Rumex japonicus Houtt. Protects Dopaminergic Neurons by Regulating Mitochondrial Function and Gut–Brain Axis in In Vitro and In Vivo Models of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-of-Flight Tandem Mass Spectrometry (UPLC-Q-TOF-MS) Analysis
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. ATP Assay
2.6. Measurement of Mitochondrial Reactive Oxygen Species (ROS)
2.7. Flow Cytometric Analysis
2.8. Animal Study Design
2.9. Behavior Test
2.10. Immunohistochemistry
2.11. Western Blot
2.12. Immunofluorescence
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. Statistical Analysis
3. Results
3.1. RJ Contains Anthraquinone Substrates
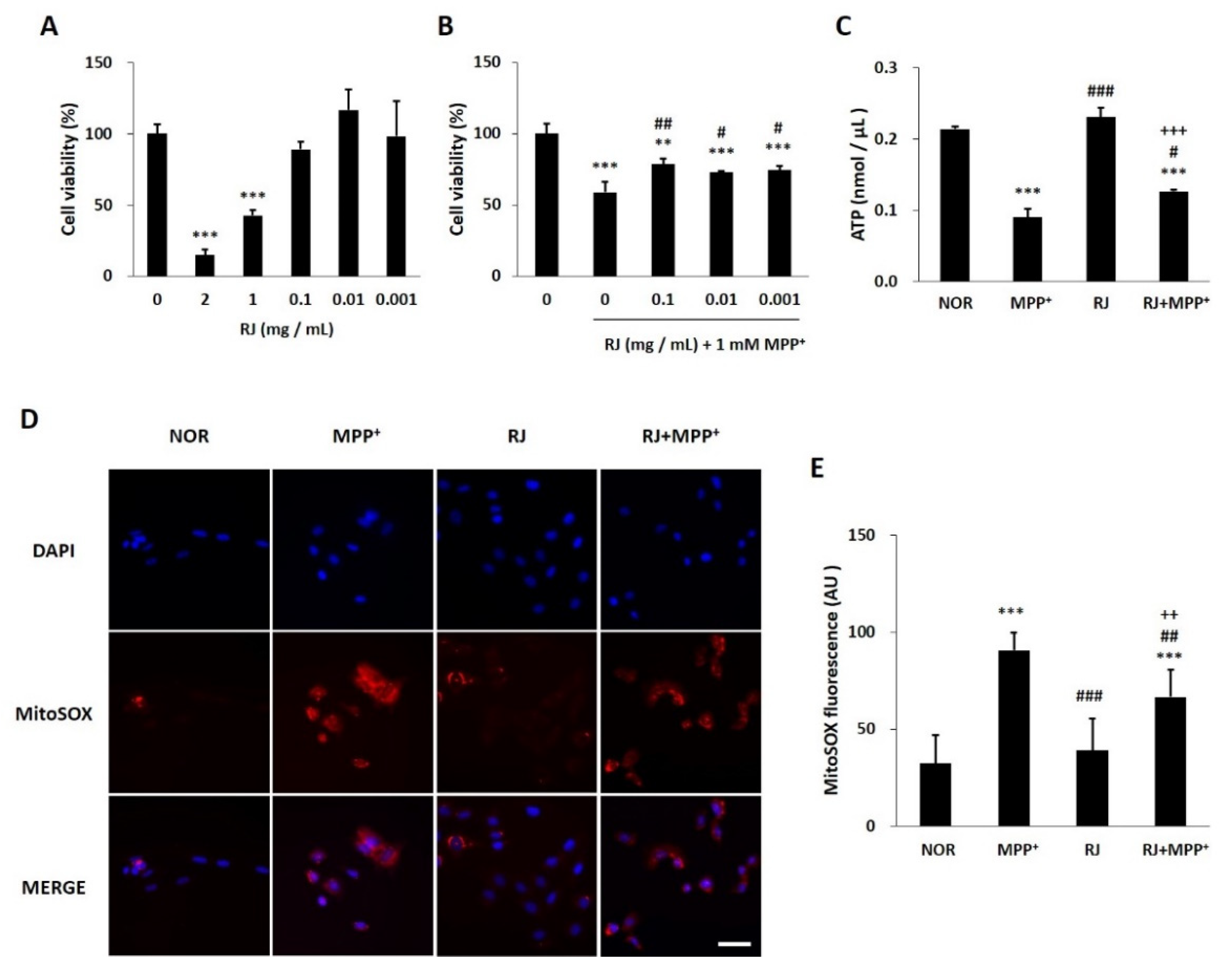
3.2. RJ Protects SH-SY5Y Cells against MPP+-Induced Cytotoxicity and Mitochondrial Dysfunction
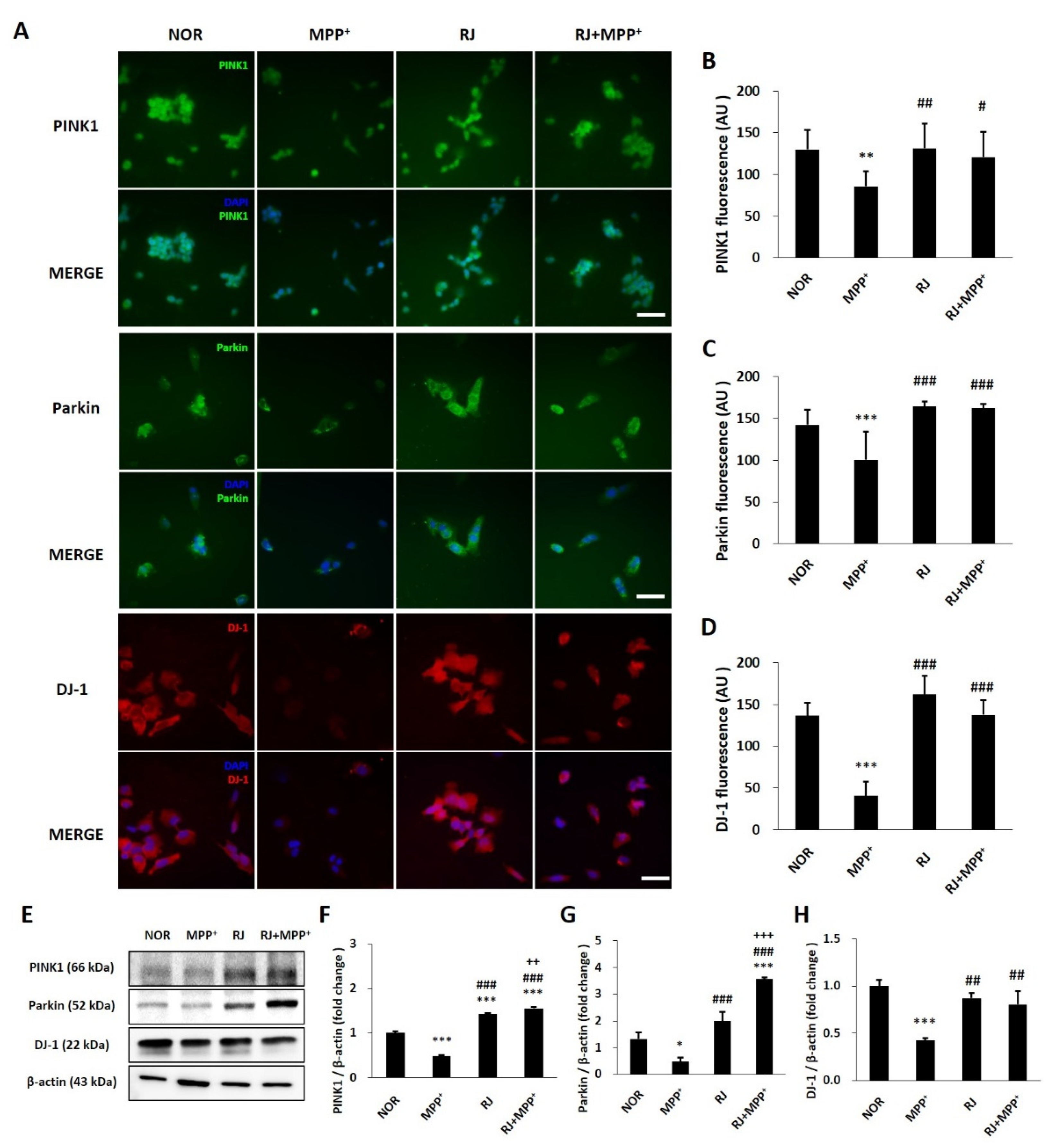
3.3. RJ Inhibits MPP+-Mediated Reduction in Parkin, PINK1, and DJ-1 in SH-SY5Y Cells
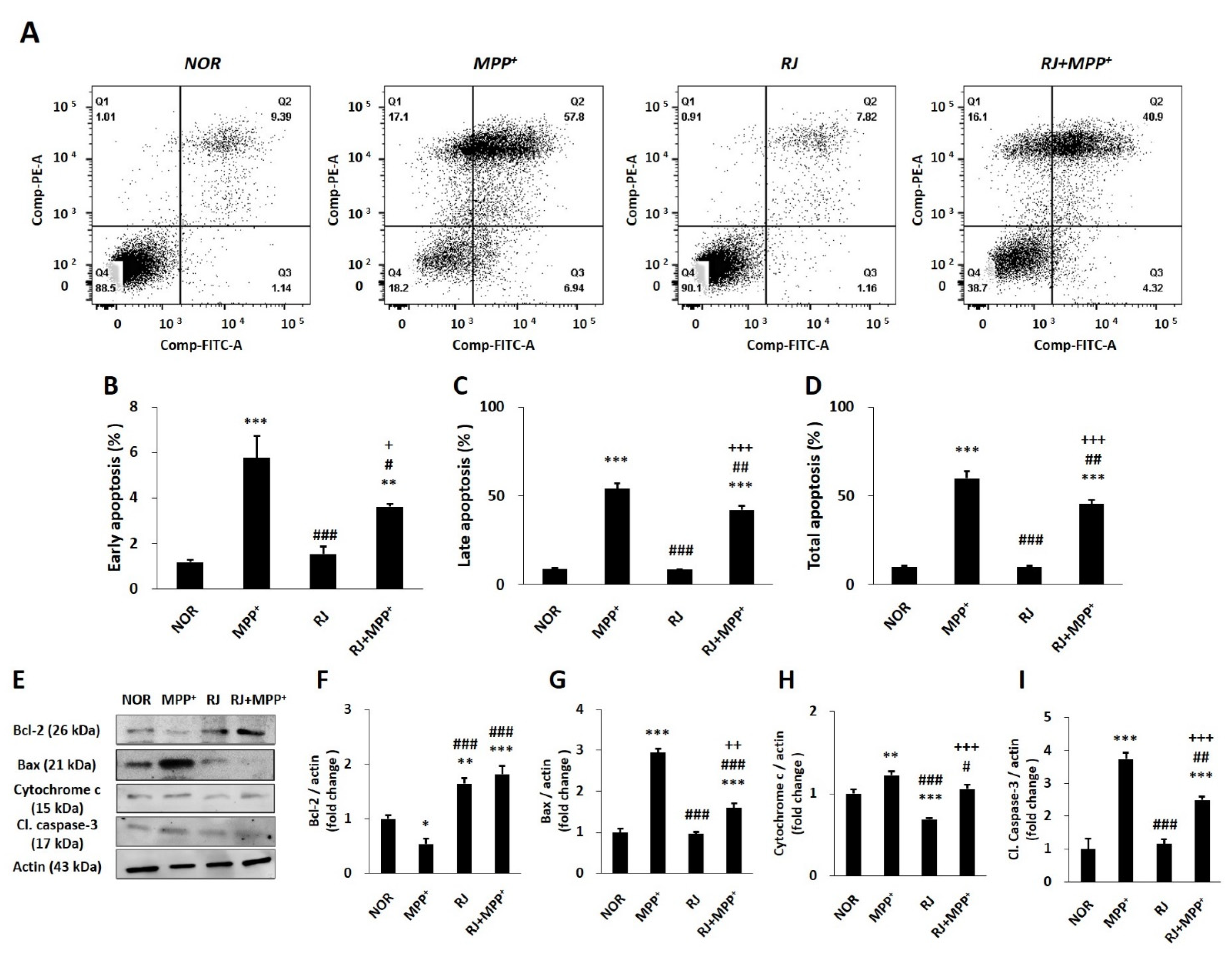
3.4. RJ Partially Protects SH-SY5Y Cells against MPP+-Induced Apoptosis
3.5. RJ Improves the Abnormal Behavior of MPTP-Treated Mice
3.6. RJ Inhibits the Loss of TH and Mitochondrial Factors of MPTP-Treated Mice
3.7. RJ Regulates Apoptosis in the Colon and the SN of MPTP-Treated Mice
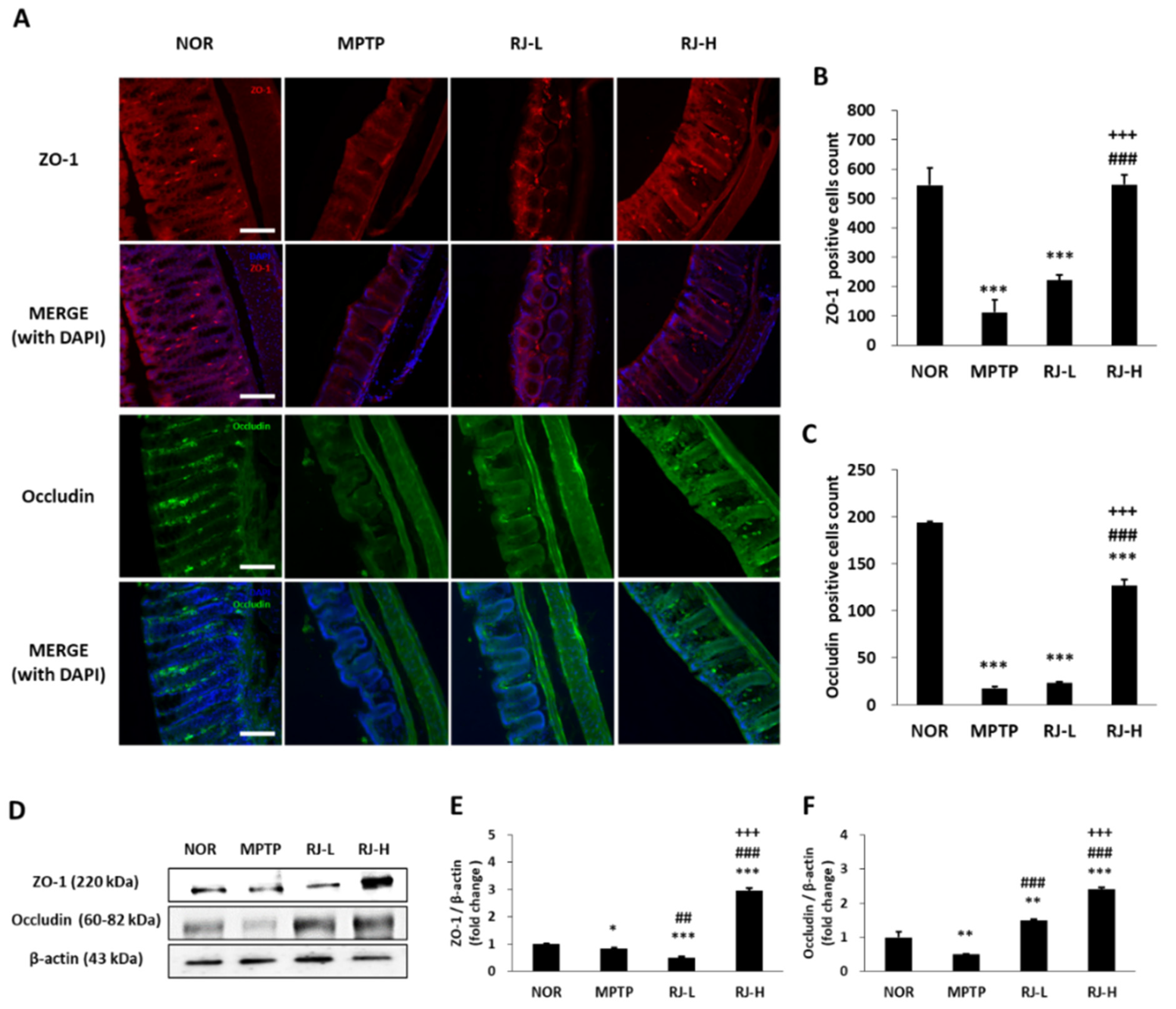
3.8. RJ Suppresses the Loss of TJs in the Colon and the SN of MPTP-Treated Mice
3.9. RJ Reduces the Levels of LPS and Pro-Inflammatory Cytokines in MPTP-Treated Mice
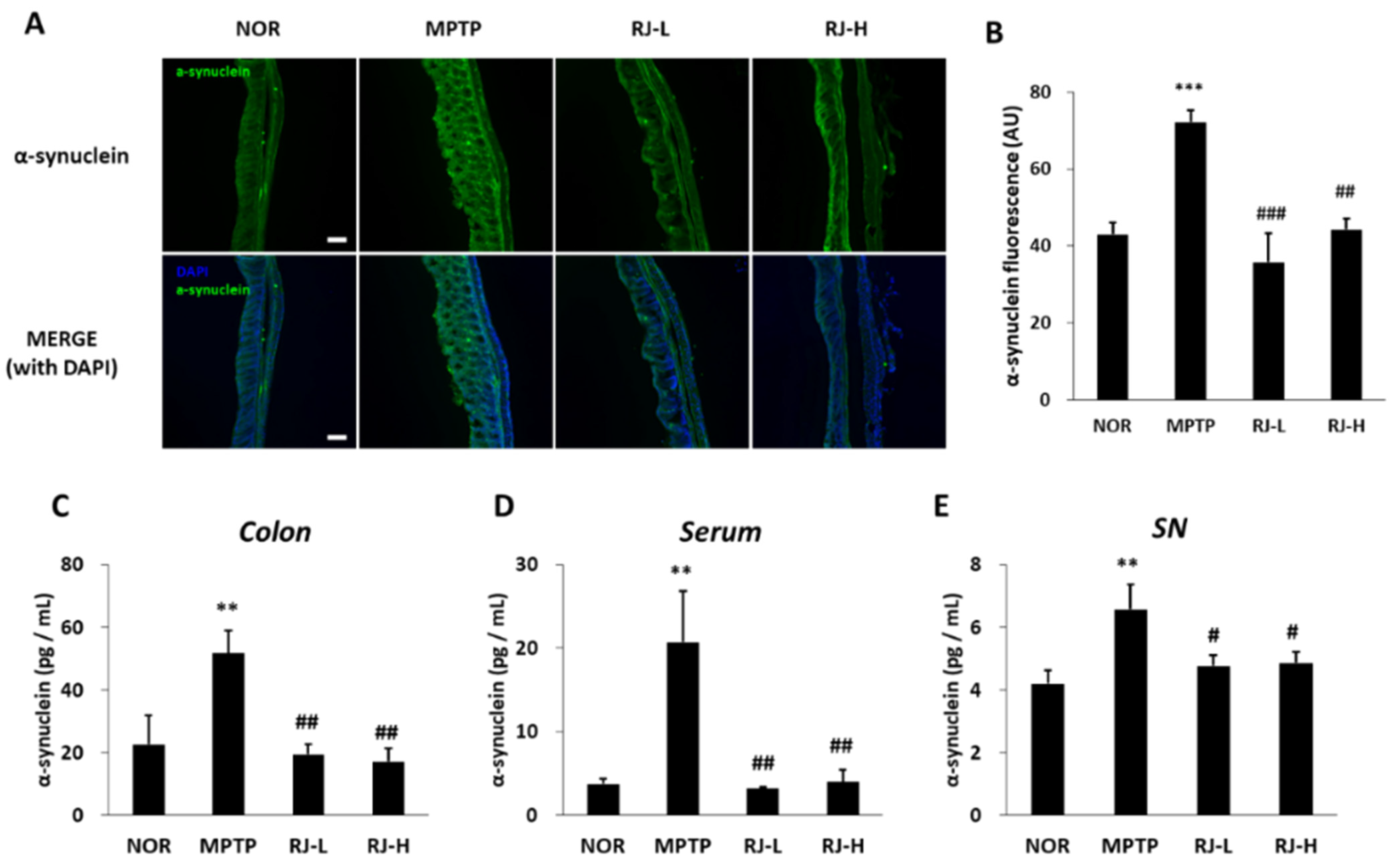
3.10. RJ Suppresses Increase in α-Synuclein in MPTP-Treated Mice
3.11. RJ Prevents MPTP-Induced Disruption of the Blood–Brain Barrier (BBB) TJs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef] [Green Version]
- Requejo-Aguilar, R.; Bolaños, J.P. Mitochondrial control of cell bioenergetics in Parkinson’s disease. Free Radic. Biol. Med. 2016, 100, 123–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloud, L.J.; Greene, J.G. Gastrointestinal features of Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2011, 11, 379–384. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Takahashi, H.; Takeda, S.; Ohama, E.; Ikuta, F. Parkinson’s disease: The presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988, 76, 217–221. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White, C.L., III; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clairembault, T.; Leclair-Visonneau, L.; Coron, E.; Bourreille, A.; Le Dily, S.; Vavasseur, F.; Heymann, M.F.; Neunlist, M.; Derkinderen, P. Structural alterations of the intestinal epithelial barrier in Parkinson’s disease. Acta Neuropathol. Commun. 2015, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Baizabal-Carvallo, J.F.; Alonso-Juarez, M. The Link between Gut Dysbiosis and Neuroinflammation in Parkinson’s Disease. Neurosci. 2020, 432, 160–173. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Ha, T.-J.; Lee, J.-R.; Lee, J.; Nam, S.-H.; Park, K.-H.; Yang, M.-S. Isolation of Anthraquinone Derivatives from the Root of Rumex japonicus H. Appl. Biol. Chem. 2004, 47, 274–278. [Google Scholar]
- Kawasaki, M.; Kanomata, T.; Yoshitama, K. Flavonoids in the leaves of twenty-eight polygonaceous plants. Bot. Mag. Tokyo 1986, 99, 63–74. [Google Scholar] [CrossRef]
- Xie, Q.-C.; Yang, Y.-P. Anti-proliferative of physcion 8-O-β-glucopyranoside isolated from Rumex japonicus Houtt. on A549 cell lines via inducing apoptosis and cell cycle arrest. BMC Complement. Altern. Med. 2014, 14, 377. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.Y.; Jeon, H.; Bae, C.H.; Lee, Y.; Kim, H.; Kim, S. Rumex japonicus Houtt. alleviates dextran sulfate sodium-induced colitis by protecting tight junctions in mice. Integr. Med. Res. 2020, 9, 100398. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, Y.; Wang, Q.; Gao, X. Metabolism and mutual biotransformations of anthraquinones and anthrones in rhubarb by human intestinal flora using UPLC-Q-TOF/MS. J. Chromatogr. B 2019, 1104, 59–66. [Google Scholar] [CrossRef]
- Luo, D.; He, M.; Li, J.; Du, H.; Mao, Q.; Pei, N.; Zhong, G.; Ouyang, H.; Yang, S.; Feng, Y. Integrating the rapid constituent profiling strategy and multivariate statistical analysis for herb ingredients research, with Chinese official rhubarb and Tibetan rhubarb as an example. Arab. J. Chem. 2021, 14, 103269. [Google Scholar] [CrossRef]
- Sun, Y.; Lenon, G.B.; Yang, A.W. Rumex japonicus Houtt.: A phytochemical, pharmacological, and pharmacokinetic review. Phytother. Res. 2020, 34, 1198–1215. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Jeon, H.; Kim, H.; Koo, S.; Kim, S. Sophora flavescens Aiton decreases MPP+-induced mitochondrial dysfunction in SH-SY5Y cells. Front. Aging Neurosci. 2018, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Kim, C.-S.; Lee, Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Jung, T.W.; Lee, J.Y.; Shim, W.S.; Kang, E.S.; Kim, S.K.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Rosiglitazone protects human neuroblastoma SH-SY5Y cells against MPP+ induced cytotoxicity via inhibition of mitochondrial dysfunction and ROS production. J. Neurol. Sci. 2007, 253, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Seaton, T.A.; Cooper, J.M.; Schapira, A.H. Free radical scavengers protect dopaminergic cell lines from apoptosis induced by complex I inhibitors. Brain Res. 1997, 777, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Liu, J.; Hong, X.; Yu, M.; Huang, Y.; Sheng, Z.; Fei, J.; Huang, F. Overexpression of parkin ameliorates dopaminergic neurodegeneration induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in mice. PLoS ONE 2012, 7, e39953. [Google Scholar] [CrossRef]
- Crompton, M. Bax, Bid and the permeabilization of the mitochondrial outer membrane in apoptosis. Curr. Opin. Cell Biol. 2000, 12, 414–419. [Google Scholar] [CrossRef]
- Strasser, A.; O’Connor, L.; Dixit, V.M. Apoptosis signaling. Annu. Rev. Biochem. 2000, 69, 217–245. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.B.; Shannon, K.M.; Kordower, J.H.; Voigt, R.M.; Shaikh, M.; Jaglin, J.A.; Estes, J.D.; Dodiya, H.B.; Keshavarzian, A. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS ONE 2011, 6, e28032. [Google Scholar] [CrossRef] [Green Version]
- Salat-Foix, D.; Tran, K.; Ranawaya, R.; Meddings, J.; Suchowersky, O. Increased intestinal permeability and Parkinson disease patients: Chicken or egg? Canad. J. Neurol. Sci. 2012, 39, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight junction proteins and signaling pathways in cancer and inflammation: A functional crosstalk. Front. Physiol. 2019, 9, 1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gassler, N.; Rohr, C.; Schneider, A.; Kartenbeck, J.; Bach, A.; Obermüller, N.; Otto, H.F.; Autschbach, F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G216–G228. [Google Scholar] [CrossRef] [PubMed]
- Bertiaux-Vandaële, N.; Youmba, S.B.; Belmonte, L.; Lecleire, S.; Antonietti, M.; Gourcerol, G.; Leroi, A.M.; Déchelotte, P.; Ménard, J.F.; Ducrotté, P.; et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 2011, 106, 2165–2173. [Google Scholar] [CrossRef]
- Choi, J.G.; Kim, N.; Ju, I.G.; Eo, H.; Lim, S.M.; Jang, S.E.; Kim, D.H.; Oh, M.S. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep. 2018, 8, 1275. [Google Scholar] [CrossRef] [Green Version]
- Powell, N.; Walker, M.M.; Talley, N.J. The mucosal immune system: Master regulator of bidirectional gut–brain communications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 143. [Google Scholar] [CrossRef]
- Santos, S.F.; de Oliveira, H.L.; Yamada, E.S.; Neves, B.C.; Pereira, A. The gut and Parkinson’s disease--a bidirectional pathway. Front. Neurol. 2019, 10, 574. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Bing, G. Lipopolysaccharide animal models for Parkinson’s disease. Parkinsons Dis. 2011, 2011, 327089. [Google Scholar] [CrossRef] [Green Version]
- Hoban, D.B.; Connaughton, E.; Connaughton, C.; Hogan, G.; Thornton, C.; Mulcahy, P.; Moloney, T.C.; Dowd, E. Further characterisation of the LPS model of Parkinson’s disease: A comparison of intra-nigral and intra-striatal lipopolysaccharide administration on motor function, microgliosis and nigrostriatal neurodegeneration in the rat. Brain Behav. Immun. 2013, 27, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Olanow, C.W.; Brundin, P. Parkinson’s disease and alpha synuclein: Is Parkinson’s disease a prion-like disorder? Mov. Disord. 2013, 28, 31–40. [Google Scholar] [CrossRef]
- Bullich, C.; Keshavarzian, A.; Garssen, J.; Kraneveld, A.; Perez-Pardo, P. Gut Vibes in Parkinson’s Disease: The Microbiota-Gut-Brain Axis. Mov. Disord. Clin. Pract. 2019, 6, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.-M.; Zhang, F.; Zhou, H.; Kam, W.; Wilson, B.; Hong, J.-S. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ. Health Perspect. 2011, 119, 807–814. [Google Scholar] [CrossRef] [Green Version]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atik, A.; Stewart, T.; Zhang, J. Alpha-Synuclein as a Biomarker for Parkinson’s Disease. Brain Pathol. 2016, 26, 410–418. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Chao, Y.X.; He, B.P.; Tay, S.S.W. Mesenchymal stem cell transplantation attenuates blood brain barrier damage and neuroinflammation and protects dopaminergic neurons against MPTP toxicity in the substantia nigra in a model of Parkinson’s disease. J. Neuroimmunol. 2009, 216, 39–50. [Google Scholar] [CrossRef]
- Kelly, L.P.; Carvey, P.M.; Keshavarzian, A.; Shannon, K.M.; Shaikh, M.; Bakay, R.A.; Kordower, J.H. Progression of intestinal permeability changes and alpha-synuclein expression in a mouse model of Parkinson’s disease. Mov. Disord. 2014, 29, 999–1009. [Google Scholar] [CrossRef]
- Zhao, C.; Ling, Z.; Newman, M.B.; Bhatia, A.; Carvey, P.M. TNF-α knockout and minocycline treatment attenuates blood–brain barrier leakage in MPTP-treated mice. Neurobiol. Dis. 2007, 26, 36–46. [Google Scholar] [CrossRef] [Green Version]





| RT (Min) | [M − H]− | [M − H]− (m/z) | [M − H]− (m/z) | Error (ppm) | Predicted Identity |
|---|---|---|---|---|---|
| Molecular Formula | Measured | Calculated | |||
| 8.612 | C21H20O10 | 431.0987 | 431.0984 | −1.57 | Emodin-8-glucoside |
| 8.706 | C21H20O9 | 415.1030 | 415.1035 | 0.35 | Chrysophanol-8-O-β-d-glucoside (pulmatin) |
| 8.756 | C15H8O6 | 283.0255 | 283.0248 | −1.49 | Rhein |
| 9.022 | C15H10O4 | 253.0505 | 253.0506 | 0.36 | Chrysophanol |
| 9.049 | C22H22O10 | 445.1140 | 445.1140 | −0.94 | Physcion-8-O-β-d-glucopyranoside |
| 10.840 | C16H12O5 | 283.0612 | 283.0612 | 1.15 | Physcion |
| 15.058 | C15H10O5 | 269.0458 | 269.0455 | −0.74 | Aloe emodin |
| 17.098 | C15H10O5 | 269.0456 | 269.0455 | −0.42 | Emodin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-Y.; Bae, C.-H.; Kim, J.; Lee, Y.; Jeon, H.; Kim, H.; Kim, S. Rumex japonicus Houtt. Protects Dopaminergic Neurons by Regulating Mitochondrial Function and Gut–Brain Axis in In Vitro and In Vivo Models of Parkinson’s Disease. Antioxidants 2022, 11, 141. https://doi.org/10.3390/antiox11010141
Kim H-Y, Bae C-H, Kim J, Lee Y, Jeon H, Kim H, Kim S. Rumex japonicus Houtt. Protects Dopaminergic Neurons by Regulating Mitochondrial Function and Gut–Brain Axis in In Vitro and In Vivo Models of Parkinson’s Disease. Antioxidants. 2022; 11(1):141. https://doi.org/10.3390/antiox11010141
Chicago/Turabian StyleKim, Hee-Young, Chang-Hwan Bae, Jayoung Kim, Yukyoung Lee, Hyongjun Jeon, Hyungwoo Kim, and Seungtae Kim. 2022. "Rumex japonicus Houtt. Protects Dopaminergic Neurons by Regulating Mitochondrial Function and Gut–Brain Axis in In Vitro and In Vivo Models of Parkinson’s Disease" Antioxidants 11, no. 1: 141. https://doi.org/10.3390/antiox11010141
APA StyleKim, H.-Y., Bae, C.-H., Kim, J., Lee, Y., Jeon, H., Kim, H., & Kim, S. (2022). Rumex japonicus Houtt. Protects Dopaminergic Neurons by Regulating Mitochondrial Function and Gut–Brain Axis in In Vitro and In Vivo Models of Parkinson’s Disease. Antioxidants, 11(1), 141. https://doi.org/10.3390/antiox11010141







