions on the Mechanisms of Formation. Surf. Coat. Technol. 2019, 380, 125085. [Google Scholar] [CrossRef]
- Boissonnet, G.; Bonnet, G.; Pasquet, A.; Bourhila, N.; Pedraza, F. Evolution of Thermal Insulation of Plasma-Sprayed Thermal Barrier Coating Systems with Exposure to High Temperature. J. Eur. Ceram. Soc. 2019, 39, 2111–2121. [Google Scholar] [CrossRef]
- Boissonnet, G.; Chalk, C.; Nicholls, J.R.; Bonnet, G.; Pedraza, F. Phase Stability and Thermal Insulation of YSZ and Erbia-Yttria Co-Doped Zirconia EB-PVD Thermal Barrier Coating Systems. Surf. Coat. Technol. 2020, 389, 125566. [Google Scholar] [CrossRef]
- Bull, S.J.; Berasetegui, E.G. An Overview of the Potential of Quantitative Coating Adhesion Measurement by Scratch Testing. Tribol. Int. 2006, 39, 99–114. [Google Scholar] [CrossRef]
- Li, Z.; Farhat, Z. The Effect of the Formation of Superelastic NiTi Phase on Static and Dynamic Corrosion Performance of Ni-P Coating. Solids 2021, 2, 278–292. [Google Scholar] [CrossRef]
- Vitry, V.; Sens, A.; Delaunois, F. Comparison of Various Electroless Nickel Coatings on Steel: Structure, Hardness and Abrasion Resistance. Mater. Sci. Forum 2014, 783–786, 1405–1413. [Google Scholar] [CrossRef]
- Taylor, R.E.; Wang, X.; Xu, X. Thermophysical Properties of Thermal Barrier Coatings. Surf. Coat. Technol. 1999, 120–121, 89–95. [Google Scholar] [CrossRef]
- Hasani, S.; Panjepour, M.; Shamanian, M. The Oxidation Mechanism of Pure Aluminum Powder Particles. Oxid. Met. 2012, 78, 179–195. [Google Scholar] [CrossRef]
- Hasani, S.; Soleymani, A.P.; Panjepour, M.; Ghaei, A. A Tension Analysis During Oxidation of Pure Aluminum Powder Particles: Non-Isothermal Condition. Oxid. Met. 2014, 82, 209–224. [Google Scholar] [CrossRef]


| Starting Atmosphere | Introduction of Synthetic Air | Sample ID * |
|---|---|---|
| Synthetic air | - | DS-air/HS-air |
| Ar | 550 °C | DS-550/HS-550 |
| Ar | 600 °C | DS-600/HS-600 |
| Ar | 650 °C | DS-650/HS-650 |
| Ar | 700 °C | DS-700/HS-700 |
| Ar | - | DS-Ar/HS-Ar |
| Atmosphere of Heat Treatment | Coating Thickness (µm) | Dmax (µm) | D0.5N (µm) | D0.03N* (µm) | ||||
|---|---|---|---|---|---|---|---|---|
| HS | DS | HS | DS | HS | DS | HS | DS | |
| Ar | 20.0 ± 5.0 | 8.5 ± 1.5 | 19.0 | 14.7 | 4.5 | 3.9 | 5.9 ± 1.4 | 6.5 ± 2.4 |
| Switch 700 °C | 35.0 ± 5.0 | 17.5 ± 2.5 | 23.4 | 25.7 | 10.2 | 4.8 | 11.0 ± 5.0 | 8.1 ± 1.1 |
| Switch 650 °C | 40.0 ± 6.0 | 27.5 ± 2.5 | 32.2 | 45.2 | 18.6 | 32.4 | 9.3 ± 6.3 | 4.8 ± 0.9 |
| Switch 600 °C | 45.0 ± 5.5 | 62.0 ± 5.0 | - | 30.6 | - | - | - | - |
| Synthetic air | 45.0 ± 5.0 | 65.0 ± 5.0 | 44.5 | 61.9 | 36.8 | 40.3 | 5.5 ± 2.2 | 5.9 ± 3.4 |
| Atmosphere of Heat Treatment | FN,av (mN) | |
|---|---|---|
| HS | DS | |
| Ar | 19.6 ± 5.1 | 21.3 ± 9.3 |
| Switch 700 °C | 8.5 ± 0.7 | 9.6 ± 2.3 |
| Switch 650 °C | 12.0 ± 1.7 | 12.6 ± 4.5 |
| Synthetic air | 17.6 ± 7.4 | 27.3 ± 4.5 |
| Other Standard Thermal Barrier Coatings | ||
| APS YSZ | 201.3 ± 22.0 | - |
| EB-PVD YSZ | 897.3 ± 229.0 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boissonnet, G.; Grosseau-Poussard, J.-L.; Bonnet, G.; Pedraza, F. Development of Thermal Barrier Coating Systems from Al Microparticles—Part II: Characterisation of Mechanical and Thermal Transport Properties. Coatings 2022, 12, 106. https://doi.org/10.3390/coatings12020106
Boissonnet G, Grosseau-Poussard J-L, Bonnet G, Pedraza F. Development of Thermal Barrier Coating Systems from Al Microparticles—Part II: Characterisation of Mechanical and Thermal Transport Properties. Coatings. 2022; 12(2):106. https://doi.org/10.3390/coatings12020106
Chicago/Turabian StyleBoissonnet, Germain, Jean-Luc Grosseau-Poussard, Gilles Bonnet, and Fernando Pedraza. 2022. "Development of Thermal Barrier Coating Systems from Al Microparticles—Part II: Characterisation of Mechanical and Thermal Transport Properties" Coatings 12, no. 2: 106. https://doi.org/10.3390/coatings12020106
APA StyleBoissonnet, G., Grosseau-Poussard, J.-L., Bonnet, G., & Pedraza, F. (2022). Development of Thermal Barrier Coating Systems from Al Microparticles—Part II: Characterisation of Mechanical and Thermal Transport Properties. Coatings, 12(2), 106. https://doi.org/10.3390/coatings12020106







