Isothermal Oxidation TGO Growth Behaviors of Laser-Remolten LZO/YSZ Thermal Barrier Coatings
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Sample Preparation
2.2. Isothermal Oxidation and Characterization
3. Results and Discussion
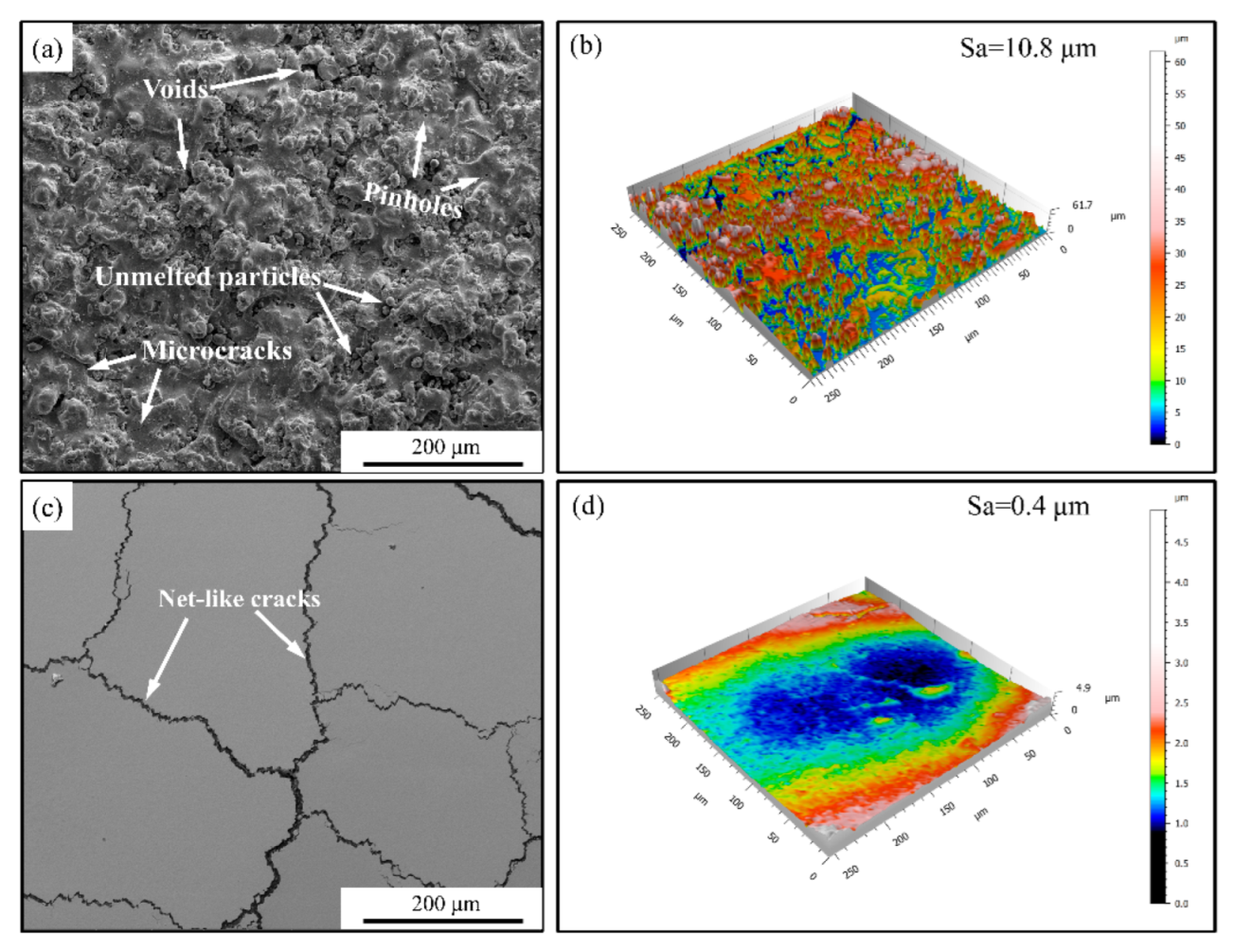
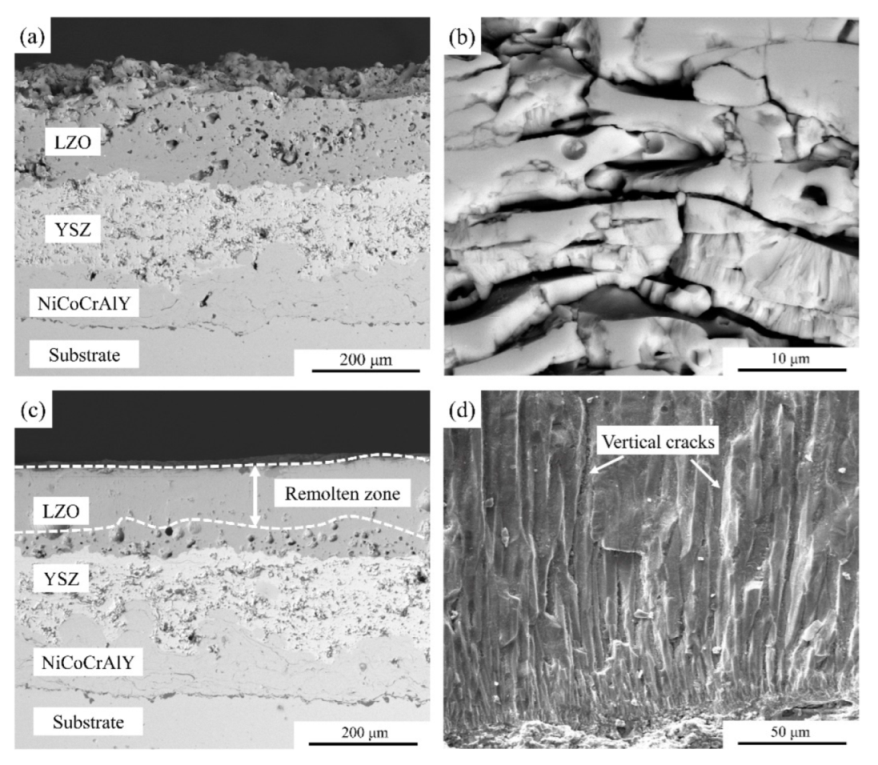
3.1. Coating Microstructure
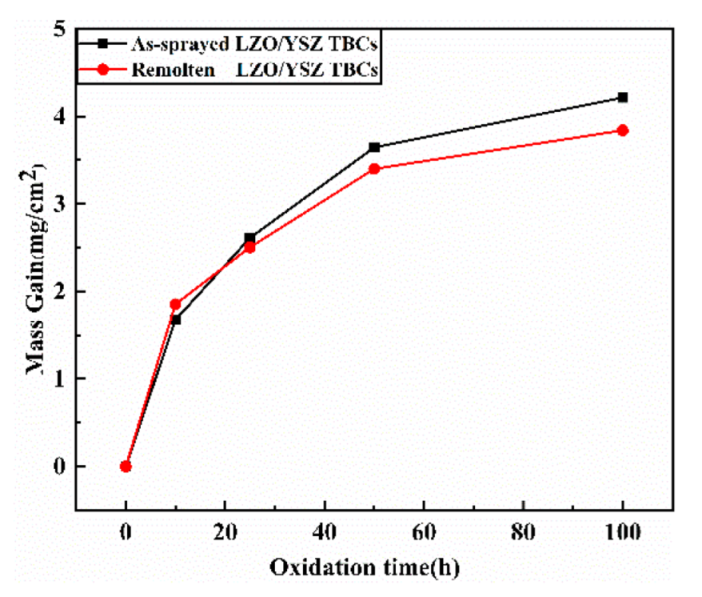
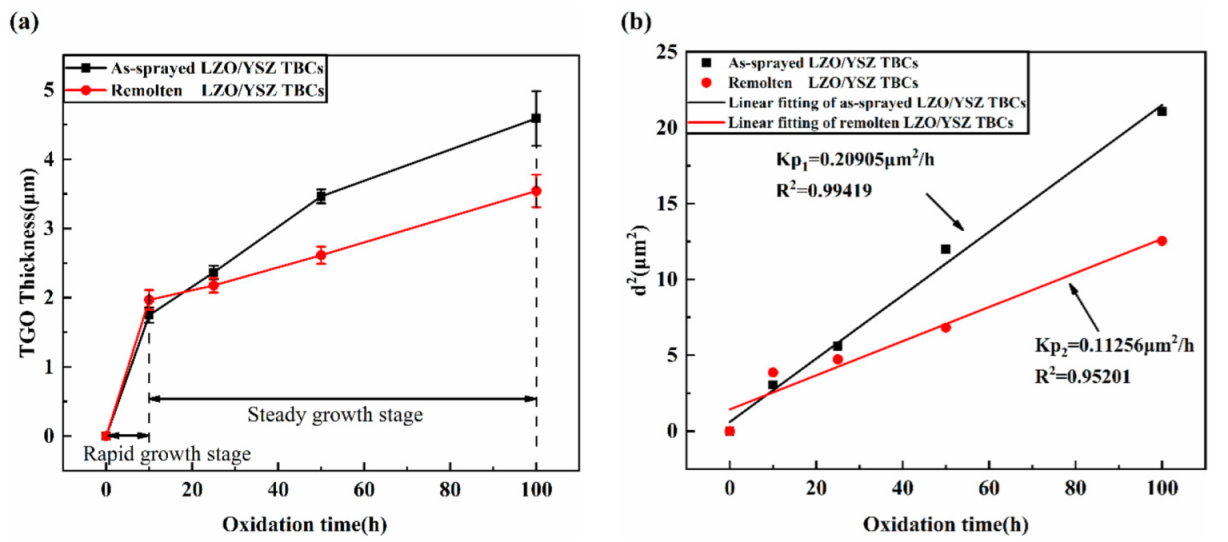
3.2. TGO Growth Kinetic
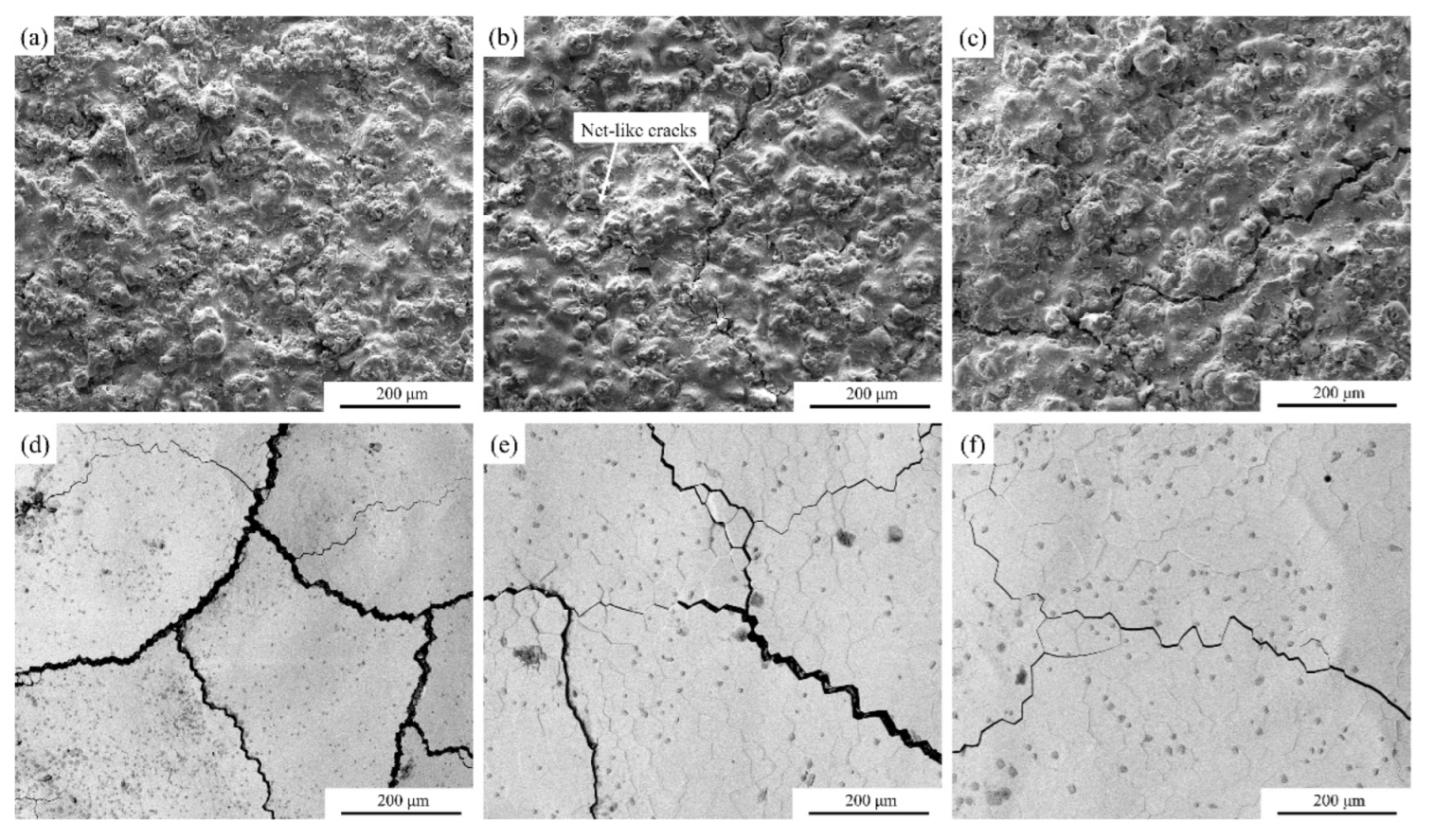
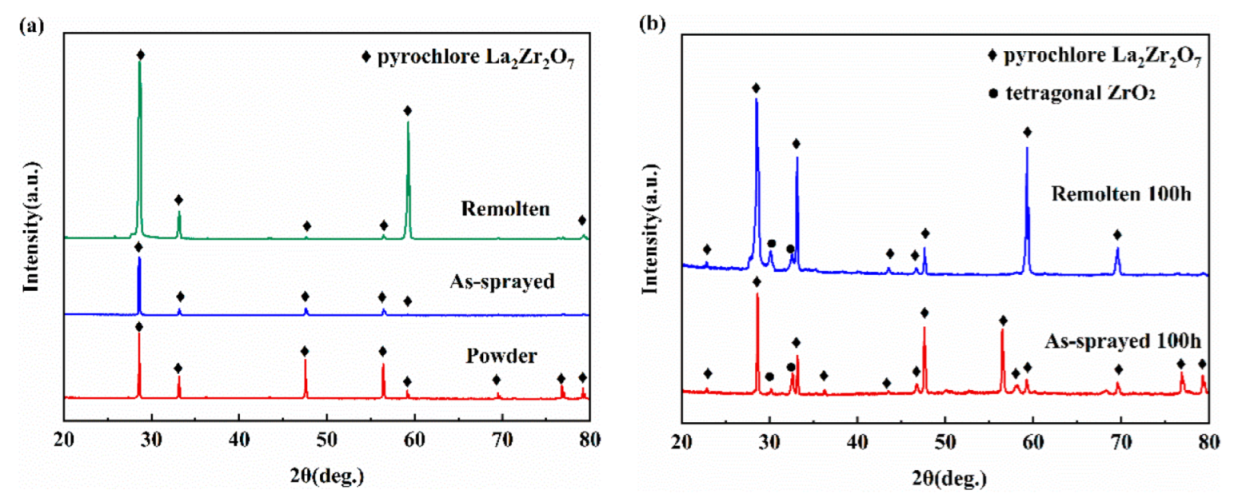
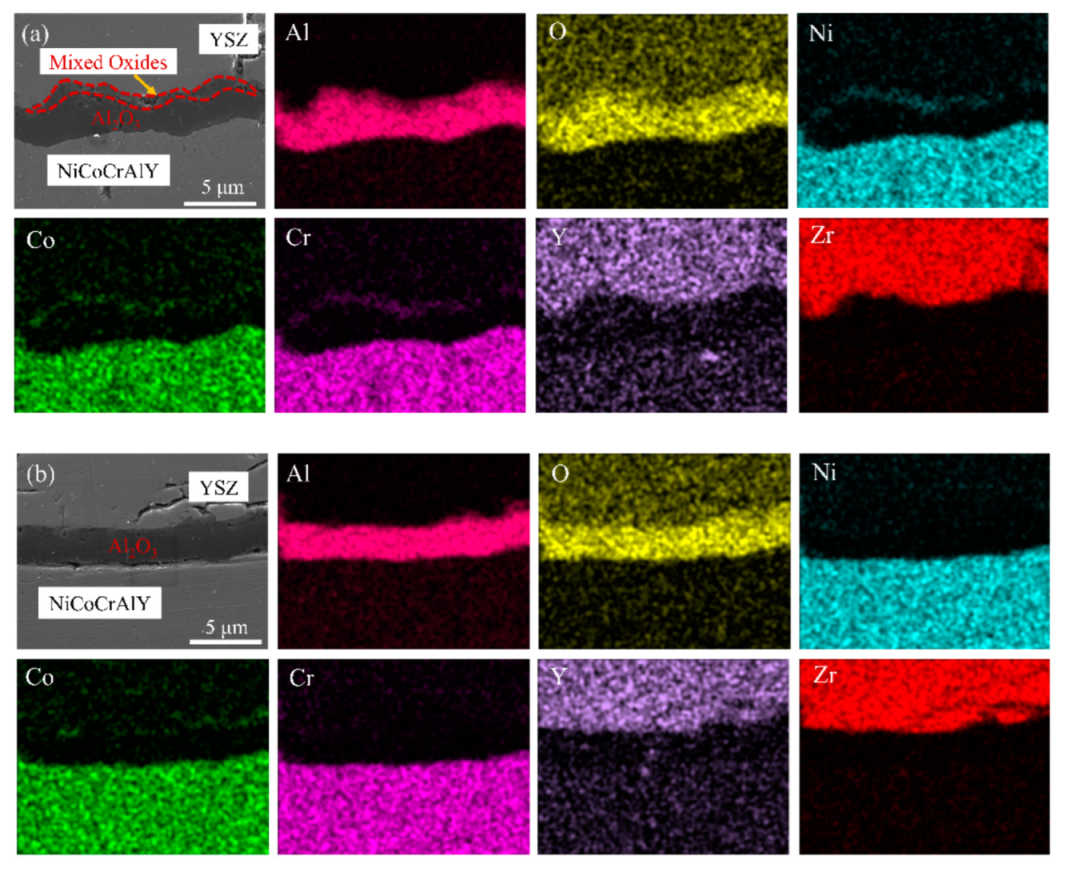
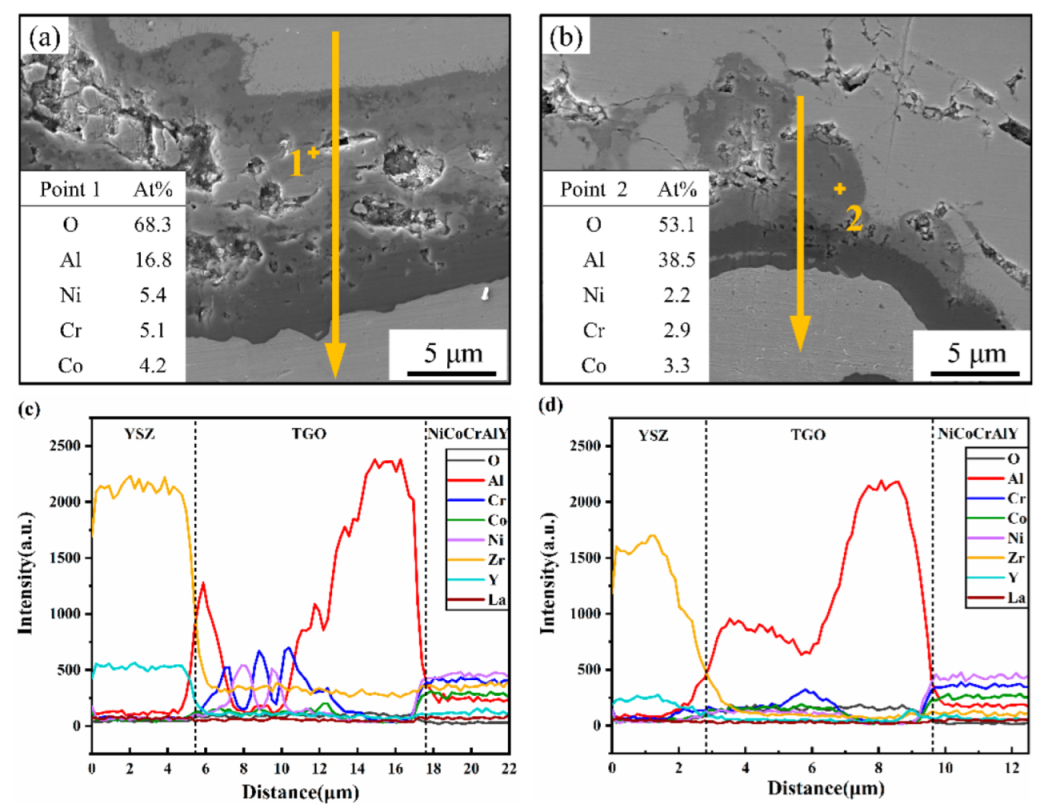
3.3. TGO Morphology and Composition Characterization
3.4. Oxidation Behavior and Mechanism
4. Conclusions
- Laser scanning remelting contributed a dense columnar structure to the LZO/YSZ TBCs, and their surface roughness and porosity decreased to 96.3% and 59.1%, respectively, compared to the as-sprayed LZO/YSZ TBCs.
- Laser scanning remelting presented a crucial inhibition effect on oxygen permeation, and the total TGO growth rate of LR TBCs (0.11256 μm2/h) was obviously 46.2% lower than that of AS TBCs (0.20905 μm2/h) during the 1100 °C isothermal oxidation.
- The Al2O3 thickness proportion of the total TGO layer in LR TBCs always remained greater than that in the AS TBCs upon the 10 h isothermal oxidization, and laser scanning remelting was effective at inhibiting TGO growth and increasing the high-temperature isothermal oxidation resistance of LZO/YSZ TBCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhomne, S.; Mahalle, A.M. Thermal barrier coating materials for SI engine. J. Mater. Res. Technol. 2018, 8, 1532–1537. [Google Scholar] [CrossRef]
- Mehboob, G.; Liu, M.; Xu, T.; Hussain, S.; Mehboob, G.; Tahir, A. A review on failure mechanism of thermal barrier coatings and strategies to extend their lifetime. Ceram. Int. 2019, 46, 8497–8521. [Google Scholar] [CrossRef]
- Lashmi, P.; Ananthapadmanabhan, P.; Unnikrishnan, G.; Aruna, S. Present status and future prospects of plasma sprayed multilayered thermal barrier coating systems. J. Eur. Ceram. Soc. 2020, 40, 2731–2745. [Google Scholar] [CrossRef]
- Tailor, S.; Modi, A.; Modi, S. Effect of controlled segmentation on the thermal cycling behavior of plasma sprayed YSZ thick coatings. Ceram. Int. 2018, 44, 6762–6768. [Google Scholar] [CrossRef]
- Darolia, R. Thermal barrier coatings technology: Critical review, progress update, remaining challenges and prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Colomban, P.; Jullian, S.; Parlier, M.; Monge-Cadet, P. Identification of the high-temperature impact/friction of aeroengine blades and cases by micro Raman spectroscopy. Aerosp. Sci. Technol. 1999, 3, 447–459. [Google Scholar] [CrossRef]
- Nath, S.; Manna, I.; Majumdar, J.D. Kinetics and mechanism of isothermal oxidation of compositionally graded yttria stabilized zirconia (YSZ) based thermal barrier coating. Corros. Sci. 2014, 88, 10–22. [Google Scholar] [CrossRef]
- Cao, X.Q. Development on New Thermal Barrier Coating Materials. J. Chin. Ceram. Soc. 2020, 48, 1622–1635. [Google Scholar]
- Velusamy, R.; Sakthinathan, G.; Vignesh, R.; Kumaraswamy, A.; Sathishkumar, D.; Priya, N.; Krishna, C.S.V. Tribological and thermal characterization of electron beam physical vapor deposited single layer thin film for TBC application. Surf. Topogr. Metrol. Prop. 2021. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.; Li, W.; Liu, W.; Wu, Y.; Liu, F. Research Progresses on Ceramic Materials of Thermal Barrier Coatings on Gas Turbine. Coatings 2021, 11, 79. [Google Scholar] [CrossRef]
- Higuero, V.; Belzunce, F.J.; Riba, J. High temperature oxidation of plasma and HVOF thermal sprayed CoNiCrAlY coatings in simulated gas turbine and furnace environments. Surf. Eng. 2009, 25, 319–325. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, T.; Sun, B.; Wang, B.; Zhang, X.; Song, L. Isothermal oxidation and TGO growth behavior of NiCoCrAlY-YSZ thermal barrier coatings on a Ni-based superalloy. J. Alloys Compd. 2020, 844, 156093. [Google Scholar] [CrossRef]
- An, G.; Li, W.; Feng, L.; Cheng, B.; Wang, Z.; Li, Z.; Zhang, Y. Isothermal oxidation and TGO growth behaviors of YAG/YSZ double-ceramic-layer thermal barrier coatings. Ceram. Int. 2021, 47, 24320–24330. [Google Scholar] [CrossRef]
- Xie, F.; Sun, Y.; Li, D.; Bai, Y.; Zhang, W. Modelling of catastrophic stress development due to mixed oxide growth in thermal barrier coatings. Ceram. Int. 2019, 45, 11353–11361. [Google Scholar] [CrossRef]
- Dong, H.; Yang, G.; Li, C.-X.; Luo, X.-T.; Li, C.-J. Effect of TGO Thickness on Thermal Cyclic Lifetime and Failure Mode of Plasma-Sprayed TBCs. J. Am. Ceram. Soc. 2014, 97, 1226–1232. [Google Scholar] [CrossRef]
- Bai, Y.; Ding, C.H.; Li, H.Q.; Han, Z.H.; Ding, B.J.; Wang, T.J.; Yu, L. Isothermal Oxidation Behavior of Supersonic Atmospheric Plasma-Sprayed Thermal Barrier Coating System. J. Therm. Spray Technol. 2013, 22, 1201–1209. [Google Scholar] [CrossRef]
- Lakiza, S.M.; Grechanyuk, M.I.; Ruban, O.K.; Redko, V.P.; Glabay, M.S.; Myloserdov, O.B.; Dudnik, O.V.; Prokhorenko, S.V. Thermal Barrier Coatings: Current Status, Search, and Analysis. Powder Met. Met. Ceram. 2018, 57, 82–113. [Google Scholar] [CrossRef]
- Kumar, V.; Balasubramanian, K. Progress update on failure mechanisms of advanced thermal barrier coatings: A review. Prog. Org. Coat. 2016, 90, 54–82. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Zhu, C.; Xiang, H.; Chen, H.; Sun, L.; Gao, Y.; Zhou, Y. Advances on strategies for searching for next generation thermal barrier coating materials. J. Mater. Sci. Technol. 2018, 35, 833–851. [Google Scholar] [CrossRef]
- Fox, A.; Clyne, T. Oxygen transport by gas permeation through the zirconia layer in plasma sprayed thermal barrier coatings. Surf. Coat. Technol. 2004, 184, 311–321. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Tietz, F.; Stoever, D. New double-ceramic-layer thermal barrier coatings based on zirconia–rare earth composite oxides. J. Eur. Ceram. Soc. 2006, 26, 247–251. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Jung, Y.-G.; Li, L.; Knapp, J. Lanthanum zirconate based thermal barrier coatings: A review. Surf. Coat. Technol. 2017, 323, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Che, J.W.; Yi, H.; Zhang, J.; Liang, G.Y. Diffusion mechanism of oxygen ions in La2Zr2O7/YSZ composite ceramics. J. Alloys Compd. 2018, 778, 522–531. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, S.Y. Thermal spraying of nanostructured La2Zr2O7(LZ)/8YSZ dual ceramic thermal barrier coatings. China Surf. Eng. 2016, 29, 16–24. (In Chinese) [Google Scholar]
- Xu, Z.; He, L.; Mu, R.; Zhong, X.; Zhang, Y.; Zhang, J.; Cao, X. Double-ceramic-layer thermal barrier coatings of La2Zr2O7/YSZ deposited by electron beam-physical vapor deposition. J. Alloys Compd. 2009, 473, 509–515. [Google Scholar] [CrossRef]
- Zaplatynsky, I. Performance of laser-glazed zirconia thermal barrier coatings in cyclic oxidation and corrosion burner rig tests. Thin Solid Films 1982, 95, 275–284. [Google Scholar] [CrossRef]
- Ahmaniemi, S.; Tuominen, J.; Vippola, M.; Vuoristo, P.; Mäntylä, T.; Cernuschi, F.; Gualco, C.; Bonadei, A.; Di Maggio, R. Characterization of Modified Thick Thermal Barrier Coatings. J. Therm. Spray Technol. 2004, 13, 361–369. [Google Scholar] [CrossRef]
- Xu, S.Q.; Zhu, C.; Zhang, Y. Effects of Laser Remelting and Oxidation on NiCrAlY/8Y2O3-ZrO2 Thermal Barrier Coatings. J. Therm. Spray Technol. 2017, 27, 412–422. [Google Scholar] [CrossRef]
- Antou, G.; Montavon, G.; Hlawka, F.; Cornet, A.; Coddet, C.; Machi, F. Modification of ceramic thermal spray deposit microstructures implementing in situ laser remelting. Surf. Coat. Technol. 2003, 172, 279–290. [Google Scholar] [CrossRef]
- Batista, C.; Portinha, A.; Ribeiro, R.; Teixeira, V.; de Oliveira, C.A.R. Evaluation of laser-glazed plasma-sprayed thermal barrier coatings under high temperature exposure to molten salts. Surf. Coat. Technol. 2006, 200, 6783–6791. [Google Scholar] [CrossRef] [Green Version]
- Balla, V.K.; Bandyopadhyay, P.P.; Bose, S.; Bandyopadhyay, A. Compositionally graded yttria-stabilized zirconia coating on stainless steel using laser engineered net shaping (LENS™). Scr. Mater. 2007, 57, 861–864. [Google Scholar] [CrossRef]
- Ahmadi-Pidani, R.; Shoja-Razavi, R.; Mozafarinia, R.; Jamali, H. Improving the thermal shock resistance of plasma sprayed CYSZ thermal barrier coatings by laser surface modification. Opt. Lasers Eng. 2012, 50, 780–786. [Google Scholar] [CrossRef]
- de Freitas, F.E.; Briguente, F.P.; dos Reis, A.G.; de Vasconcelos, G.; Reis, D.A.P. Investigation on the microstructure and creep behavior of laser remelted thermal barrier coating. Surf. Coat. Technol. 2019, 369, 257–264. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, M.; Shahid, M.; Wang, M.; Pan, W. Restrained TGO growth in YSZ/NiCrAlY thermal barrier coatings by modified laser remelting. Surf. Coat. Technol. 2017, 309, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Xin, H.; Zhang, Z.; Zhang, X.; Ye, F. Microstructure modification of Y2O3 stabilized ZrO2 thermal barrier coatings by laser glazing and the effects on the hot corrosion resistance. J. Adv. Ceram. 2020, 9, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Izadinia, M.; Soltani, R.; Sohi, M.H. Effect of segmented cracks on TGO growth and life of thick thermal barrier coating under isothermal oxidation conditions. Ceram. Int. 2019, 46, 7475–7481. [Google Scholar] [CrossRef]
- Morks, M.; Berndt, C.; Durandet, Y.; Brandt, M.; Wang, J. Microscopic observation of laser glazed yttria-stabilized zirconia coatings. Appl. Surf. Sci. 2010, 256, 6213–6218. [Google Scholar] [CrossRef]
- Chen, H.F.; Liu, Y.; Gao, Y.F.; Luo, H.J.; Tao, S.Y.; Luo, H.J. Design, Preparation, and Characterization of Graded YSZ/La2Zr2O7 Thermal Barrier Coatings. J. Am. Ceram. Soc. 2010, 93, 1732–1740. [Google Scholar]
- Cheng, B.; Yang, G.-J.; Zhang, Q.; Yang, N.; Zhang, M.; Zhang, Y.; Li, C.-X.; Li, C.-J. Gradient thermal cyclic behaviour of La2Zr2O7/YSZ DCL-TBCs with equivalent thermal insulation performance. J. Eur. Ceram. Soc. 2017, 38, 1888–1896. [Google Scholar] [CrossRef]
- Feng, Y.; Dong, T.-S.; Li, G.-L.; Wang, R.; Zhao, X.-W.; Liu, Q. High temperature oxidation resistance and TGO growth mechanism of laser remelted thermal barrier coatings. J. Alloys Compd. 2020, 828, 154266. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, C.; Wang, Y.; Yu, H.; Zhou, Y.; Zhou, G. YSZ/NiCrAlY interface oxidation of APS thermal barrier coatings. Corros. Sci. 2018, 142, 22–30. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Bao, Z.; Yu, C.; Zhu, S.; Wang, F. Effect of vacuum annealing on initial oxidation behavior and alumina transition of NiCoCrAlY coatings. Surf. Coat. Technol. 2020, 404, 126441. [Google Scholar] [CrossRef]
- Zhou, S.; Xiong, Z.; Lei, J.; Dai, X.; Zhang, T.; Wang, C. Influence of milling time on the microstructure evolution and oxidation behavior of NiCrAlY coatings by laser induction hybrid cladding. Corros. Sci. 2016, 103, 105–116. [Google Scholar] [CrossRef]
- Doleker, K.M.; Ozgurluk, Y.; Karaoglanli, A.C. Isothermal oxidation and thermal cyclic behaviors of YSZ and double-layered YSZ/La2Zr2O7 thermal barrier coatings (TBCs). Surf. Coatings Technol. 2018, 351, 78–88. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, M.C.; Chellali, R.; Bouchikhaoui, H.; Dong, J.X. Oxidation property of powder metallurgy EP741NP Ni based superalloy at elevated temperatures. Mater. Technol. 2013, 28, 122–128. [Google Scholar] [CrossRef]
- Dong, T.-S.; Wang, R.; Di, Y.-L.; Wang, H.-D.; Li, G.-L.; Fu, B.-G. Mechanism of high temperature oxidation resistance improvement of double-layer thermal barrier coatings (TBCs) by La. Ceram. Int. 2019, 45, 9126–9135. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, F.; Zhang, Z.; Wang, Y.; Li, H.; Ren, L.; Liu, M. Hot Corrosion Behavior of YSZ Thermal Barrier Coatings Modified by Laser Remelting and Al Deposition. J. Therm. Spray Technol. 2019, 28, 1225–1238. [Google Scholar] [CrossRef]
- Sun, H.-Y.; He, Q.; Zhou, Z.-J.; Wang, M.; Zhang, G.-M.; Li, S.-F. Effects of solution depletion and segregation oxidation on morphology of modified 310 austenitic stainless steel. J. Iron Steel Res. Int. 2016, 23, 393–400. [Google Scholar] [CrossRef]



| Parameters | NiCoCrAlY | YSZ | LZO |
|---|---|---|---|
| Voltage (V) | 55 | 60 | 60 |
| Current (A) | 500 | 650 | 650 |
| Spray distance (mm) | 15 | 10 | 10 |
| Primary gas flow, Ar (L/min) | 15 | 15 | 15 |
| Secondary gas flow, H2 (L/min) | 9 | 9 | 9 |
| Powder feed rate (g/min) | 33 | 30 | 30 |
| Power (W) | Scanning Speed (mm/s) | Defocus Amount (mm) | Spot Size (mm) | Overlap Rate | Energy Ratio (J) |
|---|---|---|---|---|---|
| 100 | 20 | +15 | 3 | 30% | 1.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, Z.; An, G.; Cheng, B.; Song, Q.; Sun, J.; Vaganov, V.; Wang, C.; Goransky, G. Isothermal Oxidation TGO Growth Behaviors of Laser-Remolten LZO/YSZ Thermal Barrier Coatings. Coatings 2022, 12, 107. https://doi.org/10.3390/coatings12020107
Li W, Li Z, An G, Cheng B, Song Q, Sun J, Vaganov V, Wang C, Goransky G. Isothermal Oxidation TGO Growth Behaviors of Laser-Remolten LZO/YSZ Thermal Barrier Coatings. Coatings. 2022; 12(2):107. https://doi.org/10.3390/coatings12020107
Chicago/Turabian StyleLi, Wensheng, Ziyu Li, Guosheng An, Bo Cheng, Qiang Song, Jinquan Sun, Victor Vaganov, Canming Wang, and Georg Goransky. 2022. "Isothermal Oxidation TGO Growth Behaviors of Laser-Remolten LZO/YSZ Thermal Barrier Coatings" Coatings 12, no. 2: 107. https://doi.org/10.3390/coatings12020107
APA StyleLi, W., Li, Z., An, G., Cheng, B., Song, Q., Sun, J., Vaganov, V., Wang, C., & Goransky, G. (2022). Isothermal Oxidation TGO Growth Behaviors of Laser-Remolten LZO/YSZ Thermal Barrier Coatings. Coatings, 12(2), 107. https://doi.org/10.3390/coatings12020107








