Abstract
Amino acid efflux and influx transport systems play vital roles in industrial microorganisms’ cell growth and metabolism. However, although biochemically characterized, most of them remain unknown at the molecular level in Bacillus licheniformis. In this study, three proteins, namely, YdgF, YvbW, and YveA, were predicted to be involved in the active transport of L-aspartate (L-Asp). This was verified by manipulating their encoding genes. When growing in the minimal medium with L-Asp as the only carbon and nitrogen source, the growth of strains lacking proteins YdgF, YvbW, and YveA was significantly inhibited compared with the wild-type strains, while supplementing the expression of the corresponding proteins in the single-gene knockout strains could alleviate the inhibition. Upon overexpression, the recombinant proteins mediated the accumulation of L-aspartate to varying degrees. Compared with the wild-type strains, the single knockout strains of the three protein genes exhibited reduced absorption of L-aspartate. In addition, this study focused on the effects of these three proteins on the absorption of β-alanine, L-glutamate, D-serine, D-alanine, and glycine.
1. Introduction
Amino acids are widely used in food, feed, medicine, and cosmetics, and are mainly produced by chemical synthesis, enzyme catalysis, or a combination of the two. Microbial fermentation is often used in the industrial production of amino acids, such as glutamic acid, lysine, L-valine, and threonine, given its sound economic and environmental benefits [1,2]. However, high concentrations of amino acids inside or outside the cell are often not conducive to fermentation production [3,4]. Some amino-acid-producing enzymes are inhibited by the substrate; for instance, L-aspartic acid α-decarboxylase can be irreversibly inhibited by L-aspartic acid. Excessive intracellular accumulation of certain amino acids will reduce fermentation production and have a toxic effect on cells. For example, a high intracellular concentration of glutamate will produce a negative feedback effect on related synthases, while reducing the intracellular concentration of glutamate weakens the negative feedback effect and improves glutamate production [5]. High intracellular cysteine concentration can lead to DNA damage of the host and have a toxic effect on the producing strain. In the production of alanine fermentation, the high content of intracellular alanine will inhibit cell growth [6]. When amino acids are used as substrates, they cannot be transported into the cell, and thus accumulate in considerable quantities outside the cell, leading to reduced fermentation production. Therefore, after the discovery of lysine exporter (LysE), which was the first amino acid efflux transporter to be discovered, widespread attention was paid to studies that used the transporter to change the concentration of amino acids on both sides of the cell membrane and improve the production of amino acids with recombinant bacteria [7,8]. At present, modifying transporters on the cell membrane to make bacteria more suitable for the biological production of amino acids is an efficient and well-developed bio-engineering method and a research direction with value and prospect [9,10,11,12,13,14,15,16,17,18].
Among the existing research on amino acid transporters, there is still insufficient study on L-aspartic acid and its downstream products. Further explorations in this field are significant to the development of the biological production of L-aspartic acid and its downstream products. L-aspartic acid is a common chemical raw material, and it is primarily used to produce food additives, synthetic sweeteners, synthetic biopolymer PASP, new synthetic drugs, clinical medicine, and intermediates in the synthesis of other products. In the early stages of industrial production, the traditional microbial fermentation method based on saccharide was adopted, but there was a long production cycle with many by-products and high technical risks [19,20,21]. β-alanine is a naturally occurring β-type amino acid. It is not directly involved in protein synthesis in organisms, yet it is a potential functional amino acid [22,23]. Therefore, it is of great practical value to improve the strain’s production efficiency by modifying the membrane’s ability to transport amino acids and construct engineering bacteria suitable for producing L-Asp, β-Ala, and most other amino acids.
At present, the reported L-Asp transporter, namely, YveA, in Bacillus subtilis was shown to mediate the uptake of L-Asp. Not only that, it is the main L-Asp uptake system in Bacillus subtilis. YveA is a putative transporter of the amino acid/polyamine/organocation (APC) superfamily and the first member of a new family within the APC superfamily. YveA of B. subtilis is the only characterized member of the AGT family. As reported, YveA is shown to be able to mediate the uptake of both L-aspartate and L-glutamate in B. subtilis [24,25]. Although the YveA protein was identified as an L-Asp transporter in Bacillus subtilis, the gene of this protein also exists in the genome of Bacillus licheniformis, and its functions in Bacillus licheniformis have not been studied. More than that, some proteins are predicted to be related to amino acid permeases. The genes of these proteins are in the genome of Bacillus licheniformis CICIM B1391, which can be found on NCBI. The function of certain enzymes with amino acid permeability was determined. At the same time, there are still quite a few proteins that are merely predicted to function as amino acid transporters in certain ways, indicating that they belong to amino acid permeases, but their specific functions remain unclear. The sequences of amino acid transporters found on NCBI were made into an evolutionary tree (shown in the Supplementary Materials). In this study, apart from the gene yveA, we also selected the genes ydgF and yvbW from the evolutionary tree, whose fragments are similar to yveA. Little research has been conducted on the specific functions of amino acid transporters YdgF, YvbW, and YveA expressed by these three genes in B. licheniformis. Therefore, these three proteins were the main research objects in this study [26]. The YdgF protein, a member of the small multidrug resistance (SMR) family of transporters in the inner membrane, is predicted to have the function of transporting D-serine, D-alanine, and glycine [26,27,28,29,30]. The function of the YvbW protein in B. subtilis is to transport leucine and participate in leucine metabolism, and its expression is regulated by the leucine concentration. YvbW is also a BCAA permease identified as BCAA importers, whose overexpression improves intracellular BCAA accumulations and bacitracin yields. YvbW was demonstrated to be transcribed as a monocistronic mRNA containing a prototypical T box antitermination leader and can transport a precursor for leucine, isoleucine, valine, or glutamate biosynthesis [31,32].
Bacillus licheniformis is rich in enzymes, with a moderate growth rate, full protein folding, and many advantages that other industrial strains do not have. It was recognized as a food-safe strain (GRAS) over 40 years ago [33,34]. As a platform for homologous or heterogeneous expression, it is a good industrial microbial strain that is widely used in the production of food enzymes and antibiotics. B. licheniformis is a thermophile, which can grow well at 40–50 °C. It can secrete peptides to inhibit the growth of other bacteria and fungi during the fermentation process, and thus is not easily infected by bacteria. B.licheniformis is rich in enzymes, can produce large quantities of enzymes, and has an extracellular protein secretion that can reach twice that of Bacillus subtilis. Meanwhile, it has strong stress resistance, is hardly soluble, and can be reused [35]. Nevertheless, there are many characteristics of B. licheniformis that need to be further investigated. As a food-safe strain for industrial production, B. licheniformis has a great potential in amino acid production, although the research on amino acid transporters of B. licheniformis is still scarce. It was found that CodY, which is a transcription factor that can regulate the amino acid permease, can prevent lichenysin biosynthesis under the amino-acid-rich condition in B. licheniformis, and BCAAs can relieve this inhibition. In addition, knocking out the amino acid transporter gene yhdG can improve the yield of bacitracin [10,36,37]. At present, there is still not enough research in this field. An in-depth study on the function or transport mechanism of the transporter on the cell membrane of B. licheniformis can facilitate the exploration of its future applications so that this strain can be applied to the production of food-safe amino acid products with higher efficiency and more environmental friendliness.
This study focused on the transport of L-Asp and other related amino acids by three types of amino acid permeases on the cell membrane of B. licheniformis. Using food-safe strains, such as B. licheniformis, to produce amino acids through whole-cell catalysis is an environmentally friendly and safe method with broad application prospects. To make cells adapt to amino acid production through genetic engineering, we must first comprehend the function of amino acid transporters on cell membranes. Whole-cell catalysis adopts the complete biological organism as a catalyst for chemical transformation. The reaction solution has a simple composition and is easy to purify and examine. In addition, the cell contains a complete enzyme reaction system and necessary cofactors, which makes this approach more suitable for the experiment compared to the traditional fermentation method. In this study, the whole-cell catalysis method was used to analyze the function of the protein.
2. Materials and Methods
2.1. Bacterial Strains, Plasmids, and Growth Conditions
The bacterial strains and plasmids used in this study are listed in Table S1, and the primers used are listed in Table S2. The B. licheniformis CICIM B1391 was used as a cloning host. Cells were grown at 37 °C in a lysogeny broth medium (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl, 15 g/L agar when it is solid medium), enrichment medium (FP321 (yeast peptone) 20 g/L, FM408 (yeast extract) 10 g/L, (NH4)2HPO4 75 mmol/L, K2HPO4·3H2O 40 mmol/L, KH2PO4 10 mmol/L, CaCl2 4.5 mmol/L, MgSO4·7H2O 2 mmol/L) [38] or minimal medium (NaCl 0.643 g/L, (NH4)2SO4 0.496 g/L, MgSO4·7H2O 0.102 g/L, K2SO4 0.610 g/L, NaH2PO4 2.280 g/L, Na2HPO4 4.401 g/L) with restricted carbon and nitrogen sources. When required, ampicillin (100 μg/mL), kanamycin (30 μg/mL), and tetracycline (20 μg/mL) were added. When needed, L-Asp at a final concentration of 2 g/L was added to the minimal medium for growth tests.
The shuttle plasmid pHY300-PLK was used as the vector to construct the recombinant plasmid. The ydgF, yvbW, and yveA genes on the genome of B. licheniformis CICIM B1391 were amplified through PCR using the Phanta enzyme (Vazyme Biotech Co., Ltd. 2 × Phanta Master Mix, Nanjing, China). The primers produced in this study are listed in Table S2. The PCR system (50 μL) contained 25 μL of Taq, 22 μL of redistilled water, 1 μL of upstream primer, 1 μL of downstream primer, and 1 μL of genomic DNA extracted from B. licheniformis CICIM B1391. The plasmids used and constructed in this study are listed in Table S1. E. coli JM109 was used for plasmid preparation by selecting transformants on Luria-Bertani (LB) agar plates supplemented with ampicillin (100 μg/mL). The plasmids were extracted and transformed into a competent cell of B. licheniformis with an electric shock at a voltage of about 2000 V. The transformants were cultured in a recovery medium (LB + 0.5 M sorbitol + 0.38 M mannitol) at 37 °C for 3 h and selected with a tetracycline (20 μg/mL) resistance plate [39]. This study used three promoters, i.e., mannitol inducible promoter PmtlA, constitutive promoter Pshuttle09, and Pshuttle09 with a xylose inducible site Pshuttle09-XBS, which can mediate protein overexpression in B. licheniformis [40,41].
2.2. Method of Gene Knockout in Bacillus licheniformis
In this study, FLP/FRT recombination system was used to knock out related genes. The constructed knockout plasmids pNZTT-FFKF, pNZTT-WFKF, and pNZTT-AFKF were transferred into B. licheniformis to obtain the strains BlpNZTT-FFKF, BlpNZTT-WFKF, and BlpNZTT-AFKF. These three strains were pre-incubated at a temperature of 30 °C and 200 rpm. After that, 300 μL of the culture was transferred to 15 mL fresh LB for 14~20 h at 42 °C, with a shaking speed of 250 rpm. The bacterial solution was streaked with kanamycin, cultured at 37 °C until the single colony grew, and one more generation was inoculated before each plate streaking. If there were no single colonies on the LB plate, the corresponding strain needed to be reactivated. Then, whether the colonies obtained on the LB plate containing kanamycin did not contain the target gene was verified. There are three PCR products called the left exchange, right exchange, and double exchange each obtained using a different pair of primers. The left exchange used the pair of primers that sequenced at the upstream of the left homologous arm of the knocked-out gene and sequence in the middle of the gene used for replacement, the right exchange used the pair of primers that sequenced at the downstream of the right homologous arm of the knocked-out gene and sequence in the middle of the gene used for replacement, the double exchange used the pair of primers that sequenced at the upstream and downstream of the homologous arms of the knocked-out gene. When all the PCR products showed clear bands and correct sizes regarding the nucleic acid electrophoresis, the single colony verified using PCR may be the strain without the target gene. Then, the single colony was streaked and amplified on the LB plate containing kanamycin, with colonies not growing on the LB plate containing tetracycline verified one by one. The genome of the strain was extracted, amplified, and sequenced with the upstream and downstream primers of the knockout gene to confirm whether the target gene was removed. If the nucleic acid electrophoresis of the three PCR and the DNA sequencing results were correct, the double-exchange strains BldF, BldW, and BldA were obtained. If the double-exchange strains were not obtained, the strains BlpNZTT-FFKF, BlpNZTT-WFKF, and BlpNZTT-AFKF should be reactivated [42,43].
2.3. Method of Cell Density Determination
In this study, the OD value of the culture at 600 nm was used to express the cell concentration. The bacterial solution was shaken and diluted to a certain concentration. Its OD value was measured at 600 nm with an ultraviolet spectrophotometer and a blank medium with the same dilution ratio as the control was used. The absorption value of the control was subtracted and the result was multiplied by the dilution ratio to obtain the OD600 [38].
2.4. Method of Whole-Cell Catalyst
The strain was pre-incubated by streaking on the corresponding antibiotic-resistant plate and cultured for 20 h. A single colony was selected and inoculated into an LB medium for 16 h. The activated bacterial solution was inoculated into an enrichment medium and cultured until the OD600 was about 20. The bacterial solution was poured into a 50 mL centrifuge tube, washed twice with minimal medium cooled with ice in advance, and then suspended in the minimal medium of the same volume. If the cells had antibiotic resistance, certain antibiotics could be added to prevent bacterial infection. The cell suspension was pre-cultured at a centrifugation speed of 120 rpm and a temperature of 37 °C for 30 min before the addition of the amino acid solution of a certain final concentration. The orifice of the centrifuge tube was then sealed with sealing film and cultured at 120 °C for 16 h [44,45,46,47,48].
2.5. HPLC
The bacterial solution was centrifuged and the supernatant was taken. Then, 0.22 μM of the membrane was used to filtrate the supernatant before the filtered liquid was diluted to an amino acid concentration of 1 g/L. The OPA (o-phthaldialdelhyde) pre-column derivatization method was used to separate the different components in the sample via gradient elution. Most amino acids could be detected at the 338 nm UV wavelength. This study adopted external standards. A 1 g/L amino acid standard solution was prepared, and the standard curves are made by gradient dilutions of it. The retention time was measured to determine the amino acid, and the peak area was measured to obtain the concentration of the amino acid in the solution.
3. Results
3.1. Construction of Plasmids and Strains
Since the functions of the amino acid permeases YdgF, YvbW, and YveA are still not clear, these three proteins were selected as the research objects, where their encoding genes are designated as ydgF, yvbW, and yveA, respectively. Using the genome of B. licheniformis CICIM B1391 as a template, the genes ydgF, yvbW, and yveA were amplified with PCR. In order to overexpress the proteins YdgF, YvbW, and YveA, the amplified fragments were connected to the shuttle plasmid pHY300-PLK, which can replicate in both E. coli and B. licheniformis. The mannitol inducible promoters PmtlA [40], Pshuttle09 [41], and shuttle09 with xylose-inducible site Pshuttle09-XBS were chosen to mediate the overexpression of the above proteins in B. licheniformis. After all the constructed plasmids were transformed into B. licheniformis, nine strains with overexpressed permease genes were constructed, namely, BlpMF, BlpS09F, BlpS09XF, BlpMW, BlpS09W, BlpS09XW, BlpMA, BlpS09A, and BlpS09XA, as shown in Table S1. The restriction enzyme analysis results are shown in Figure S1A–C. Each of the three genes ydgF, yvbW, and yveA was successfully removed from the chromosome of the strain B1391, according to diagnostic PCR (shown in Figure S1E,F). The resulting strains were designated as BldF, BldW, and BldA, respectively.
The corresponding transporter genes were supplemented and expressed in the single-gene knockout strains. Plasmids that demonstrated transporter overexpression mediated by promoter shuttle09 were constructed. The plasmids were transformed into the corresponding single-gene knockout strains BldF, BldW, and BldA listed in Table S1 to obtain strains BldFpS09F, BldWpS09W, and BldApS09A, which are also listed in Table S1. For comparison, the empty plasmid was also transformed into three single-gene knockout strains, and strains BldFpHY, BldWpHY, and BldWpHY were constructed. The transporter gene and promoter gene on the plasmid in the strain were amplified to ensure the transformation of the plasmid into the strain, which was verified using nucleic acid electrophoresis. The results are shown in Figure S1D.
3.2. Effects of Transport Protein Expression Changes on Strain Growth in a Minimal Medium with Free L-Asp as the Sole Carbon and Nitrogen Source
3.2.1. Growth Curves of Single-Gene Knockout Strains in a Minimal Medium with Free L-Asp as the Only Carbon and Nitrogen Source
The single-gene knockout strains BldF, BldW, and BldA that lacked genes ydgF, yvbW, and yveA, respectively, were pre-incubated and inoculated into 30 mL of minimal medium with L-Asp at a concentration of 2 g/L as the only carbon and nitrogen source. The strains were grown at 37 °C at a centrifugation speed of 250 rpm, and the wild-type strain was used as a control. The absorbance at 600 nm of the fermentation broth was measured every 3 h. With the obtained data, Origin 9 was used to make a line graph of OD600 over time, and then nonlinear fitting was performed on the line graph. The curve (growth curve) of OD600 over time is shown in the Supplementary Materials. The derivative of the fitted curve was obtained and divided by OD600 to obtain the specific growth rate curves of the strains. The results are shown in Figure 1.
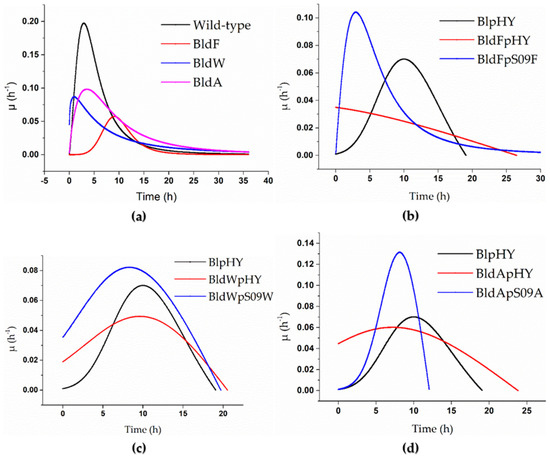
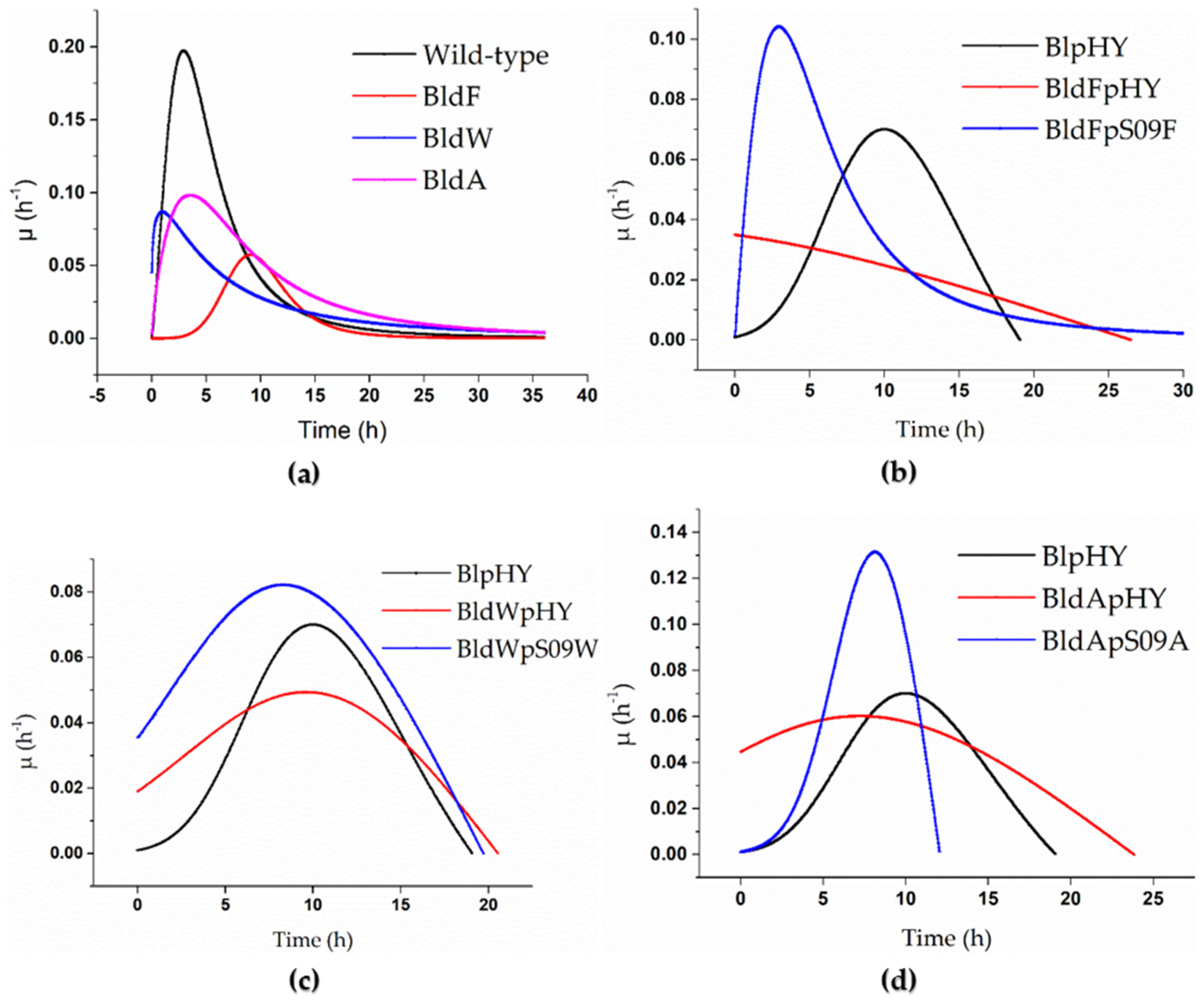
Figure 1.
Specific growth rate curves of the strains when using a minimal medium with free L-Asp as the only carbon and nitrogen source. (a) The specific growth rate curves of the wild-type strain (black), strain BldF (red), strain BldW (blue), and strain BldA (pink). (b–d) Strains BlpHY (black), BldFpHY (red), and transporter gene single-knockout strains with plasmids with overexpressed transporter mediated by promoter Pshuttle09, which means the transport protein gene was supplemented and expressed in the strains with single knockout transporter gene (blue): (b) specific growth rate curve of strain BldFpS09F, (c) specific growth rate curve of strain BldWpS09W, and (d) specific growth rate curve of strain BldApS09A.
It is clear from Figure 1a that the specific growth rates of the strains without a transport protein were significantly lower than that of the wild-type strain when the concentration of L-Asp was 2 g/L. The highest specific growth rate of the wild-type strain could reach 0.197 h−1. The highest specific growth rates of the three strains lacking transport proteins were all lower than 0.1 h−1. The highest specific growth rate of BldF was 0.057 h−1, 0.086 h−1 for BldW, and 0.098 h−1 for BldA, which were much lower than that of the wild-type strain, indicating that the growth rate of the wild-type strain was higher than the strains BldF, BldW, and BldA, and the growth of the strains was inhibited to varying degrees after the transporter gene was knocked out, which meant the optimal growth of the strains required the existence of these three transport proteins. It also showed that the proteins YdgF, YvbW, and YveA had different effects on L-Asp absorption.
3.2.2. Growth Curves of Single-Knockout Strains Supplemented with Genes Expressing Corresponding Transporters in a Minimal Medium with Free L-Asp as the Only Carbon and Nitrogen Source
The strains BldFpS09F, BldWpS09W, and BldApS09A were pre-incubated and inoculated into 30 mL of minimal medium with L-Asp at a concentration of 2 g/L as the only carbon and nitrogen source. The strains were grown at 37 °C at a centrifugation speed of 250 rpm, and the strains BldFpHY, BldWpHY, BldApHY, and BlpHY were used as controls. The absorbance of the fermentation broth at 600 nm was measured every 3 h. The obtained data were processed in the same way as described in Section 3.2.1. The results are shown in Figure 1b–d, and the growth curves are shown in the Supplementary Materials.
It can be found from Figure 1 that supplementing the corresponding protein in a strain lacking transport proteins can significantly alleviate the inhibition and return the cell proliferation to normal to some extent. The three proteins’ abilities regarding alleviating the inhibition were different. The highest specific growth rate of the wild-type strain with the empty plasmid was 0.07 h−1. The maximum specific growth rate of the strain BldFpS09F was 0.104 h−1, 0.082 h−1 for the strain BldWpS09W, and 0.131 h−1 for the strain BldApS09A, which were significantly higher than that of BlpHY, indicating that the biomass concentrations of the strains with retro-complementation of the corresponding transport proteins increased more rapidly than the wild-type strain with an empty plasmid. The strain BldFpS09F proliferated rapidly in the early stages, partly making the growth of the strain return to normal (Figure 1b). Although it was not significantly higher than the complementary expression of YdgF and YveA, the specific growth rate of the strain BldWpS09W was higher than that of the control group, and the growth of the strain had returned to normal (Figure 1c). The maximum specific growth rate of the strain BldApS09A was significantly higher than that of the strain BlpHY, and the peak appeared earlier, indicating that the cell count of the strain BldApS09A increased rapidly in the early stages, which alleviated the inhibitory effect caused by the absence of the transport protein (Figure 1d).
3.3. Effect of Strains Overexpressing Amino Acid Permease Gene on L-Asp Absorption
In order to study the influence of the expression of ydgF, yvbW, and yveA on the absorption of L-aspartate, the nine strains listed in Table S1 overexpressed the three proteins: BlpMF, BlpS09F, BlpS09XF, BlpMW, BlpS09W, BlpS09XW, BlpMA, BlpS09A, and BlpS09XA, which were pre-incubated and inoculated into the fermentation medium. All of the fermentation liquid was centrifugated for 15 min at 9000 rpm in a 50 mL centrifuge tube. The cells were collected when the OD600 was about 10. The effects of the overexpression of YdgF, YvbW, and YveA on the absorption of L-Asp in the wild-type strains with plasmid pHY300-PLK were used as a comparison according to Section 2.4. The OD600 of the cell suspension was about 150. The high cell concentration had a strong impact on the absorption of amino acids. As such, it is not appropriate to compare the results which only use the amino acids absorbed by cells. Therefore, the ratio of the amino acid absorption concentration to OD600 of bacterial suspension was used as the result for comparison [44]. The results are shown in Figure 2.
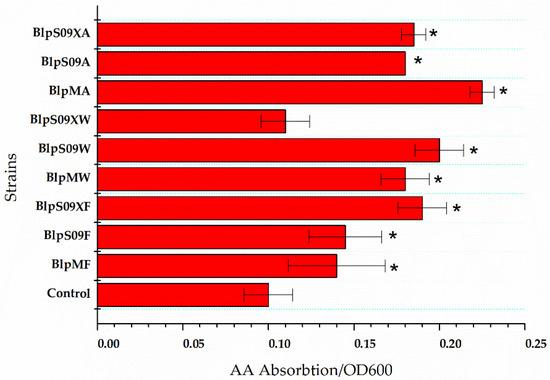
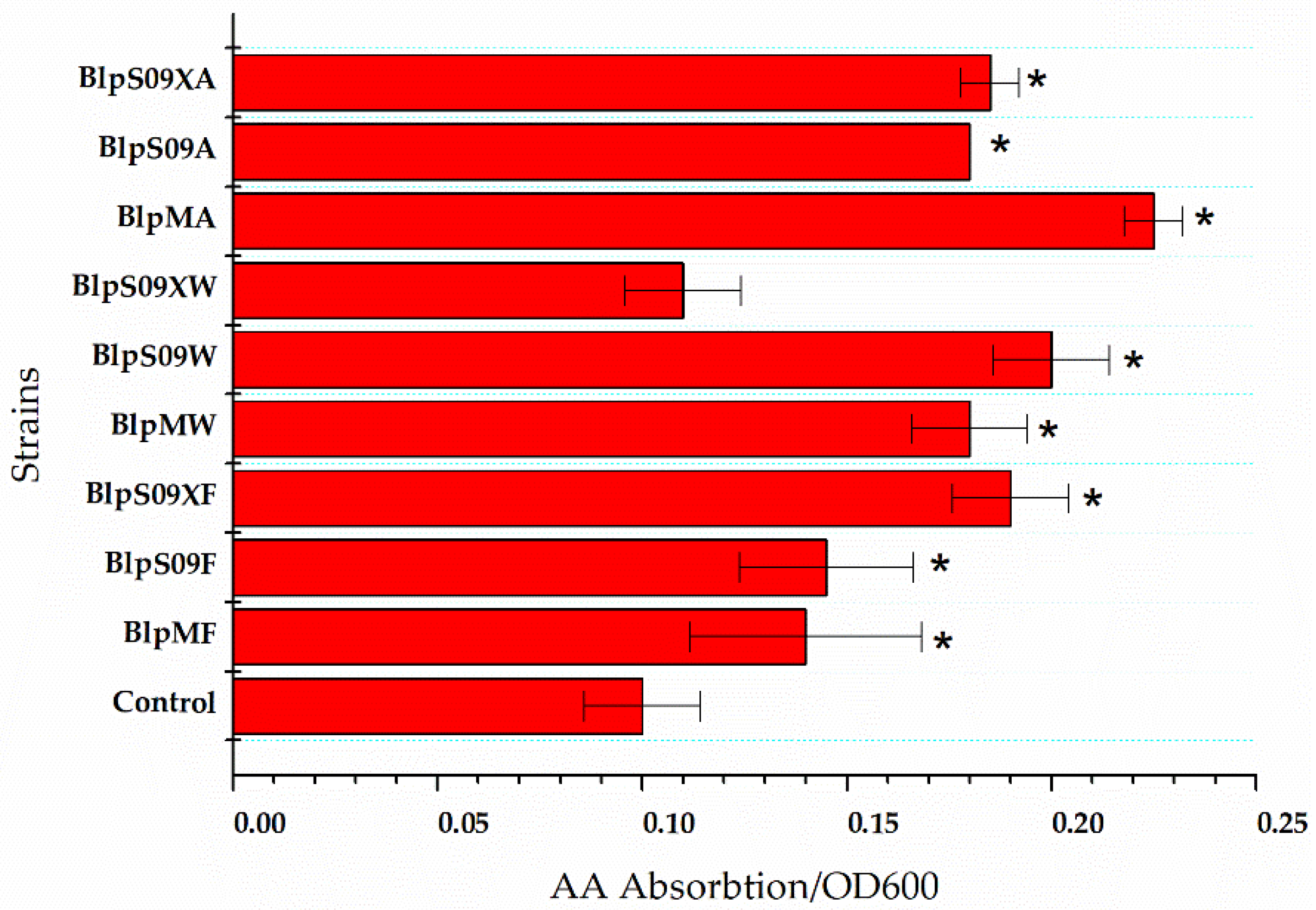
Figure 2.
The ratio of extracellular L-aspartic acid concentration to the cell concentration of the strains that overexpressed the amino acid permease. A wild-type strain with plasmid pHY300-PLK was used as the control. Each experiment was repeated at least three times; data are represented as the means of three replicates and bars represent the standard deviations. * p < 0.05 indicates the significance levels between the recombinant strains and the control strain.
The Figure 2 shows how the L-Asp absorption was affected by the overexpression of the three transporters mediated by different promoters when the L-Asp concentration was 20 g/L and the reaction time was 16 h. The strain BlpHY was used as a comparison.
It was found that the ratio of extracellular L-Asp concentration to the cell concentration of the strain overexpressing protein ydgF was significantly lower than that of a wild-type strain with an empty plasmid, indicating that the overexpression of the protein YdgF could improve the absorption of L-Asp by the cells (the ratio of extracellular L-Asp concentration to cell concentration was 62.5% higher than that of the wild-type strain with an empty plasmid when the Pshuttle09-XBS promoter was used to mediate protein overexpression). The overexpression of both YvbW and YveA proteins could improve the absorption of L-Asp to a certain extent. The Figure 2 also shows that when the Pshuttle09-XBS promoter functioned as the mediator, the expression of the protein was the highest.
3.4. Effects of the Absence of Amino Acid Permease Genes on L-Asp Absorption
The three strains overexpressing the three proteins pre-incubated and collected cells in the same way, as shown in Section 3.3. According to Section 2.4, the strain BlpHY was used as a control to study the effect of the absence of the amino acid permease genes ydgF, yvbW, and yveA on the L-Asp absorption. The OD600 of the cell suspension was about 150. Figure 3 shows how knocking out three amino acid permease genes from the strain affected the L-Asp absorption when the L-Asp concentration was 20 g/L and the reaction time was 16 h. The wild-type strain was used as a comparison.
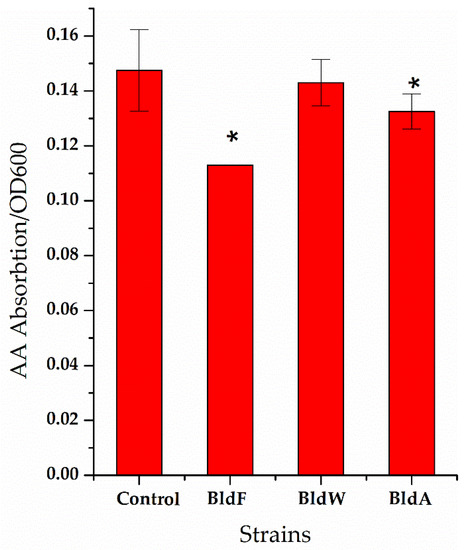
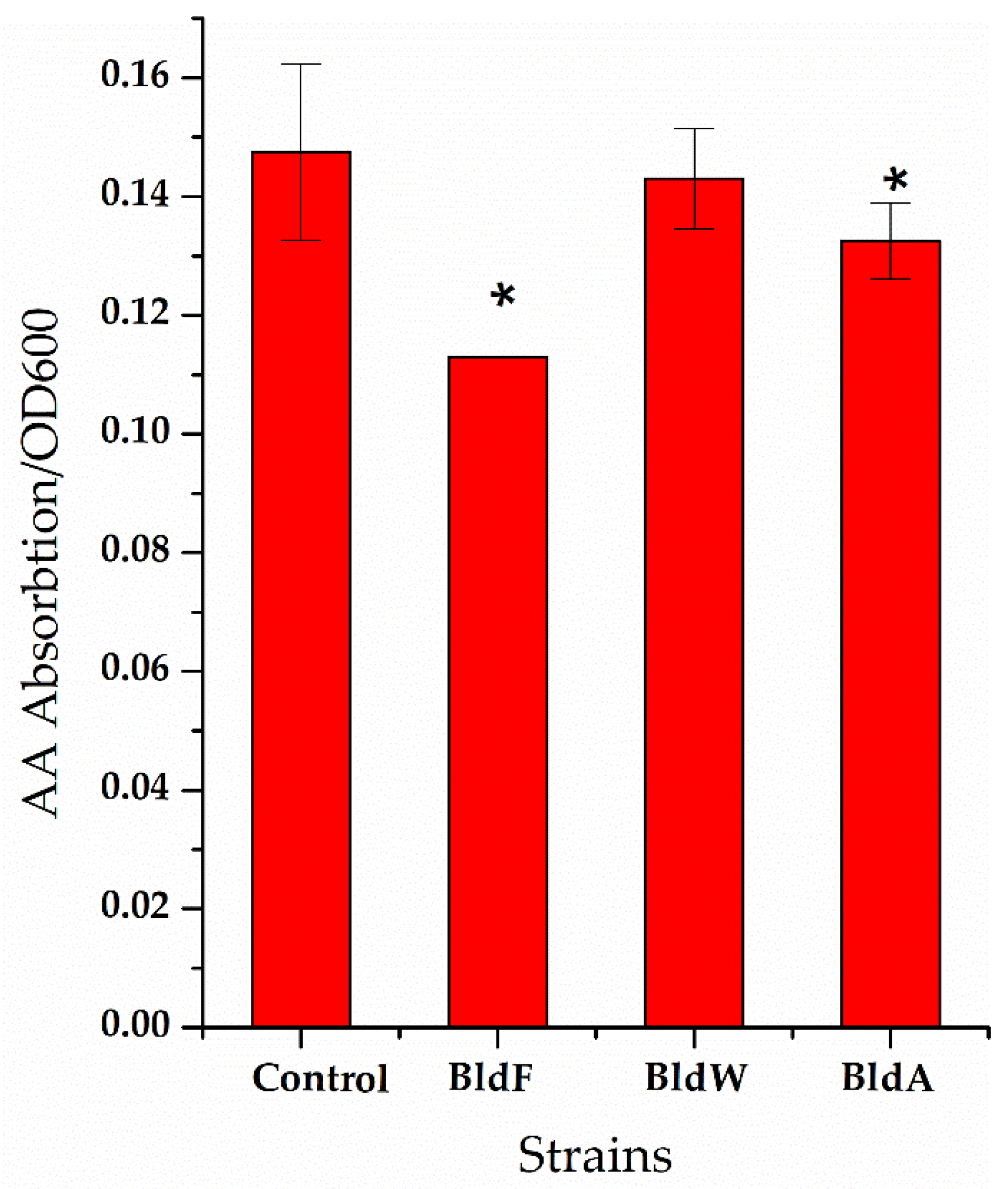
Figure 3.
The ratio of the concentration of extracellular L-aspartic acid to the cell concentration of the strain lacking amino acid permease. A wild-type strain was used as a comparison. Each experiment was repeated at least three times; data are represented as the means of three replicates and bars represent the standard deviations. * p < 0.05 indicates the significance levels between recombinant strains and the control strain.
It can be seen that in the strain lacking the expression of the three proteins, the ratio of extracellular L-Asp concentration to cell concentration was significantly higher than that of the wild-type strain, indicating that the absence of the expression of the three proteins weakened the absorption of L-Asp, which is generally consistent with the effect of the overexpression of permease proteins on L-Asp absorption.
3.5. Effects of the Three Transport proteins on the Transportation of Other Amino Acids by Bacillus licheniformis
In order to test whether these three proteins can transport other amino acids, the amino acids predicted to be transported by the three transporters were selected as the research objects. The strains BlpHY, BlpS09XF, BlpS09XW, and BlpS09XA were pre-incubated and collected according to Section 2.4 Separate solutions containing each of β-alanine, D-serine, D-alanine, glycine, and L-glutamic acid were added to make the final concentration of amino acid 20 g/L. The effects of the overexpression of three proteins on the absorption of each of these four amino acids in wild-type strains were used as a comparison. The results are shown in Figure 4.
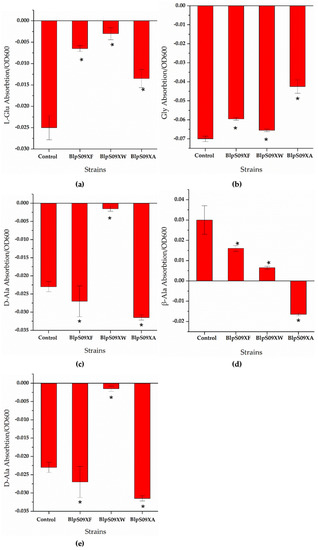
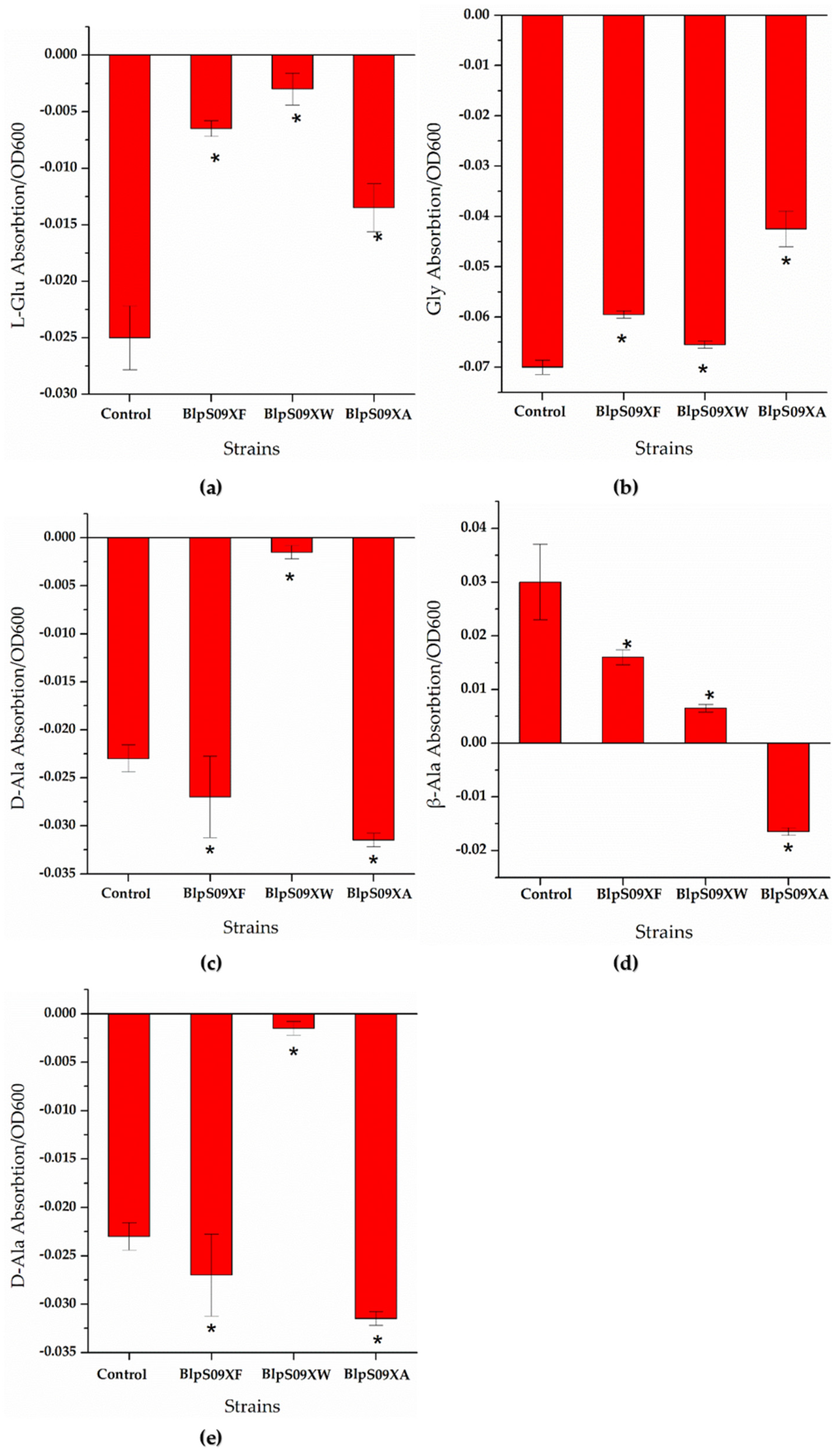
Figure 4.
(a) The ratio of the extracellular L-Glu concentrations of the strains overexpressing amino acid permease mediated by promoter Pshuttle09-XBS to the cell concentration; (b) the ratio of the extracellular Gly concentrations of the strains overexpressing amino acid permease mediated by promoter Pshuttle09-XBS to the cell concentration; (c) the ratio of the extracellular D-Ala concentrations of the strains overexpressing amino acid permease mediated by promoter Pshuttle09-XBS to the cell concentration; (d) the ratio of the extracellular β-Ala concentrations of the strains overexpressing amino acid permease mediated by promoter Pshuttle09-XBS to the cell concentration; (e) the ratio of the extracellular D-Ser concentrations of the strains overexpressing amino acid permease mediated by promoter Pshuttle09-XBS to the cell concentration. A wild-type strain with plasmid pHY300-PLK was used as a comparison. Each experiment was repeated at least three times; data are represented as the means of three replicates and bars represent the standard deviations. * p < 0.05 indicates the significance levels between recombinant strains and the control strain.
It can be seen from Figure 4d that the ratio of the extracellular β-Ala concentration of strain BlpS09XA to the cell concentration was higher than that of strain BlpHY, indicating that the strain overexpressing YveA had a lower β-Ala absorption than the wild-type strain, i.e., the overexpression of yveA hindered the absorption of β-Ala. The overexpression of YdgF and YvbW proteins hardly affected the β-Ala absorption of the B. licheniformis cells. As for the other amino acids, it can be seen from Figure 4a,b,e that the ratio of the concentration of L-Glu, Gly, and D-Ser absorbed by the cells to the cell concentration was significantly higher than that of the wild-type strains with empty plasmids, indicating that the overexpression of protein YdgF could improve the absorption of D-Ser and L-Glu. However, the ratio of D-Ala absorbed by the cells to the cell concentration was lower than that of strain BlpHY, indicating that the absorption of D-Ala was weakened. The overexpression of YvbW protein could enhance the absorption of D-Ser and D-Ala, but changes in the transport of L-Glu and Gly were not obvious. The overexpression of the YveA protein could improve the absorption of D-Ser and L-Glu and weaken the absorption of Gly and D-Ala.
4. Discussion
At present, there is still not enough research on the amino acid transporters on the cell membrane of B. licheniformis and on using B. licheniformis as a whole-cell catalyst. B. licheniformis is an excellent production strain with significant research value and huge potential for further explorations. From current research and predictions, it is known that the protein YdgF can transport D-serine and D-alanine. The function of the protein YvbW in B. subtilis is to transport leucine; it participates in leucine metabolism while its expression is regulated by the leucine concentration. Protein YveA is known to be related to the transport of L-aspartate and L-glutamate in other Bacilli. In this study, the three transporter genes ydgF, yvbW, and yveA in the genome of B. licheniformis CICIM B1391 were cloned, and their expression changed in B. licheniformis. The changes in amino acid transport capacity were studied through whole-cell catalysis. We also knocked out these three genes from the genome of B. licheniformis CICIM B1391, constructed three single knockout strains, and observed their growth with the designated amino acid as the only carbon and nitrogen source. Finally, the transport effects of the three proteins on some other amino acids were also studied.
We concluded that the proteins encoded by these three genes constituted amino acid transporters and the final results were not entirely consistent with the prediction. In addition, the permeases could transport more than one type of amino acid. The three proteins had a particular absorption effect on L-aspartic. The protein YdgF had a more pronounced ability regarding L-Asp absorption after being overexpressed in the cell. The YveA protein had an efflux effect on β-Ala, and the absence of the yveA gene could enhance β-Ala absorption. The protein YdgF could absorb D-Ser and L-Glu, and it may have an efflux effect on D-Ala; the YvbW protein could absorb D-Ser and D-Ala; the YveA protein could absorb D-Ser and L-Glu, and it may have had an efflux effect on Gly and D-Ala. The growth defects caused by the removal of these three genes from the chromosome led to reduced uptake ability of free amino acids by the single knockout strains, indicating that these three proteins had a specific effect on the uptake of amino acids in the strain. However, different transport proteins produced different effects regarding alleviating the growth defect. In strains where the YdgF gene was knocked out, the overexpressed protein YdgF alleviated the growth defect in later stages of growth, while the complementary expression of YveA alleviated the growth defect in the early stages. The specific growth rate was always higher than that of the strain BlpHY, while the complementary expression of the protein YvbW and strain growth returned to normal. The reason may have been the different uptake mechanisms of L-Asp of the three proteins. In addition, after knocking out the single gene, the bacteria could still grow slowly, which indicated that apart from these three proteins, there were other unreported L-Asp transport mechanisms on the cell membrane of B. licheniformis, and L-Asp was absorbed for cell growth.
B. licheniformis has significant advantages in biological production and is a very suitable object for whole-cell catalysis research. At present, there is little research on B. licheniformis as a whole-cell catalyst. As an efficient production method, whole-cell catalysis essentially involves catalysis by enzymes in cells [44]. It is a biocatalytic process that falls between fermentation and extracted enzyme catalysis. Compared with free enzymes, whole-cell catalysis has many advantages; for instance, the cell has a complete multi-enzyme system that can realize enzyme cascade reactions. The reaction solution after cell removal is simple in composition and is easy to purify and examine. Moreover, the cell membrane functions as a barrier, which has the ability to control the contact between substrate and enzyme. However, there are still many problems regarding whole-cell catalysis, such as the permeability of the cell membrane to substrates or enzymes, the accumulation of by-products and degradation caused by by-products, and the uncertainties in the catalytic reaction process, which hinder its further adoption in the industry. Transforming the ability of the cell membrane to transport amino acids, improving the efficiency of whole-cell catalysts, and using genetic engineering to screen and transform various cell catalysts will be the most effective way to resolve the issues [45,46,47,48,49]. In this study, the function of transporters was studied through whole-cell catalysis. The cells that changed the expression of the transporters were collected, and the whole-cell catalyst with changed amino acid transport capacity was obtained, which can be used as a catalyst when generating other products in the future. This paper also puts forward some ideas on obtaining the whole-cell catalyst with a modified cell membrane for amino acid production and provides insights into how B. licheniformis is used as a whole-cell catalyst.
Supplementary Materials
The following materials are available at https://www.mdpi.com/article/10.3390/fermentation8010022/s1, Figure S1: DNA fragments and nucleic acid electrophoresis in the construction of the strain. Figure S2: Evolutionary tree of amino acid transporter gene in Bacillus licheniformis from NCBI. Figure S3: Cell growth in minimal medium with free L-Asp as the only carbon and nitrogen source. Table S1: Bacterial strains and plasmids used in this study. Table S2: Oligonucleotides used in this study.
Author Contributions
Conceptualization, Y.L. and G.S.; methodology, H.W.; validation, F.X. and Y.Z.; data curation, H.W.; writing—original draft preparation, H.W.; writing—review and editing, Y.L.; visualization, L.Z.; supervision, S.X.; project administration, Z.D.; funding acquisition, Z.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2020YFA0907700, 2018YFA0900300, and 2018YFA0900504), the National Natural Foundation of China (32172174, 31401674), Postgraduate Research and Practice Innovation Program of Jiangsu Province (SJCX20_0746), the National First-Class Discipline Program of Light Industry Technology and Engineering (LITE2018-22), and the Top-Notch Academic Programs Project of Jiangsu Higher Education Institutions.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, J.H.; Lee, K.H.; Kim, T.Y.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of L-valine based on transcriptome analysis and in silico gene knockout simulation. Proc. Natl. Acad. Sci. USA 2007, 104, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Zakataeva, N.P.; Aleshin, V.V.; Tokmakova, I.L.; Troshin, P.V.; Livshits, V.A. The novel transmembrane Escherichia coli proteins involved in the amino acid efflux. FEBS Lett. 1999, 452, 228–232. [Google Scholar] [CrossRef]
- Trötschel, C.; Deutenberg, D.; Bathe, B.; Burkovski, A.; Krämer, R. Characterization of Methionine Export in Corynebacterium glutamicum. J. Bacteriol. 2005, 187, 3786–3794. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Hirasawa, T. Regulation and Metabolic Engineering. In Amino Acid Biosynthesis~Pathways, Regulation and Metabolic Engineering; Wendisch, V.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–38. [Google Scholar]
- Radmacher, E.; Stansen, K.C.; Besra, G.; Alderwick, L.; Maughan, W.N.; Hollweg, G.; Sahm, H.; Wendisch, V.F.; Eggeling, L. Ethambutol, a cell wall inhibitor of Mycobacterium tuberculosis, elicits l-glutamate efflux of Corynebacterium glutamicum. Microbiology 2005, 151, 1359–1368. [Google Scholar] [CrossRef]
- Park, S.; Imlay, J.A. High Levels of Intracellular Cysteine Promote Oxidative DNA Damage by Driving the Fenton Reaction. J. Bacteriol. 2003, 185, 1942–1950. [Google Scholar] [CrossRef]
- Vrljic, M.; Sahm, H.; Eggeling, L. A new type of transporter with a new type of cellular function: L-lysine export from Corynebacterium glutamicum. Mol. Microbiol. 1996, 22, 815–826. [Google Scholar] [CrossRef]
- BRÖER, S.; KRÄMER, R. Lysine excretion by Corynebacterium glutamicum. Eur. J. Biochem. 1991, 202, 137–143. [Google Scholar] [CrossRef]
- Liu, Q.; Liang, Y.; Zhang, Y.; Shang, X.; Liu, S.; Wen, J.; Wen, T. YjeH Is a Novel Exporter of l -Methionine and Branched-Chain Amino Acids in Escherichia coli. Appl. Environ. Microbiol. 2015, 81, 7753–7766. [Google Scholar] [CrossRef]
- Den Hengst, C.D.; Groeneveld, M.; Kuipers, O.P.; Kok, J. Identification and functional characterization of the Lactococcus lactis CodY-regulated branched-chain amino acid permease BcaP (CtrA). J. Bacteriol. 2006, 188, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Belitsky, B.R. Role of Branched-Chain Amino Acid Transport in Bacillus subtilis CodY Activity. J. Bacteriol. 2015, 197, 1330–1338. [Google Scholar] [CrossRef]
- Ihara, K.; Sato, K.; Hori, H.; Makino, Y.; Shigenobu, S.; Ando, T.; Isogai, E.; Yoneyama, H. Expression of the alaE gene is positively regulated by the global regulator Lrp in response to intracellular accumulation of l -alanine in Escherichia coli. J. Biosci. Bioeng. 2017, 123, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kennerknecht, N.; Sahm, H.; Yen, M.R.; Patek, M.; Saier, M.H., Jr.; Eggeling, L. Export of L-isoleucine from Corynebacterium glutamicum: A two-gene-encoded member of a new translocator family. J. Bacteriol. 2002, 184, 3947–3956. [Google Scholar] [CrossRef]
- Kim, S.; Ihara, K.; Katsube, S.; Hori, H.; Ando, T.; Isogai, E.; Yoneyama, H. Characterization of the l -alanine exporter AlaE of Escherichia coli and its potential role in protecting cells from a toxic-level accumulation of l -alanine and its derivatives. Microbiology 2015, 4, 632–643. [Google Scholar] [CrossRef]
- Kruse, D.; Kramer, R.; Eggeling, L.; Rieping, M.; Pfefferle, W.; Tchieu, J.H.; Chung, Y.J.; Saier, M.H., Jr.; Burkovski, A. Influence of threonine exporters on threonine production in Escherichia coli. Appl. Microbiol. Biotechnol. 2002, 59, 205–210. [Google Scholar]
- Park, J.H.; Oh, J.E.; Lee, K.H.; Kim, J.Y.; Lee, S.Y. Rational design of Escherichia coli for L-isoleucine production. ACS Synth. Biol. 2012, 1, 532–540. [Google Scholar] [CrossRef]
- Dassler, T.; Maier, T.; Winterhalter, C.; Bock, A. Identification of a major facilitator protein from Escherichia coli involved in efflux of metabolites of the cysteine pathway. Mol. Microbiol. 2000, 36, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, A.; Vrljić, M.; Pátek, M.; Sahm, H.; Krämer, R.; Eggeling, L. Expression control and specificity of the basic amino acid exporter LysE of Corynebacterium glutamicum. Microbiology 2001, 147, 1765–1774. [Google Scholar] [CrossRef]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chibata, I.; Tosa, T.; Sato, T. Continuous production of L-aspartic acid. Appl. Biochem. Biotechnol. 1986, 13, 231–240. [Google Scholar] [CrossRef]
- Chibata, I.; Tosa, T.; Sato, T. Immobilized aspartase-containing microbial cells: Preparation and enzymatic properties. Appl. Microbiol. 1974, 27, 878–885. [Google Scholar] [CrossRef]
- Song, C.W.; Lee, J.; Ko, Y.-S.; Lee, S.Y. Metabolic engineering of Escherichia coli for the production of 3-aminopropionic acid. Metab. Eng. 2015, 30, 121–129. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, J.; Chen, G.; Ge, Y.; Zhang, X.; Zhu, H. Extracellular Expression of L-Aspartate-α-Decarboxylase from Bacillus tequilensis and Its Application in the Biosynthesis of β-Alanine. Appl. Biochem. Biotechnol. 2019, 189, 273–283. [Google Scholar] [CrossRef]
- Lorca, G.; Winnen, B.; Saier, M.H., Jr. Identification of the l -Aspartate Transporter in Bacillus subtilis. J. Bacteriol. 2003, 185, 3218–3222. [Google Scholar] [CrossRef]
- Saier, J.; Milton, H. Families of transmembrane transporters selective for amino acids and their derivatives. The information presented in this review was initially prepared for presentation at the FASEB meeting on amino acid transport held in Copper Mountain, Colorado, June 26–July 1, 1999 and was updated in January 2000 following the meeting of the Transport Nomenclature Panel of the International Union of Biochemistry and Molecular Biology (IUBMB) in Geneva, November 28–30, 1999. The system of classification described in this review reflects the recommendations of that panel. Microbiology 2000, 146, 1775–1795. [Google Scholar]
- Kikukawa, T.; Miyauchi, S.; Araiso, T.; Kamo, N.; Nara, T. Anti-parallel membrane topology of two components of EbrAB, a multidrug transporter. Biochem. Biophys. Res. Commun. 2007, 358, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Zheng, H.; Hao, P.; Konno, T.; Kume, H.; Ye, L.; Oda, M.; Suzuki, K.; Ji, Z.-S. Acquisition of amino acids by Lactobacillus delbrueckii subsp. bulgaricus 2038 when grown in the presence of casein. Int. Dairy J. 2014, 35, 145–152. [Google Scholar] [CrossRef]
- Duran, A.M.; Meiler, J. INVERTED TOPOLOGIES IN MEMBRANE PROTEINS: A MINI-REVIEW. Comput. Struct. Biotechnol. J. 2013, 8, e201308004. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, E.; Zheng, H.; Shi, T.; Ye, L.; Konno, T.; Oda, M.; Shen, H.; Ji, Z.-S. Relationship between Lactobacillus bulgaricus and Streptococcus thermophilus under whey conditions: Focus on amino acid formation. Int. Dairy J. 2016, 56, 141–150. [Google Scholar] [CrossRef]
- Daley, D.O.; Rapp, M.; Granseth, E.; Melén, K.; Drew, D.; von Heijne, G. Global Topology Analysis of the Escherichia coli Inner Membrane Proteome. Science 2005, 308, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Rollins, S.M. Induced Transcriptional Expression of Bacillus subtilis Amino Acid Permease yvbW in Response to Leucine Limitation. Adv. Microbiol. 2014, 4, 484–492. [Google Scholar] [CrossRef][Green Version]
- Cai, D.; Zhu, J.; Li, Y.; Li, L.; Zhang, M.; Wang, Z.; Yang, H.; Li, J.; Yang, Z.; Chen, S. Systematic engineering of branch chain amino acid supply modules for the enhanced production of bacitracin from Bacillus licheniformis. Metab. Eng. Commun. 2020, 11, e00136. [Google Scholar] [CrossRef] [PubMed]
- Phengnuam, T.; Suntornsuk, W. Detoxification and anti-nutrients reduction of Jatropha curcas seed cake by Bacillus fermentation. J. Biosci. Bioeng. 2013, 115, 168–172. [Google Scholar] [CrossRef]
- Voigt, B.; Schroeter, R.; Schweder, T.; Jürgen, B.; Albrecht, D.; van Dijl, J.M.; Maurer, K.-H.; Hecker, M. A proteomic view of cell physiology of the industrial workhorse Bacillus licheniformis. J. Biotechnol. 2014, 191, 139–149. [Google Scholar] [CrossRef]
- Shi, J.; Zhan, Y.; Zhou, M.; He, M.; Wang, Q.; Li, X.; Wen, Z.; Chen, S. High-level production of short branched-chain fatty acids from waste materials by genetically modified Bacillus licheniformis. Bioresour. Technol. 2019, 271, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xiao, F.; Qiu, Y.; Wang, Q.; He, Z.; Chen, S. Lichenysin production is improved in codY null Bacillus licheniformis by addition of precursor amino acids. Appl. Microbiol. Biotechnol. 2017, 101, 6375–6383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cai, D.; Xu, H.; Liu, Z.; Zhang, B.; Wu, F.; Li, J.; Chen, S. Enhancement of precursor amino acid supplies for improving bacitracin production by activation of branched chain amino acid transporter BrnQ and deletion of its regulator gene lrp in Bacillus licheniformis. Synth. Syst. Biotechnol. 2018, 3, 236–243. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. Transcriptional Changes in the Xylose Operon in Bacillus licheniformis and Their Use in Fermentation Optimization. Int. J. Mol. Sci. 2019, 20, 4615. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Y.; Zhang, Y.; Wang, H.; Zhang, L.; Ding, Z.; Gu, Z.; Xu, S.; Shi, G. A new CcpA binding site plays a bidirectional role in carbon catabolism in Bacillus licheniformis. iScience 2021, 24, 102400. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, Y.; Zhang, Y.; Wang, H.; Zhang, L.; Ding, Z.; Gu, Z.; Xu, S.; Shi, G. Construction of a novel sugar alcohol-inducible expression system in Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2020, 104, 5409–5425. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Ji, S.; Cao, P.; Chen, Y.; Zhao, X. Generation of an Artificial Double Promoter for Protein Expression in Bacillus subtilis through a Promoter Trap System. PLoS ONE 2013, 8, e56321. [Google Scholar] [CrossRef]
- Tan, X.; Liang, F.; Cai, K.; Lu, X. Application of the FLP/FRT recombination system in cyanobacteria for construction of markerless mutants. Appl. Microbiol. Biotechnol. 2013, 97, 6373–6382. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, C.; Pérez-Martín, J. Site-specific targeting of exogenous DNA into the genome of Candida albicans using the FLP recombinase. Mol. Genet. Genom. 2002, 268, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yoneyama, H.; Tobe, R.; Ando, T.; Isogai, E.; Katsumata, R. Inducible l -Alanine Exporter Encoded by the Novel Gene ygaW ( alaE ) in Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 4027–4034. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Noronha, S.B. Utilization of Bacillus subtilis cells displaying a glucose-tolerant beta-glucosidase for whole-cell biocatalysis. Enzym. Microb. Technol. 2020, 132, 109444. [Google Scholar] [CrossRef]
- Pinto, A.; Contente, M.L.; Tamborini, L. Advances on whole-cell biocatalysis in flow. Curr. Opin. Green Sustain. Chem. 2020, 25, 100343. [Google Scholar] [CrossRef]
- Talukder, M.R.; Min, P.S.; Jae, C.W. Integration of cell permeabilization and medium engineering for enhanced enantioselective synthesis of ethyl-S-3-hydroxy-3-phenylpropanoate (S-EHPP). Biochem. Eng. J. 2019, 148, 24–28. [Google Scholar] [CrossRef]
- Wachtmeister, J.; Rother, D. Recent advances in whole cell biocatalysis techniques bridging from investigative to industrial scale. Curr. Opin. Biotechnol. 2016, 42, 169–177. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Guo, D.; Shi, C.; Zhang, C.; Peng, X.; Yang, H.; Xia, X. Disinfectant Resistance Profiles and Biofilm Formation Capacity of Escherichia coli Isolated from Retail Chicken. Microb. Drug Resist. 2019, 25, 703–711. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).