1. Introduction
Herpes simplex virus type 2 (HSV-2) is the primary cause of genital herpes and one of the most common sexually transmitted infections. After establishing latency in the sacral ganglia, HSV-2 reactivates, leading to intermittent shedding of virus in the genital tract [
1]. Global prevalence of HSV-2 is high: as the majority of HSV-2 cases are subclinical and asymptomatic, viral shedding results in high rates of viral transmission. In 2012, an estimated 417 million people aged 15–49 (11%) were living with HSV-2 infection, of whom ~50% were women [
2]. Epidemiological and clinical studies showed that genital herpes is positively linked to the risk of acquiring and transmitting HIV [
3]. Antiviral drugs, which target viral DNA polymerase, can partially control the signs and symptoms of genital herpes when used to treat first clinical and recurrent episodes or when used as daily suppressive therapy. However, these drugs neither eradicate latent virus nor affect the risk, frequency, or severity of recurrences after the drug is discontinued [
1].
Several natural antimicrobial proteins and peptides have demonstrated the ability to inhibit viral infection and block viral entry into the host cell or influence later stages of viral growth [
4]. Lactoferrin (LF) is a glycoprotein present in external secretions, having multiple functions, including antiviral activity and stimulation of immune response [
5,
6]. LF has shown antiviral activity against several viruses, including rotavirus, respiratory syncytial virus, hepatitis C virus (HCV), herpes virus, and HIV [
4,
7,
8,
9]. LF exerts its anti-HSV effect by inhibiting viral attachment and entry into the host cell [
4,
10,
11]. Antiviral activity of LF has been showed against both HSV-1 and HSV-2, although the exact antiviral mechanism is slightly different, due to the difference between the two viruses in the initial binding with target cells [
1,
4,
6].
Silver nanoparticles (AgNPs) have been demonstrated to exert an antiviral activity against several viruses [
12]. There are two ways the AgNP interacts with the pathogenic virus: (1) the AgNP will bind to the outer coat of the virus thus inhibit the attachment of virus towards cell receptors and (2) the AgNP will bind to the DNA or the RNA of the virus thus inhibiting the replication or propagation of the virus inside the host cells [
12,
13].
We have previously described anti-HSV-2 activity of tannic acid modified AgNPs (TA-AgNPs) by using both in vitro and in vivo models [
14]. The mechanisms of antiviral action of TA-AgNPs included blocking of virus attachment, entry, cell-to-cell spread as well as induction of anti-viral cytokine and chemokine production [
1]. Moreover, anti-viral effect was size-dependent, with smaller nanoparticles showing stronger activation of inflammatory reaction [
14].
Recently, LF has been attached onto the surface of biosynthesized AgNPs showing higher antibacterial activity against selected bacterial strains as than their single components [
15]. In this study, we tested whether combined activities of lactoferrin and silver or gold nanoparticles of different sizes can lead to development of a novel microbicide using both in vitro and in vivo model of HSV-2 infection.
2. Materials and Methods
2.1. Synthesis of Nanoparticles
The nanoparticles colloids were synthesized in water according to chemical reduction method. The following reagents for the nanoparticles and nanoconjugates preparation were used: tetrachloroauric acid (HAuCl4, Sigma-Aldrich, ≥49%), tannic acid (C76H52O46, Sigma-Aldrich), sodium citrate (C6H5Na3O7·2H2O) purity 99.0%, Sigma-Aldrich), silver nitrate (AgNO3, purity 99.999%, Sigma-Aldrich, St. Louis, MO, USA), sodium borohydride (NaBH4, purity ≥ 96%, Sigma-Aldrich), lactoferrin (CAS no. 936541-36-5. Sigma Aldrich ≥ 85%). For all preparations, deionized water obtained from Deionizer Millipore Simplicity UV system (specific resistivity of water was 18.2 MΩ·cm) was used. 10 nm AgNPs were prepared by reduction of silver nitrate with sodium borohydride in the presence of sodium citrate and tannic acid. The molar ratio of silver nitrate: sodium citrate: tannic acid: sodium borohydride reagents was equal to 1:7:2:4. For the synthesis process, silver nitrate (1.574 g, 1%) and deionized water (92.914 g) were mixed in the flask for 5 min, then a mixture of sodium citrate (4.181 g, 4%) and tannic acid (0.630 g, 5%) was added. After ~2s, sodium borohydride (0.701 g, 2%) was added and the solution was mixed for additional 15 min. The final concentration of metal NPs was equal 100 ppm.
10 nm AuNPs were prepared by reduction of tetrachloroauric acid with a mixture of sodium citrate and tannic acid with the molar ratio of the reagents equal 1:3:2. Briefly, tetrachloroauric acid (12.691 g, 0.136%) and deionized water (81.655 g) were warmed to the boiling point under reflux and then a mixture of reducing and stabilizing agents was added (mixture of sodium citrate 3.929 g, 1% and tannic acid 1.725 g, 1%). The mixture was boiled for additional 15 min and then cooled down to room temperature. The final concentration of metal NPs was equal 100 ppm. 30 nm AgNPs were prepared by reduction of silver nitrate with a mixture of sodium citrate and tannic acid (the molar ratio of reagents was equal 1:7:2). The synthesis procedure was as follows: silver nitrate (1.574 g, 1%) and deionized water (93.615 g) were mixed and boiled under reflux. Next, a warm mixture of sodium citrate (4.181 g, 4%) and tannic acid (0.630 g, 5%) was added to the boiling solution and heated for an additional 15 min, and then the colloid was cooled down to room temperature. The final concentration of metal NPs was equal 100 ppm. Nanoconjugates were prepared by incubation of nanoparticles with lactoferrin (1% aqueous solution, human recombinant, iron-saturated, Sigma-Aldrich) at room temperature for 24 h. Briefly, 2.475 g of each colloid was incubated with 27.76 mg of 1% aqueous solution of lactoferrin to obtain the final lactoferrin concentration equal 110 µg/mL for each sample. Attempts to prepare 30 nm AuNPs conjugated with lactoferrin failed due to instability of the obtained colloid. For all conjugates a reference sample was prepared as an aqueous solution of lactoferrin in concentration corresponding to concentration present in colloids. The nanoparticles after modification with lactoferrin (LF) were denoted as LF-Ag/AuNPs, while unmodified as Ag/AuNPs.
Nanoparticles before and after lactoferrin functionalization were characterized using the following techniques: high-resolution scanning electron microscopy (HR-SEM equipped with STEM II detector; NovaNanoSEM 450, FEI, Hillsboro, Oregon, USA; images acquired at bright field (BF), dark field (DF), high-angle annular dark-field (HAADF) detector mods; samples for measurements prepared by drop-casting of colloid on carbon coated copper grids), DLS and Zeta (Lifesizer 500, Particle Analyzer, Anton Paar, Graz, Austria), UV–Vis (spectrophotometer UV-5600, Biosens, METASH, Shanghai, China).
2.2. Virus and Anti-Viral Tests
HSV-2 (strain 333) was grown and titrated in Vero cells (ATCC
® CCL-81, Rockville, MD, USA) and kept at −80 °C until use. Virus was diluted in MEM (Thermo Fisher Scientific, Waltham, MA, USA) and maintained on ice until administered to mice or mixed with nanoparticles maximum one hour later. To analyze how
LF-NPs can influence viral attachment, cells were pre-chilled at 4 °C for 15 min, then co-treated with NPs and HSV-2 for 1 h. After this time, virus and NPs were removed and cell monolayers were washed with ice-cold PBS, and further incubated at 37 °C. At 24 h post infection, virus titers were determined by plaque assays or qPCR (see below), as described previously [
14]. The viral penetration assay started by pre-chilling cells at 4 °C for 15 min and then cells were infected for 2 h at 4 °C to allow virus binding but not entry. The inoculum was removed, and cells were washed with ice-cold PBS before adding
LF-NPs for 2 h at 37 °C. The
LF-NPs were afterwards removed, and cells were washed twice with cold PBS. After another 18 h at 37 °C, virus titers were determined by qPCR. For examining post viral entry effects of
LF-NPs use, cells were infected at 37 °C, then the virus was removed, cells washed, and
LF-NPs were added 6 h post infection (p.i.). At 24 h post infection, the infected cultures were analyzed by qPCR. Pre-treatment was performed by incubating cells with
LF-NPs (at 37 °C) for 6 h then cells were washed, infected and further titered by qPCR.
2.3. Cell Lines and Infection
Human VK2-E6/E7 vaginal epithelial cells were obtained from ATCC (CRL-2616) and propagated in EpiLife™ medium supplemented with EpiLife™ Defined Growth Supplement (EDGS) (Thermo Fisher Scientific) and 10 U/mL penicillin and 100 μg/mL streptomycin (GIBCO by Thermo Fisher Scientific). Human HaCaT keratinocytes were from CLS Cell Lines Service GmbH (Eppelheim, Germany) and propagated in Dulbecco’s modified EMEM (DMEM) supplemented with 10% fetal calf serum, 10 U/mL penicillin, and 100 μg/mL streptomycin (GIBCO). Both cell lines were maintained in standard conditions (37 °C, 5% CO2) and handled according to ATCC and CLS recommendations.
2.4. Cell Viability Assay
Cell viability was measured using the MTT assay ((-(4, 5-Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide, Sigma-Aldrich). Cultures of Vero, HaCaT and VK-2-E6/E7 A were incubated for 24 h at 37 °C in a 96-well plate with lactoferrin modified NPs and lactoferrin in a range of NPs concentrations (1–50 μg/mL) in a final volume of 100 µL. The control cells were untreated. Thereafter, 20 µL MTT at a concentration of 5 mg/mL in PBS was added to each well, and the cells were incubated for 4 h at 37 °C. Subsequently, 100 µL of 10% Triton X-100, 0.1 M HCl in isopropanol solution was added, and the cells were incubated for 30 min at room temperature. After the incubation, the optical density (OD) was measured at a wavelength of 570 nm using Omega Microplate Spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA). The cellular viability was calculated as the percentage of viable cells relative to the control. All experiments were performed in triplicate. The 50% cytotoxic concentration (CC50) was defined as the compound’s concentration (μg/mL) required for the reduction of cell viability by 50%, which were calculated by regression analysis, plotting cytotoxicity percentage to NPs concentration. MNTC (maximum non-toxic concentrations) was calculated as NPs concentration with ≤20% of non-viable cells and these concentrations were used for in vitro studies.
2.5. Virus Titration
Total DNA and RNA from vaginal tissues and spinal cords were isolated at day 7 post HSV-2 infection using RNA/DNA Extracol kit (Eurx, Gdansk, Poland). DNA from infected cell cultures was isolated with Universal RNA/DNA extraction kit (Eurx). HSV-2 was detected by qPCR with primers and probe for the viral envelope glycoprotein (gB), as described by Namvar et al. [
16] in Rotor-Gene Q (Qiagen, Hilden, Germany) with GoTaq
® Probe qPCR Master Mix (Promega, Madison, WI, USA). Forward primer: ‘TGCAGTTTACGTATAACCACATACAGC’, reverse primer: ‘AGCTTGCGGGCCTCGTT’ and probe: 5′ FAM-CGCCCCAGCATGTCGTTCACGT’. Standard curve analysis was based on Ct values and serial of 10-fold dilutions of the plasmid standard containing the gB gene with an initial concentration of 2.62 × 10
6 HSV-2 genome copy numbers per reaction. A standard curve was included in each PCR run. Data are expressed as the HSV-2 copy number per ng of the total DNA in the tissue.
2.6. Ethical Statement
The study was performed in accordance with the recommendations of the Polish Act of 21 January 2005 on animal experiments (OJ no 33, item 289) and Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes. The protocol was approved by the Local Committee on the Ethics of Animal Experiments in Warsaw, Poland (permit Number: WAW2/69/2021).
2.7. Genital HSV-2 Infection and Virus Challenge
Female C57BL/6 mice, 6 to 8 weeks old, were injected s.c. with 2.0 mg/mouse of medroxyprogesterone (Depo-Provera; Pfizer, Puurs, Belgium) in 200 µL of PBS. Five days later the mice were anesthetized and inoculated intravaginally with 20 μL of virus solution, containing 10
3 PFU of HSV-2 strain 333 in MEM medium [
14,
17]. Vaginal washings were performed at 24, 48 and 72 h after infection with 100 μL of 0.9% NaCl solution containing 10 μg/mL LF-Ag/AuNPs or 0.9% NaCl solution. Illness was scored according to the scale: no signs = 0; slight inflammation of anogenital area = 1; gross inflammation = 2; gross inflammation and hair loss = 3; and gross inflammation, ulceration and neurological signs = 4. Animal scores were averaged in each group to obtain a single representative value. At day 7 post infection, mice were sacrificed by cervical dislocation and the spinal cords and vaginas were collected for further tests.
2.8. Flow Cytometry Phenotypic Analysis
Cell suspensions from vaginal tissues were prepared as follows: tissues were cut into small pieces and treated with Liberase TL (Roche, Indianapolis, IN, USA) in MEM medium at 37 °C for 40 min. Tissues were next pressed through a 70 µm cell strainer and washed in PBS/1%FBS. Cell suspensions were pretreated with the Fc receptors block-rat anti-CD16/32 antibody (2.4G2) (BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s protocol. The following antibodies were used: anti-CD3-FITC (145-2C11, ThermoFisher Scientific), anti-CD4-PE (clone RM4-5, BD Biosciences), anti-CD8-PerCP (clone 53-6.7., BD Biosciences), anti-NK1.1-APC (clone PK136, BD Biosciences), anti-CD11b-FITC or PE (clone M1/70) (BD Biosciences), anti-CD69-APC antibody (H1.2F3, eBioscience), anti-CD11c-APC (HL3, eBioscience), rat anti-F4/80-FITC (BM8, eBioscience) and rat anti-I-A-PE (M5/114.15.2, eBioscience) antibodies, and rat anti-Gr-1-PE (RB6-8C5, BD Biosciences). Following the immunolabeling for the extracellular markers, cells were fixed with Perm/Wash buffer (BD Bioscience) and were incubated with anti-IFN-γ APC (eBioscience, clone-XMG1.2). The stained cell suspensions were analyzed in FACS Calibur for the percentage of positively stained cells and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
2.9. qPCR for Cytokines and Chemokines
Approximately 0.5 μg of total RNA isolated from vaginal tissues and spinal cords, as described above, was converted to cDNA using MLV Reverse transcriptase (Invitrogen, Thermofisher Scientific). qPCR reactions for cytokines and chemokines were carried out using GoTaq® Probe qPCR Master Mix (Promega) and TaqMan® probes for the detection of IL-1β (Mm00434228_m1), IFN-γ (Mm01168134_m1), TNF-α (Mm00443258_m1), CXCL9 (Mm00434946_m1), CXCL10 (Mm00445235_m1), and GADPH (Mm99999915_g1) in Rotor-Gene Q (Qiagen). Cytokines and chemokines were normalized to the mean threshold cycle (CT) of GADPH housekeeping gene. The 2−∆∆Ct method was used in calculating the relative ratio to control uninfected tissue.
2.10. Statistical Methods
For statistical analysis, GraphPad Prism version 7 (GraphPad software) was used. Data were analyzed using an unpaired Student’s t-test (normal distribution) or the Mann–Whitney U test and the results are reported as mean ± standard error of the mean (SEM) unless indicated otherwise. The p < 0.05 was considered statistically significant.
4. Discussion
This study demonstrates anti-HSV-2 activity of lactoferrin functionalized silver and gold nanoparticles in the in vitro infection of different types of human keratinocytes but also in the treatment of in vivo HSV-2 infection in mice. The in vitro and in vivo models of HSV-2 infection were chosen on the basis of the results previously obtained in our group [
14,
17,
18,
19]. We have demonstrated that combination of antiviral compound, tannic acid (TA), with silver nanoparticles boosts antiviral properties several times [
14,
17,
18]. This effect was due to a better interaction between tannic-acid modified silver nanoparticles and the virus surface [
17,
18], in comparison to tannic acid itself. Furthermore, TA-AgNPs worked efficiently when applied both as a colloid or in an appropriate mucoadhesive gel to treat HSV-2 infection in the mouse model [
19]. However, the antiviral properties of TA-AgNPs depended strongly on the size of nanoparticles with 20–40 nm silver nanoparticles being the most effective without undesired toxic and proinflammatory properties when applied in vivo [
14,
20].
Here, we observed the same characteristics of small AgNPs (10 nm) as being more toxic than 30 nm AgNPs and 10 nm AuNPs. Toxicity of AgNPs is related with internalization of NPs, which leads to production of ROS, oxidative stress and genotoxic effects. If produced in sufficiently high amounts, ROS can induce cell death by either apoptosis or necrosis [
20,
21]. The toxicity of silver nanoparticles is also directly related with the ability of a particular cell type to internalize NPs [
21]. As previously shown, HaCaT keratinocytes internalize AgNPs very poorly, thus are less sensitive to AgNPs induced toxicity than VK-2-E6/E7 keratinocytes [
20]. Here, the differences observed between HaCaT and VK-2 cells sensitivity to AgNPs were insignificant. On the other hand, small (10 nm) AuNPs functionalized with lactoferrin showed very little toxicity for all tested cell types (
Table 1). These results were very advantageous as AuNPs sized < 10 nm are more readily accumulated into cell nuclei and organelles, leading to DNA damage [
22]. Our results indicate that functionalization of AuNPs sized 10 nm with lactoferrin does not result in toxicity.
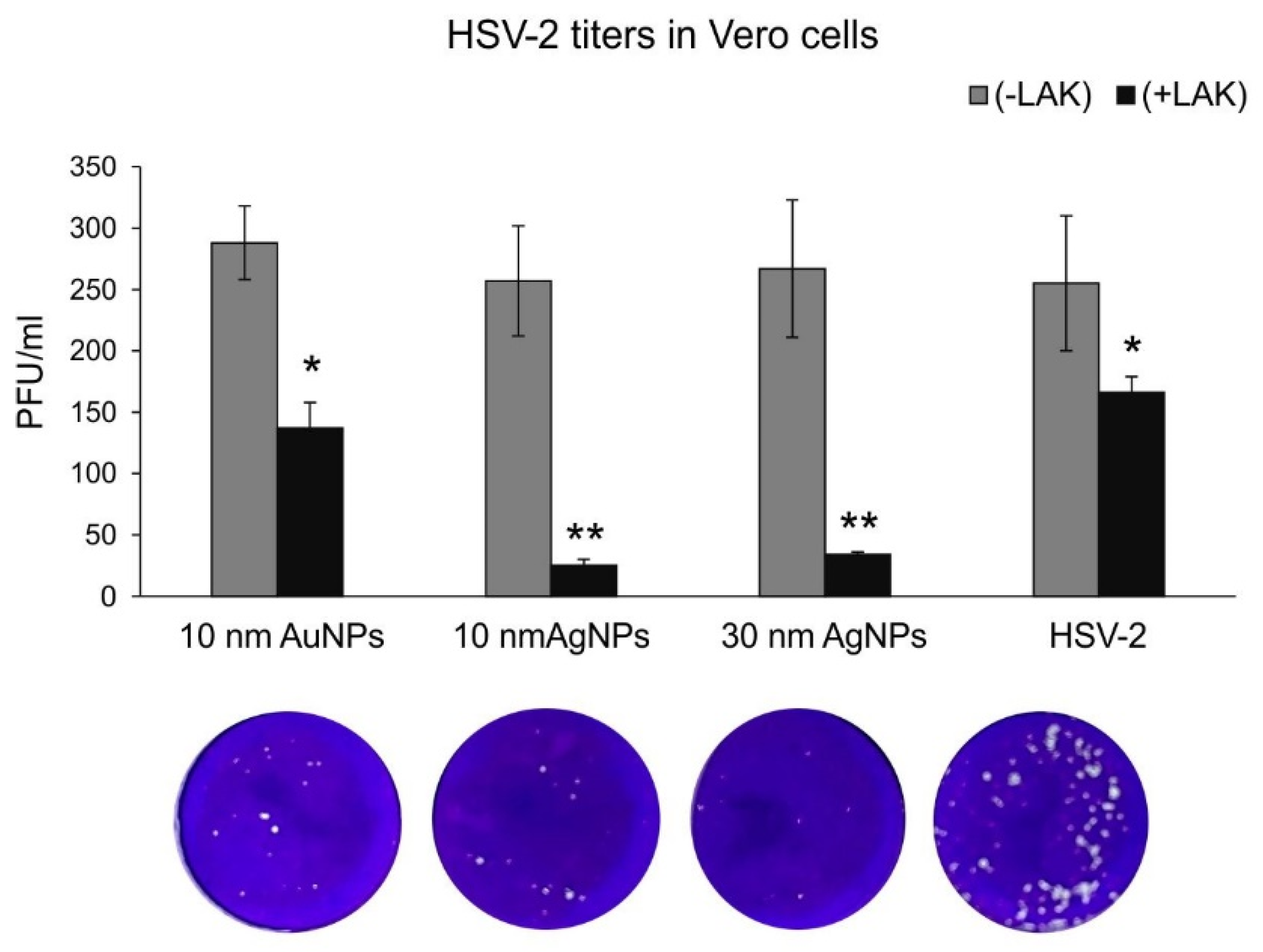
As lactoferrin inhibits different stages of HSV-2 infection, such as attachment, penetration, and cell-to-cell spread of HSV-2, we decided to first perform classical plaque reduction assay in Vero cells (
Figure 2). Functionalized silver nanoparticles appeared the most effective in comparison to 10 nm AuNPs (
Figure 2). As this assay was performed first by incubating virus with functionalized nanoparticles in RT, we suggest that the presence of silver metal may provide better local environment for interactions between HSV-2 and lactoferrin present on the surface of a metal nanoparticle. Antiviral effects of several cationic peptides, such as LF is dependent on their structure. LF has a relative high net positive charge and its N-terminal region is extremely basic [
23]. Therefore, the binding of silver lactoferrin conjugates to the virus surface may be stronger and less prone to dissociation. More research is necessary to decipher the nature of lactoferrin–nanoparticle interaction. Nanoparticles can bind a variety of proteins, forming so called an interfacial corona that provides biological identity and activity to nanoparticles. Kayak et al. demonstrated that adsorption of bovine lactoferrin (BLf) onto AgNP interface is driven by van der Waals interactions and hydrogen bonds, without any significant effect on the protein conformation and stability [
24]. BLf also ameliorated the particle-mediated cytotoxicity [
24].
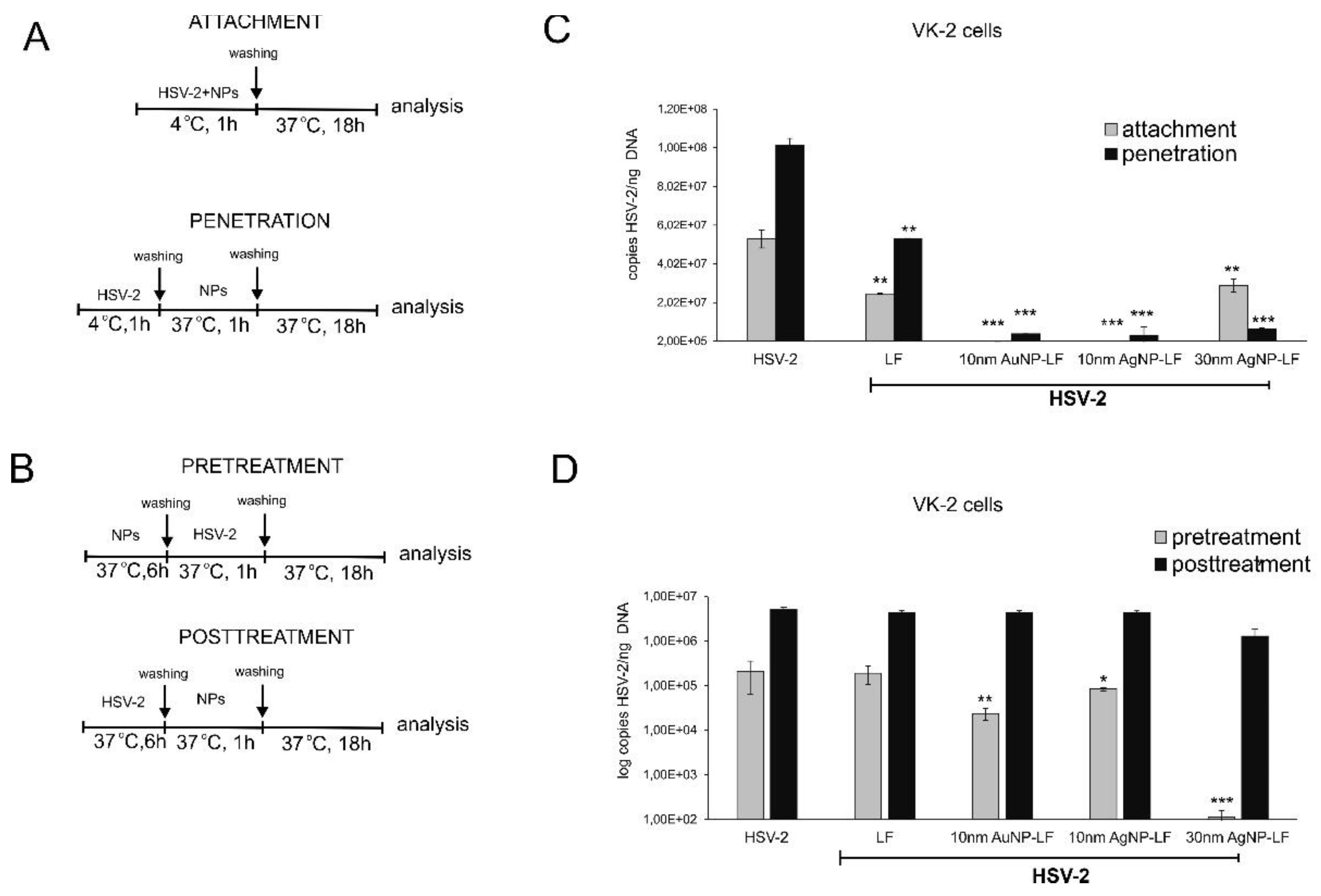
Lactoferrin inhibits HSV infection by interfering with virus binding to the target cells but also disturbs intracellular trafficking of HSV virions [
25]. We decided to use two different cells lines in order to compare if conjugates can work better than lactoferrin itself in inhibiting of virus attachment and penetration to human keratinocytes. We chose human HaCaT keratinocytes, commonly used to grow HSV and human vaginal VK-2-E6/E7 keratinocytes representing the target cells for HSV-2. Surprisingly, lactoferrin significantly blocked both attachment and penetration of HSV-2 to vaginal keratinocytes rather than to skin keratinocytes (
Figure 3 and
Figure 4). All lactoferrin conjugates showed very efficient inhibition of HSV-2 attachment and penetration in vaginal keratinocytes (except for 30 nm AgNPs, showing the same inhibition of penetration as lactoferrin itself) but also very good inhibition properties for HaCaT cells in comparison to lactoferrin (
Figure 3 and
Figure 4). These results prove that lactoferrin conjugates with noble metal nanoparticles can be better antivirals than peptides, especially for cells insensitive to LF activity.
To further check if lactoferrin conjugates with noble metal nanoparticles can improve inhibition of cell-to-cell spread by lactoferrin, we incubated the infected keratinocytes with conjugates but no significant inhibition was obtained. This is in contrast with previous results showing that lactoferrin can inhibit post-infection spread by interfering with viral glycoprotein complex (gE–gI) [
4] or with intracellular pathways [
4,
6]. Therefore, we can assume that physical form of the conjugates can impede interaction of lactoferrin with glycoproteins involved in cell-to-cell spread or intracellular pathways.
However, we observed a very interesting effect of pre-treatment with lactoferrin conjugates, which led to significant decrease of HSV-2 infection in VK-2 cells with all tested conjugates and only for conjugated AgNPs in HaCaT cells. Such effect for lactoferrin has not been shown before. Upon cell treatment, lactoferrin is mainly found on the cell surface of cells expressing heparan. This may result from interactions with a highly specific receptor for LF, characterized on several mammalian cell types and tissues [
26], but the surface localization of LF is believed to be less important for its antiviral activity [
4]. We can speculate that lactoferrin in conjugates may still bind to LF receptors on the cell surface creating in this manner a kind of barrier preventing from HSV-2 infection. This is an additional, advantageous effect of lactoferrin conjugates, which can further contribute to protection of neighboring cells from infection.
There are numerous studies showing that lactoferrin protects against several, other than HSV-2, viruses. These studies were performed mostly in vitro but there are a few examples where lactoferrin has demonstrated a potent antiviral effect in vivo. For example, lactoferrin given intraperitoneally protects mice against enterovirus and murine cytomegalovirus [
27,
28]. When deposited in the conjunctival sac it could protect mice against ocular HSV-1 infection [
29]. On the other hand, lactoferricin but not lactoferrin was a potent inhibitor of HSV-2 infection in a mouse mode of genital herpes [
30].
Here, we found that treatment with lactoferrin in 24, 48, and 72h post infection resulted in significant reduction of virus titers both at 3 and 7 day post infection in the vaginal lavages and tissue, respectively (
Figure 5). Lactoferrin-functionalized nanoparticles reduced viral titers better than lactoferrin, also in the spinal cords (
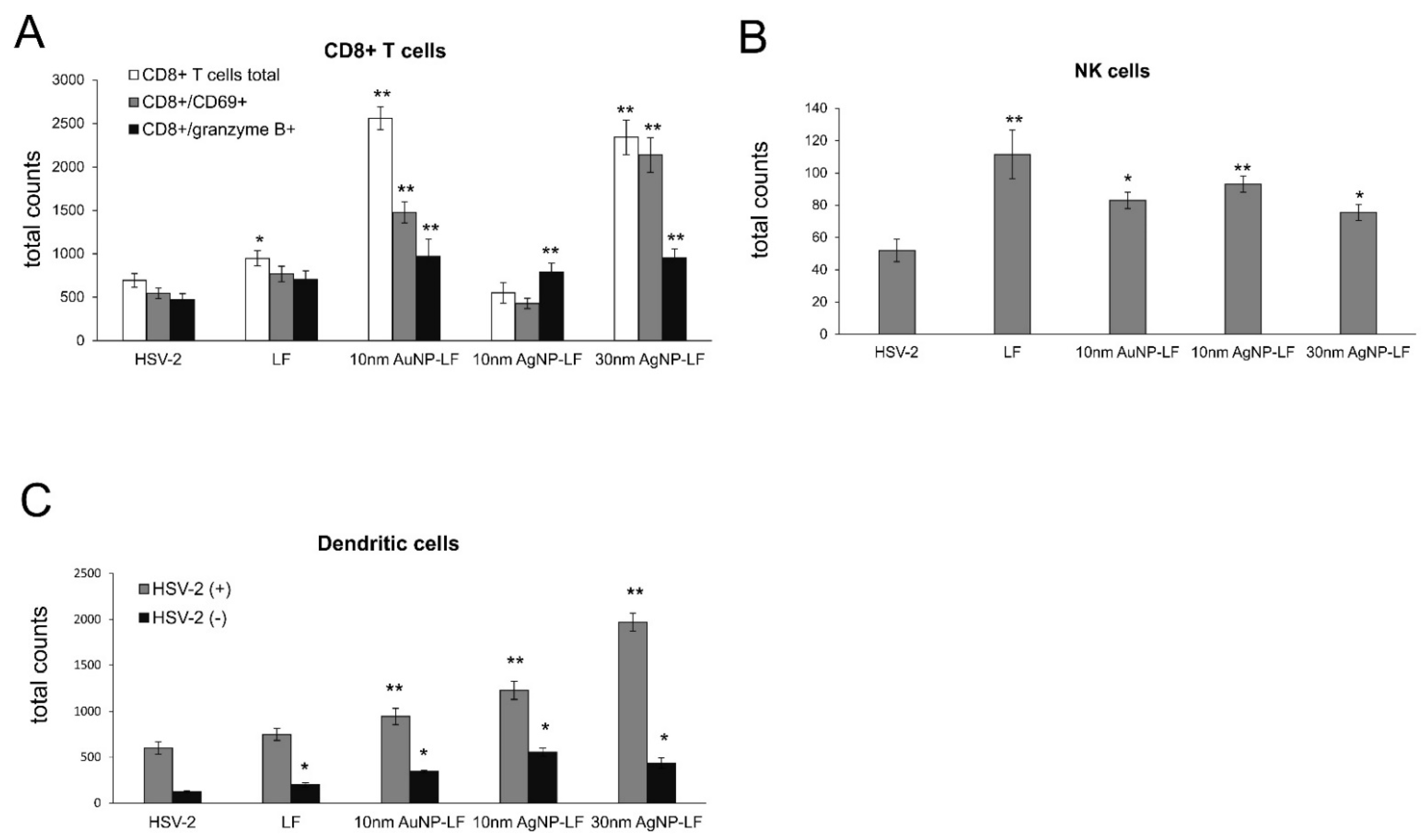
Figure 5), which makes them promising microbicides. Previously, we demonstrated that tannic acid-modified AgNPs stimulate migration of dendritic cells (DCs) into the vaginal tissue not only early during primary infection but also later, upon re-challenge with the virus [
17]. This helped to present the virus antigens both to naïve cytotoxic T cells in the lymph nodes and to memory T cells resulting in the activation of the specific antiviral response [
18]. In this study, we also observed significant migration of DCs to both infected and uninfected vaginal tissues stimulated with lactoferrin-modified NPs. It is possible that functionalized nanoparticles can retain lactoferrin present in the vaginal tissues for longer times, thus helping to stimulate both innate antiviral response to HSV-2 (NK cells) and virus-specific CD8+ T cell response (
Figure 6). Furthermore, while lactoferrin and lactoferrin-modified nanoparticles differed in the amount of activated innate antiviral response, only nanoparticle conjugates with lactoferrin were able to significantly activate cytotoxic CD8+/granzyme B+ T cells. As shown previously, the aggregates of modified NPs with viral antigens may be better internalized and processed by antigen-presenting cells (APC), thus leading to better activation of immune competent cells downstream. The role of silver and gold nanoparticles as adjuvants for infectious agents is attracting more and attention as well as possible practical uses in vaccines [
31,
32].
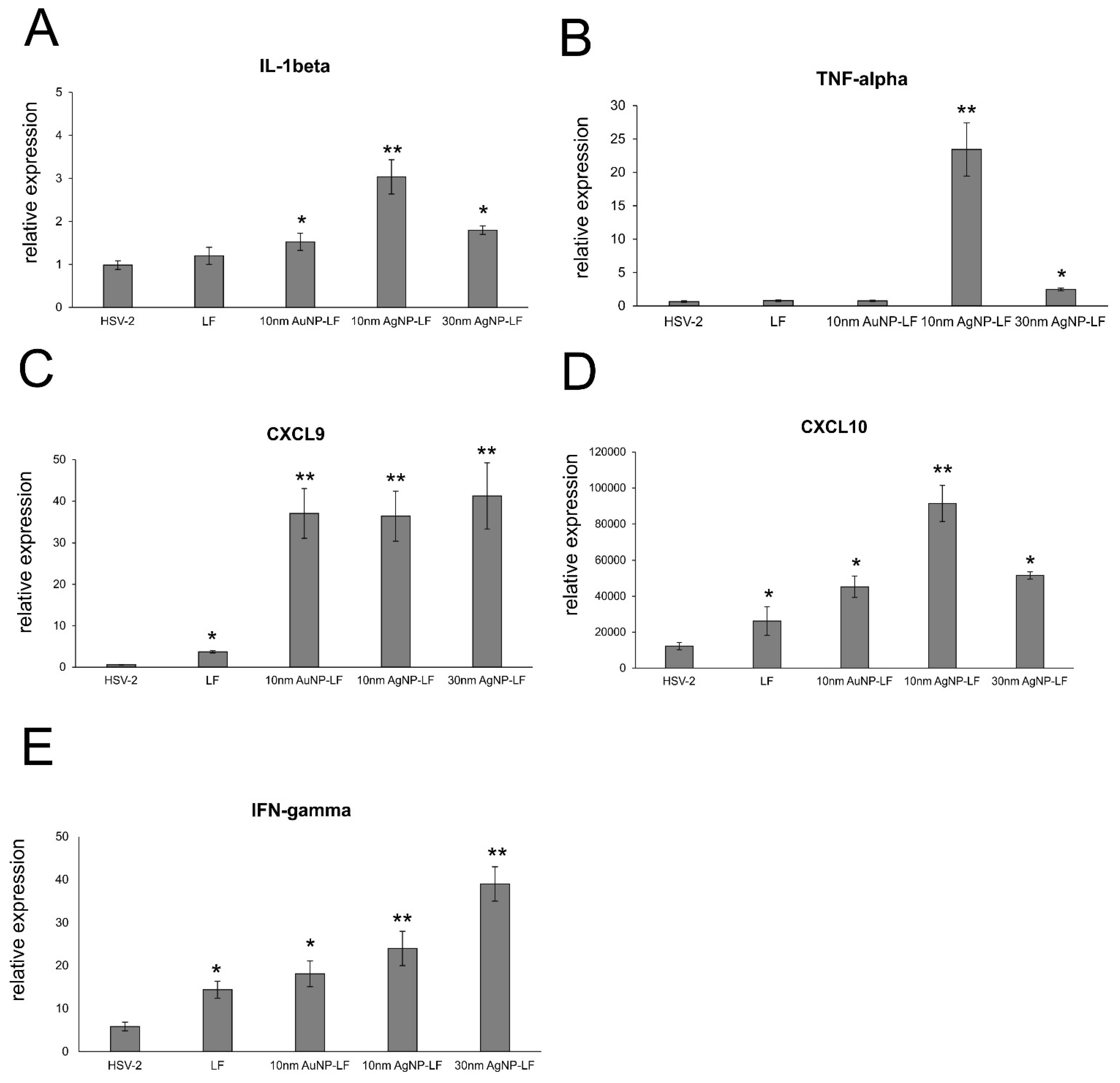
Lactoferrin is able to induce chemokine responses in the genital tract, such as CCL5, one of the major T cell- and NK cell-attracting chemokines [
30]. Here, we found that while lactoferrin induced upregulation of CXCL9, CXCL10, and IFN-γ in the vaginal tissues, lactoferrin functionalized nanoparticles induced several times more expression of these chemoattractants for NK cells as well as CD8+ T cells [
33], further reflected by increased NK and T cell numbers in the vaginal tissues (
Figure 6). Taking into account a well-known fact that small AgNPs can induce cellular toxicity, we also checked for production of inflammatory cytokines, such as IL-1β and TNF-α. The vaginal tissued treated with lactoferrin functionalized 10 nm AgNPs showed significant increase of these two cytokines (
Figure 7). TNF-α and IL-1 β are cytokines important for mounting a proper adaptive antiviral response to HSV-2 [
34,
35]. TNF-α can also induce CCL2 expression and mobilize monocytes/macrophages and T cells through the chemokine receptor CCR2 [
35]. However, it is also important to remember that excessive production of proinflammatory cytokines can amplify inflammation triggered by the virally infected cells and result in deregulation of the antiviral response [
36]. Therefore, certain caution should be taken when planning in vivo treatment with AgNPs sized < 10 nm.