GOx/Hb Cascade Oxidized Crosslinking of Silk Fibroin for Tissue-Responsive Wound Repair
Abstract
1. Introduction
2. Results and Discussion
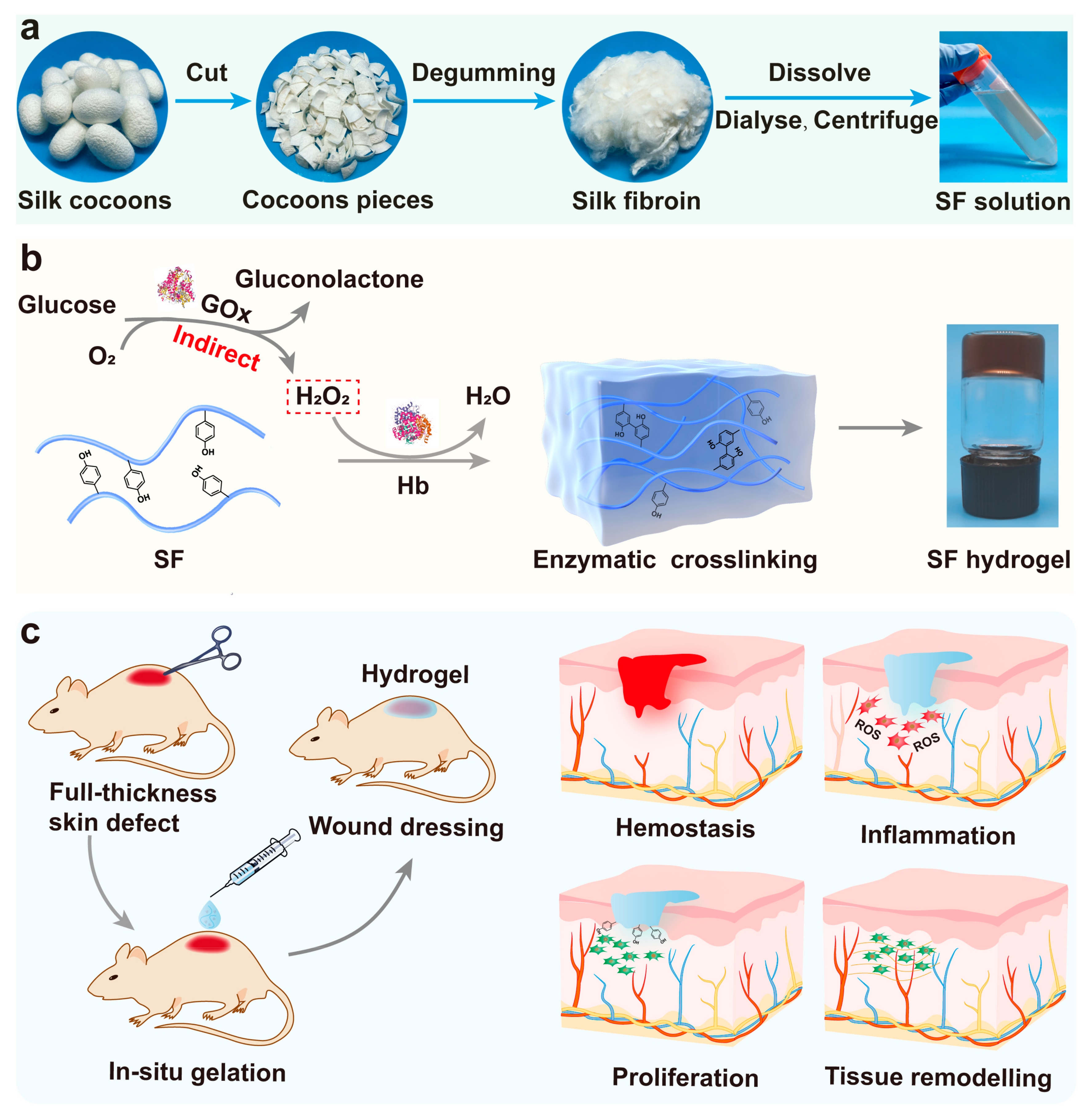
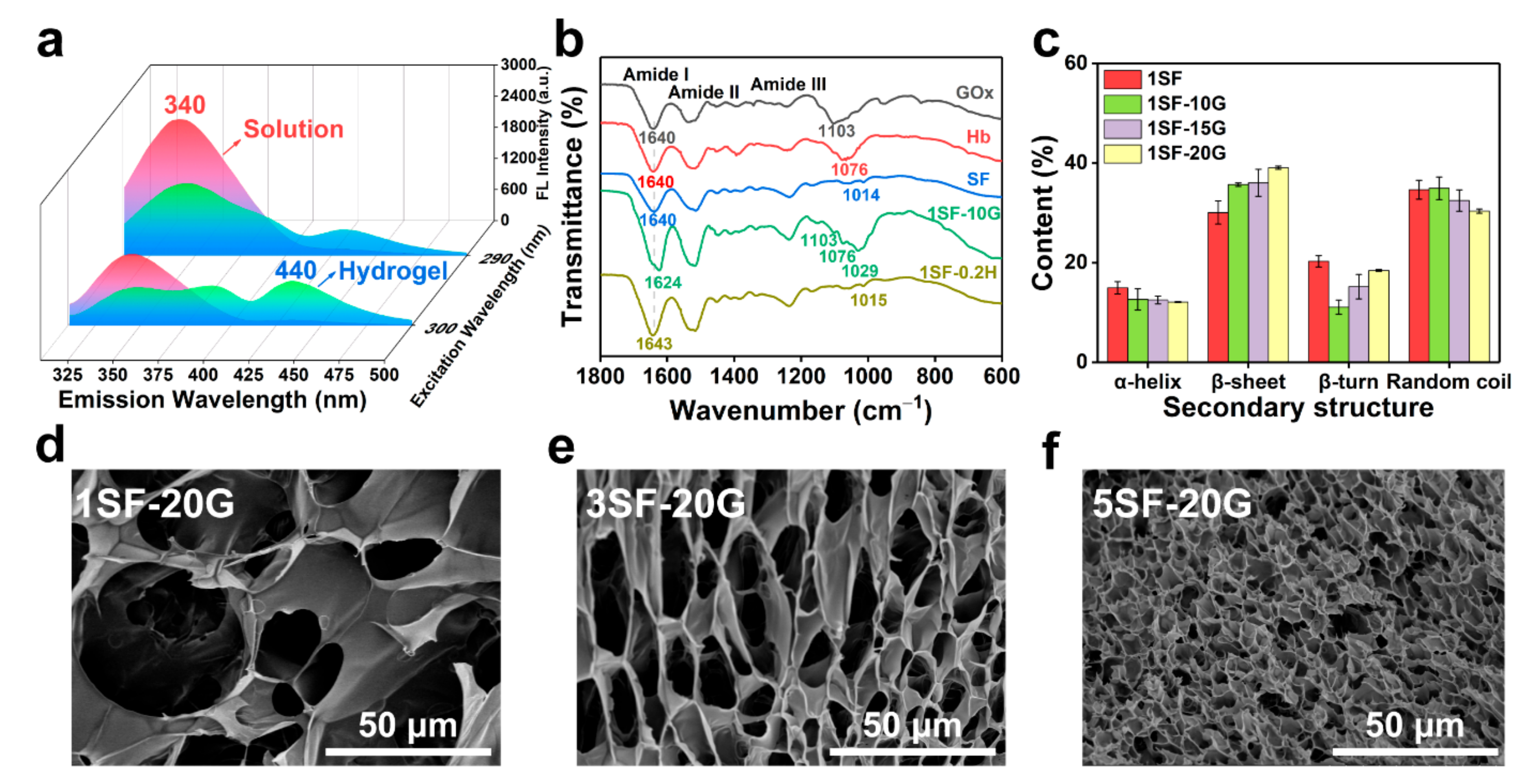
2.1. Fabrication and Structural Characterisation of Hb-Mediated SF Gelation
2.2. Rheological and Mechanical Properties of SF Hydrogels
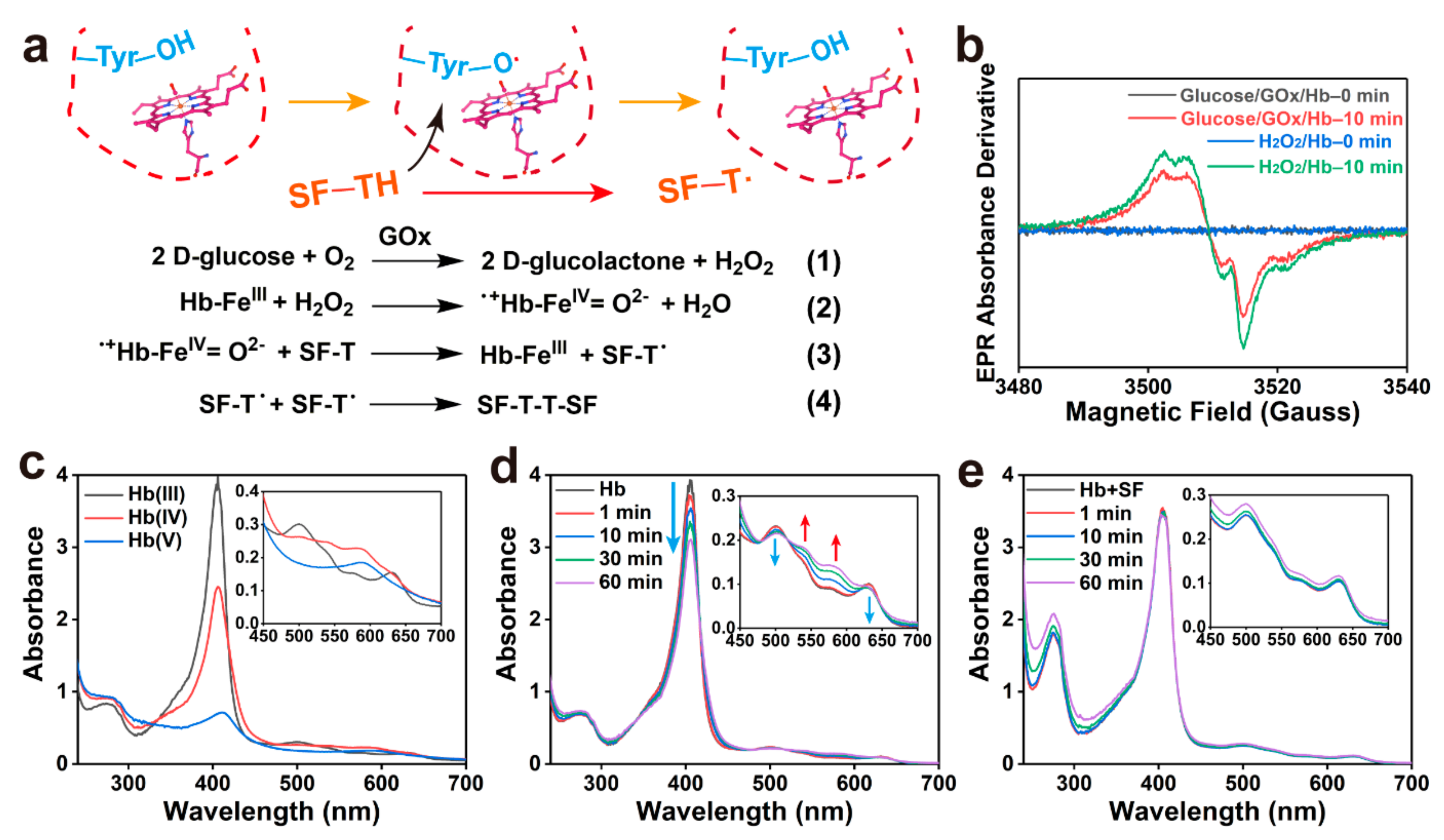
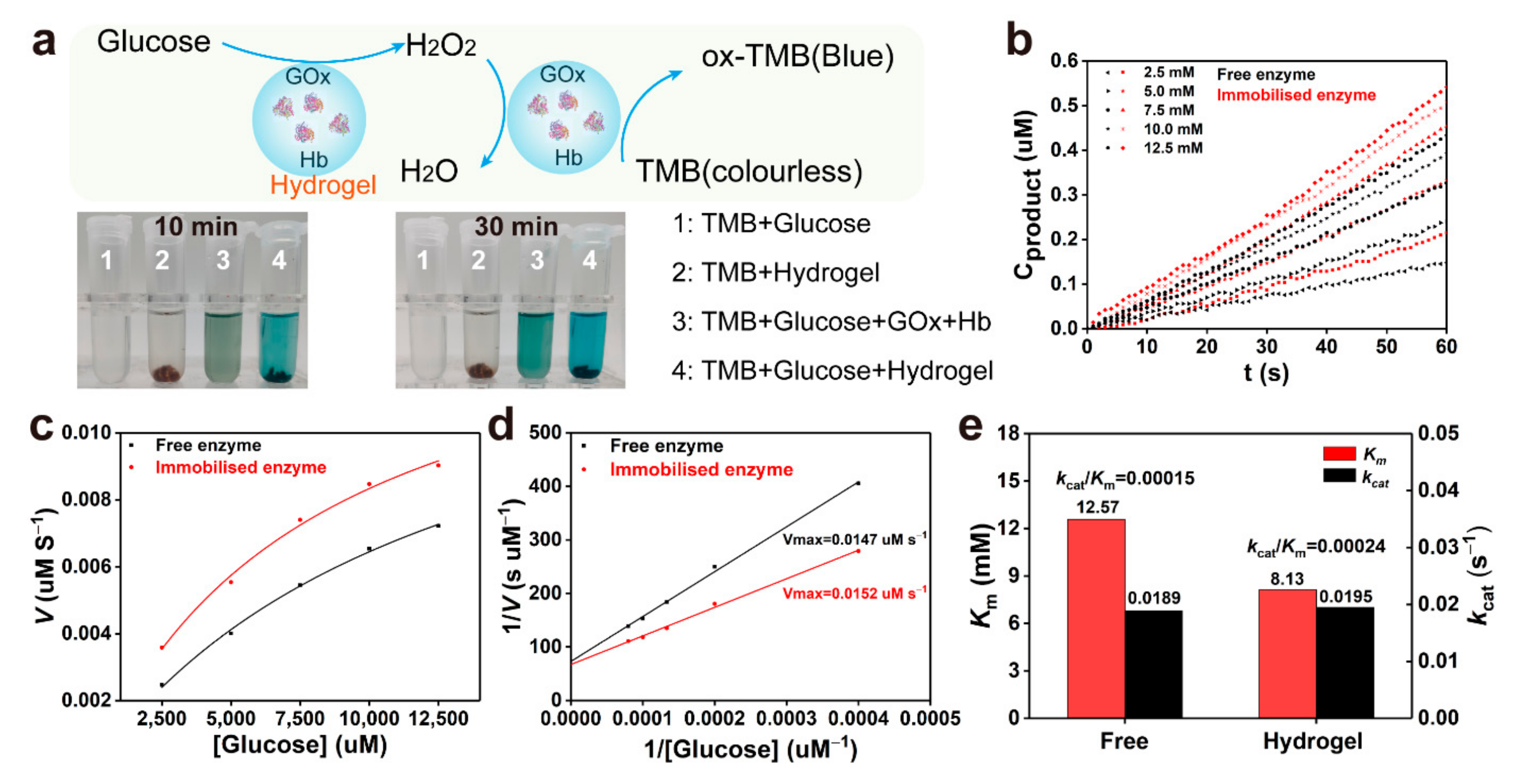
2.3. Mechanism of Enzymatic Crosslinking and Antioxidant
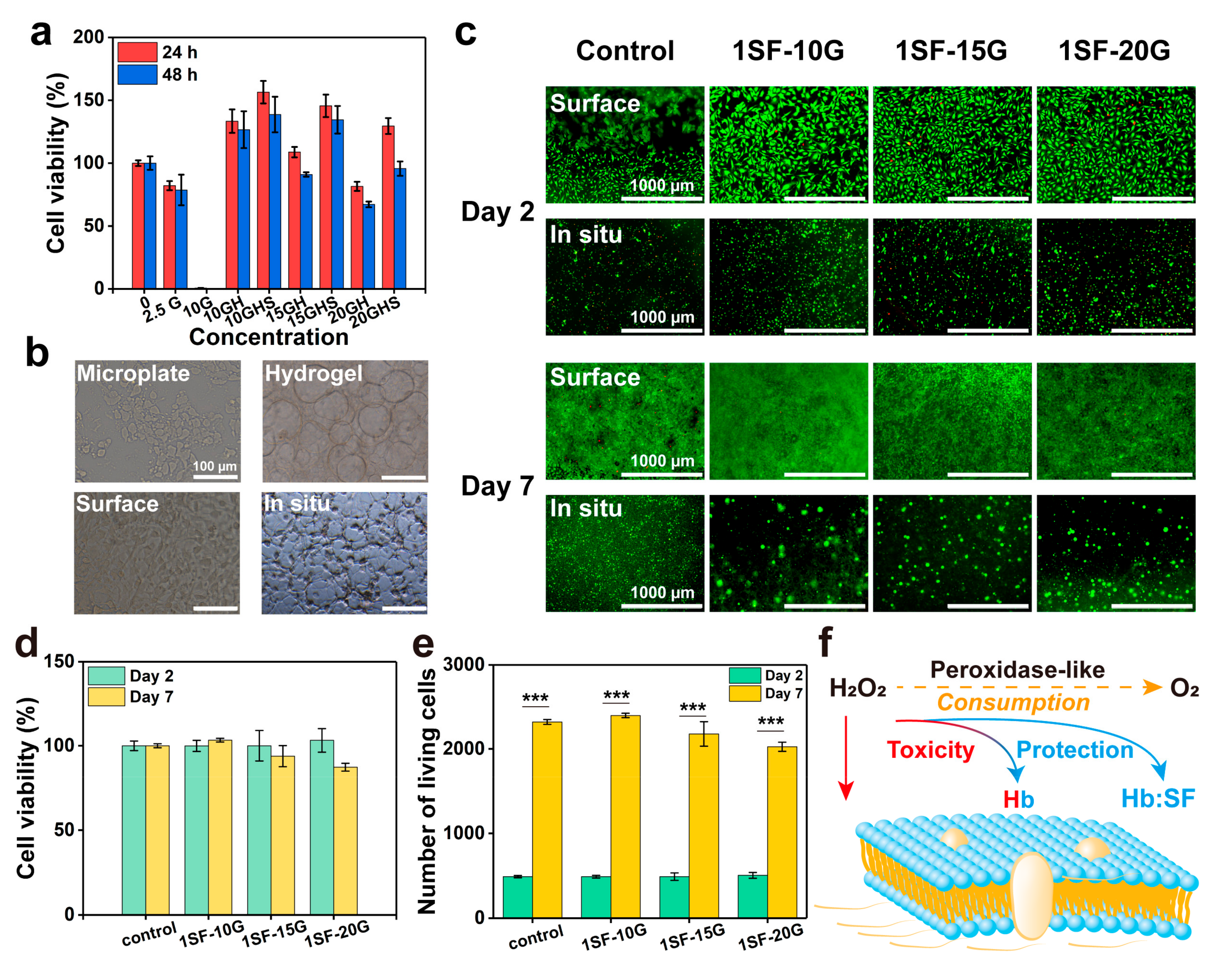
2.4. Cell Viability of Enzymatic System and Hydrogels
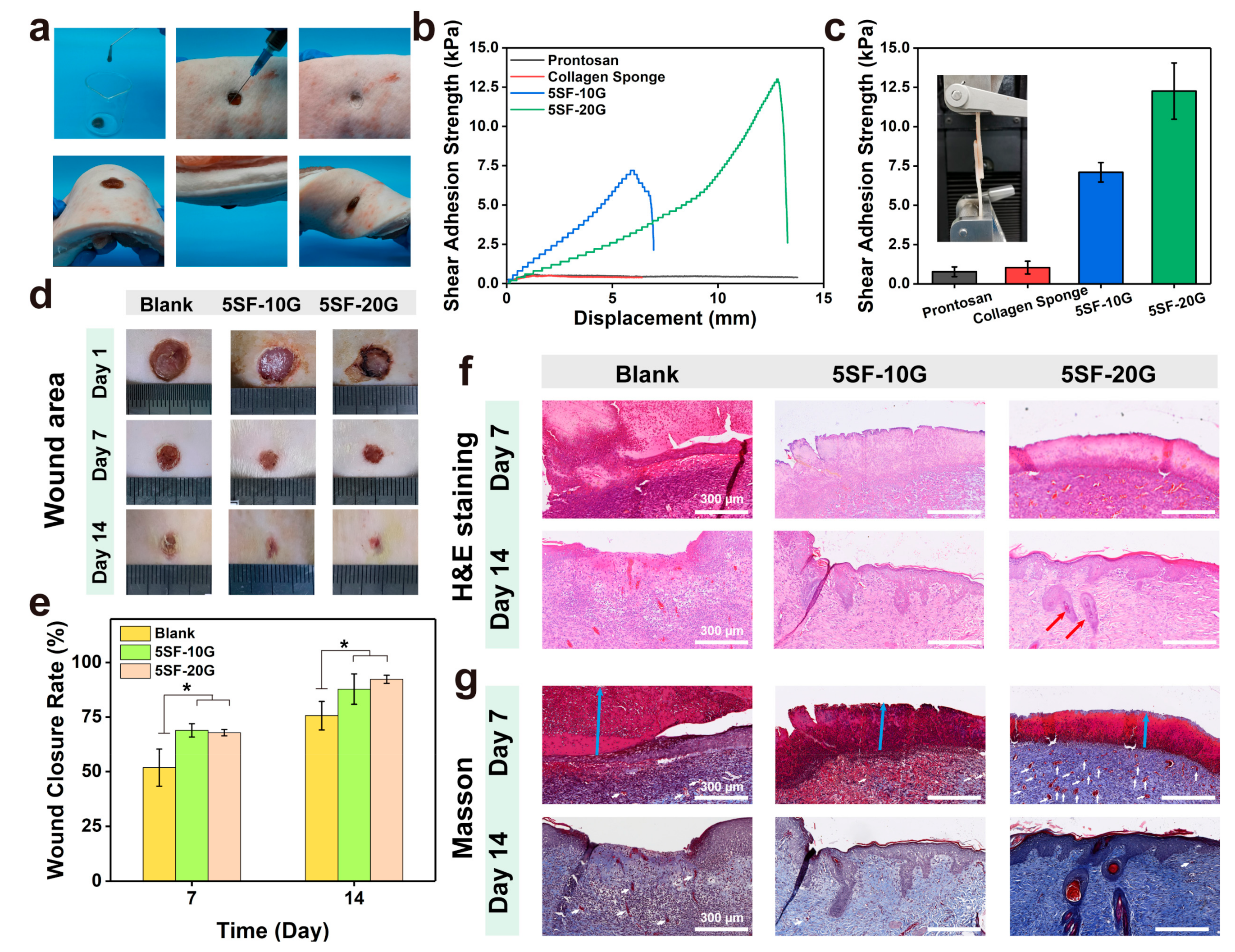
2.5. Enzymatically Crosslinked SF Hydrogels for Full-Thickness Wound Regeneration
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of SF Solution
4.3. Preparation of SF Hydrogels
4.4. Fluorescence Spectroscopy
4.5. Fourier Transform Attenuated Total Reflection Infrared Spectroscopy (ATR–FTIR)
4.6. Scanning Electron Microscopy (SEM)
4.7. Rheological Measurements
4.8. Mechanical and Lap-Shear Testing
4.9. The Degree of Hb Oxidation
4.10. Electron Paramagnetic Resonance (EPR) Spectroscopy
4.11. Catalytic Activity of Cascade Enzyme
4.12. Hydrogels In Vitro Free Radical Scavenging
4.13. Evaluation of Swelling Ratio and In Vitro Enzymatic Degradation
4.14. Cell Survival and Proliferations
4.15. Intracellular ROS Scavenging Ability of SF Hydrogels
4.16. Injectability Test
4.17. In Vivo Wound Healing
4.18. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Farahaniv, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, D.; Zhao, W.Y.; Zhang, R.; Yu, B.R.; Ma, G.P.; Li, Y.; Hao, D.F.; Xu, F.-J. Engineering Platelet-Rich Plasma Based Dual-Network Hydrogel as a Bioactive Wound Dressing with Potential Clinical Translational Value. Adv. Funct. Mater. 2020, 31, 2009258. [Google Scholar] [CrossRef]
- Mehrotra, S.; de Melo, B.A.G.; Hirano, M.; Keung, W.; Li, R.A.; Mandal, B.B.; Shin, S.R. Nonmulberry Silk Based Ink for Fabricating Mechanically Robust Cardiac Patches and Endothelialized Myocardium-on-a-Chip Application. Adv. Funct. Mater. 2020, 30, 1907436. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, Y.; Ryu, H.A.; Jang, Y.; Lee, K.M.; Choi, Y.; Choi, W.J.; Lee, M.; Park, K.M.; Park, K.D.; et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016, 38, 59–68. [Google Scholar] [CrossRef]
- Xu, X.Y.; Xia, X.F.; Zhang, K.Y.; Rai, A.; Li, Z.; Zhao, P.C.; Wei, K.C.; Zou, L.; Yang, B.G.; Wong, W.K.; et al. Bioadhesive hydrogels demonstrating pH-independent and ultrafast gelation promote gastric ulcer healing in pigs. Sci. Transl. Med. 2020, 12, eaba8014. [Google Scholar] [CrossRef]
- Teixeira, L.S.M.; Feijen, C.J.; van Blitterswijk, A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef]
- Sun, F.F.; Bu, Y.Z.; Chen, Y.R.; Yang, F.; Yu, J.K.; Wu, D.C. An Injectable and Instant Self-Healing Medical Adhesive for Wound Sealing. ACS Appl. Mater. Interfaces 2020, 12, 9132–9140. [Google Scholar] [CrossRef]
- Dong, Y.X.; Sigen, A.; Rodrigues, M.; Li, X.L.; Kwon, S.H.; Kosaric, N.; Khong, S.; Gao, Y.S.; Wang, W.X.; Gurtner, G.C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27, 1606616. [Google Scholar] [CrossRef]
- Lu, Z.H.; Liu, S.J.; Le, Y.G.; Qin, Z.N.; He, M.W.; Xu, F.B.; Zhu, Y.; Zhao, J.M.; Mao, C.B.; Zheng, L. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials 2019, 218, 119190. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.H.; Lee, J.E.; Park, S.J.; Kim, K.; Kim, I.S.; Lee, Y.S.; Hwang, N.S.; Kim, B.G. Tissue adhesive, rapid forming, and sprayable ECM hydrogel via recombinant tyrosinase crosslinking. Biomaterials 2018, 178, 401–412. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.P.; Zhang, T.L.; Ma, P.X.; Guo, B.L. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, M.J.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-Responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef]
- Yang, X.W.; Wang, Y.B.; Mao, T.J.; Wang, Y.; Liu, R.L.; Yu, L.; Ding, J.D. An oxygen-enriched thermosensitive hydrogel for the relief of a hypoxic tumor microenvironment and enhancement of radiotherapy. Biomater. Sci. 2021, 9, 7471–7482. [Google Scholar] [CrossRef]
- Li, Z.; Fu, Q.R.; Ye, J.M.; Ge, X.G.; Wang, J.; Song, J.B.; Yang, H.H. Ag+-Coupled Black Phosphorus Vesicles with Emerging NIR-II Photoacoustic Imaging Performance for Cancer Immune-Dynamic Therapy and Fast Wound Healing. Angew. Chem. Int. Edit. 2020, 59, 22202–22209. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Costa, J.B.; Morais, A.d.S.; Lopez-Cebral, R.; Silva-Correia, J.; Reis, R.L.; Miguel Oliveira, J. Tunable Enzymatically Cross-Linked Silk Fibroin Tubular Conduits for Guided Tissue Regeneration. Adv. Healthc. Mater. 2018, 7, 1800186. [Google Scholar] [CrossRef]
- Chen, Y.S.; Li, Y.; Yang, X.X.; Cao, Z.J.; Nie, H.L.; Bian, Y.G.; Yang, G. Glucose-triggered in situ forming keratin hydrogel for the treatment of diabetic wounds. Acta Biomater. 2021, 125, 208–218. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Z.; Liu, Y.X.; Shao, C.M.; Bian, F.K.; Zhao, Y.J. Biomimetic enzyme cascade reaction system in microfluidic electrospray microcapsules. Sci. Adv. 2018, 4, eaat2816. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.G. Enzyme-Laden Bioactive Hydrogel for Biocatalytic Monitoring and Regulation. Acc. Chem. Res. 2021, 54, 1274–1287. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.S.; Wu, D.B.; Wu, Q.; Wei, Q.C.; He, B.; Lu, Q.H.; Wang, Q.G. Oxidoreductase-Initiated Radical Polymerizations to Design Hydrogels and Micro/Nanogels: Mechanism, Molding, and Applications. Adv. Mater. 2018, 30, 1705668. [Google Scholar] [CrossRef]
- Gao, J.; Zhan, J.; Yang, Z.M. Enzyme-Instructed Self-Assembly (EISA) and Hydrogelation of Peptides. Adv. Mater. 2020, 32, 1805798. [Google Scholar] [CrossRef]
- He, H.J.; Tan, W.Y.; Guo, J.Q.; Yi, M.H.; Shy, A.N.; Xu, B. Enzymatic Noncovalent Synthesis. Chem. Rev. 2020, 120, 9994–10078. [Google Scholar] [CrossRef]
- Widmer, C.C.; Pereira, C.P.; Gehrig, P.; Vallelian, F.; Schoedon, G.; Buehler, P.W.; Schaer, D.J. Hemoglobin Can Attenuate Hydrogen Peroxide-Induced Oxidative Stress by Acting as an Antioxidative Peroxidase. Antioxid. Redox Signal. 2010, 12, 185–198. [Google Scholar] [CrossRef]
- Koh, L.D.; Cheng, Y.; Teng, C.P.; Khin, Y.W.; Loh, X.J.; Tee, S.Y.; Low, M.; Ye, E.; Yu, H.D.; Zhang, Y.W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Reeder, B.J.; Svistunenko, D.A.; Cooper, C.E.; Wilson, M.T. The radical and redox chemistry of myoglobin and hemoglobin: From in vitro studies to human pathology. Antioxid. Redox Signal. 2004, 6, 954–966. [Google Scholar] [CrossRef]
- Moreau, S.; Davies, M.J.; Mathieu, C.; Herouart, D.; Puppo, A. Leghemoglobin-derived radicals—Evidence for multiple protein-derived radicals and the initiation of peribacteroid membrane damage. J. Biol. Chem. 1996, 271, 32557–32562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly Tunable Elastomeric Silk Biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Mollan, T.L.; Jia, Y.P.; Banerjee, S.; Wu, G.; Kreulen, R.T.; Tsai, A.L.; Olson, J.S.; Crumbliss, A.L.; Alayash, A.I. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free. Radic. Bio. Med. 2014, 69, 265–277. [Google Scholar] [CrossRef]
- Sanz, V.A.; de Marcos, S.; Galban, J. Analytical applications of the optical properties of ferric hemoglobin: A theoretical and experimental study. Microchem. J. 2014, 114, 175–181. [Google Scholar] [CrossRef]
- Andre, R.; Natalio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schroder, H.C.; Muller, W.E.G.; Tremel, W. V2O5 Nanowires with an Intrinsic Peroxidase-Like Activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- van Schijndel, J.W.P.M.; Vollenbroek, E.G.M.; Wever, R. The chloroperoxidase from the fungus Curvularia inaequalis; a novel vanadium enzyme. Biochim. Biophys. Acta 1993, 1161, 249–256. [Google Scholar] [CrossRef]
- Wang, S.Q.; Zheng, H.; Zhou, L.; Cheng, F.; Liu, Z.; Zhang, H.P.; Wang, L.L.; Zhang, Q.Y. Nanoenzyme-Reinforced Injectable Hydrogel for Healing Diabetic Wounds Infected with Multidrug Resistant Bacteria. Nano Lett. 2020, 20, 5149–5158. [Google Scholar] [CrossRef]
- Yildirimer, L.; Seifalian, A.M. Three-dimensional biomaterial degradation—Material choice, design and extrinsic factor considerations. Biotechnol. Adv. 2014, 32, 984–999. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Schaer, C.A.; Deuel, J.W.; Bittermann, A.G.; Rubio, I.G.; Schoedon, G.; Spahn, D.R.; Wepf, R.A.; Vallelian, F.; Schaer, D.J. Mechanisms of haptoglobin protection against hemoglobin peroxidation triggered endothelial damage. Cell Death Differ. 2013, 20, 1569–1579. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Toivola, D.M.; Boor, P.; Alam, C.; Strnad, P. Keratins in health and disease. Curr. Opin. Cell. Biol. 2015, 32, 73–81. [Google Scholar] [CrossRef]
- Mao, X.Y.; Cheng, R.Y.; Zhang, H.B.; Bae, J.H.; Cheng, L.Y.; Zhang, L.; Deng, L.F.; Cui, W.G.; Zhang, Y.G.; Santos, H.A.; et al. Self-Healing and Injectable Hydrogel for Matching Skin Flap Regeneration. Adv. Sci. 2019, 6, 1901124. [Google Scholar] [CrossRef]
- Kilarski, W.W.; Samolov, B.; Petersson, L.; Kvanta, A.; Gerwins, P. Biomechanical regulation of blood vessel growth during tissue vascularization. Nat. Med. 2009, 15, 657–664. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.Q.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, H.; Wang, P.; Han, X.; Ma, M.; Shang, Y.; Ju, Y.; Shen, S.; Yin, F.; Wang, Q. GOx/Hb Cascade Oxidized Crosslinking of Silk Fibroin for Tissue-Responsive Wound Repair. Gels 2022, 8, 56. https://doi.org/10.3390/gels8010056
Shen H, Wang P, Han X, Ma M, Shang Y, Ju Y, Shen S, Yin F, Wang Q. GOx/Hb Cascade Oxidized Crosslinking of Silk Fibroin for Tissue-Responsive Wound Repair. Gels. 2022; 8(1):56. https://doi.org/10.3390/gels8010056
Chicago/Turabian StyleShen, Hongdou, Pei Wang, Xiaoke Han, Mengli Ma, Yinghui Shang, Ye Ju, Saiji Shen, Feng Yin, and Qigang Wang. 2022. "GOx/Hb Cascade Oxidized Crosslinking of Silk Fibroin for Tissue-Responsive Wound Repair" Gels 8, no. 1: 56. https://doi.org/10.3390/gels8010056
APA StyleShen, H., Wang, P., Han, X., Ma, M., Shang, Y., Ju, Y., Shen, S., Yin, F., & Wang, Q. (2022). GOx/Hb Cascade Oxidized Crosslinking of Silk Fibroin for Tissue-Responsive Wound Repair. Gels, 8(1), 56. https://doi.org/10.3390/gels8010056




