Testing the Accuracy of a Bedside Screening Tool Framework to Clinical Records for Identification of Patients at Risk of Malnutrition in a Rural Setting: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Analysis
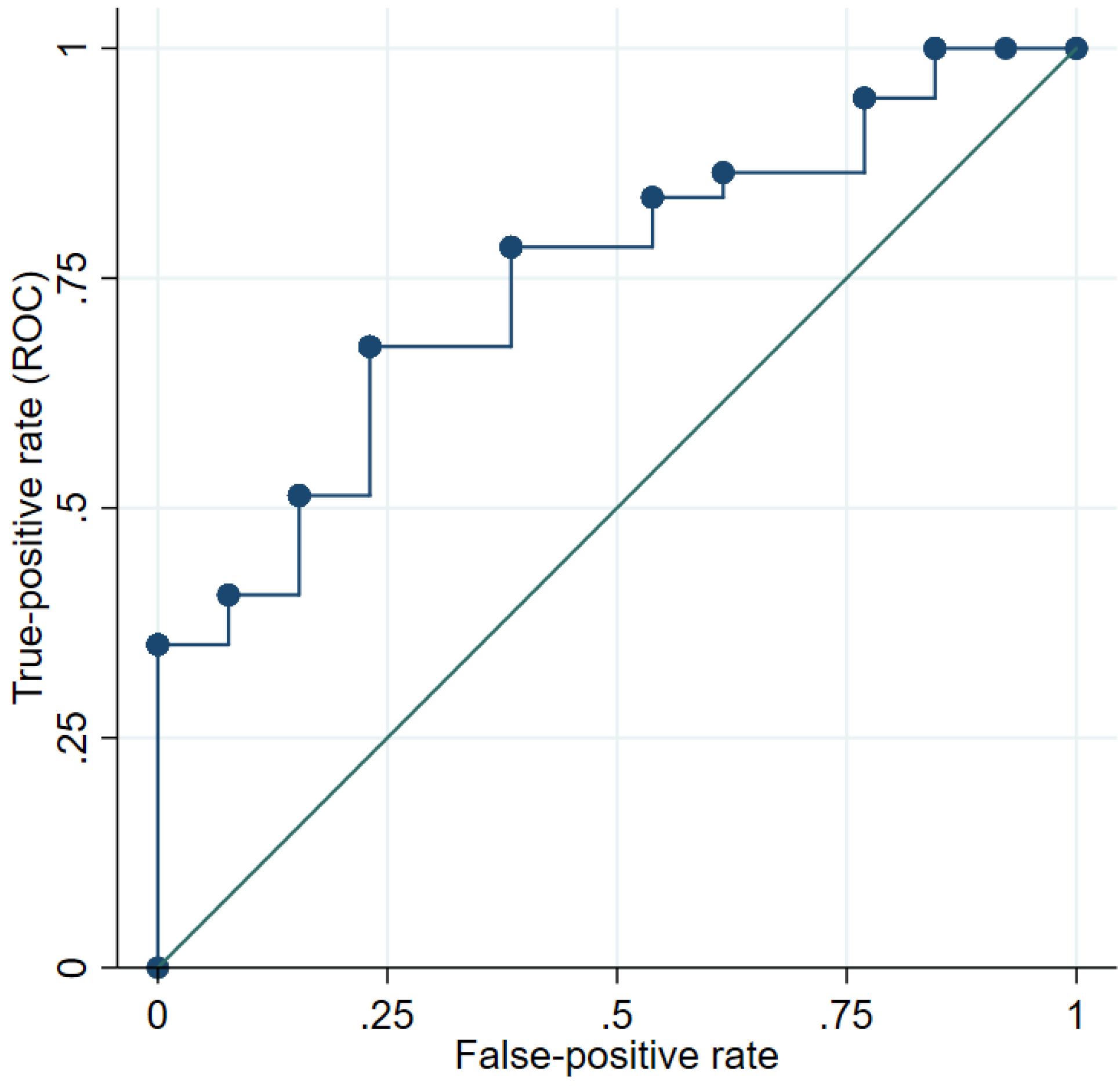
3. Results
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Geiker, N.R.W.; Larsen, S.M.H.; Stender, S.; Astrup, A. Poor performance of mandatory nutritional screening of in-hospital patients. Clin. Nutr. 2012, 31, 862–867. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Marques-Vidal, P. Impact of nutritional risk screening in hospitalized patients on management, outcome and costs: A retrospective study. Clin. Nutr. 2016, 35, 1340–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, E.; Ferguson, M.; Banks, M.; Batterham, M.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: Results from the Nutrition Care Day Survey 2010. Clin. Nutr. 2013, 32, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital Malnutrition: Prevalence, Identification and Impact on Patients and the Healthcare System. Int. J. Environ. Res. Public Health 2011, 8, 514–527. [Google Scholar] [CrossRef] [Green Version]
- Marco, J.; Barba, R.; Zapatero, A.; Matía, P.; Plaza, S.; Losa, J.E.; Canora, J.; De Casasola, G.G. Prevalence of the notification of malnutrition in the departments of internal medicine and its prognostic implications. Clin. Nutr. 2011, 30, 450–454. [Google Scholar] [CrossRef]
- Tappenden, K.A.; Quatrara, B.; Parkhurst, M.L.; Malone, A.M.; Fanjiang, G.; Ziegler, T.R. Critical role of nutrition in improving quality of care: An interdisciplinary call to action to address adult hospital malnutri-tion. J. Parenter. Enter. Nutr. 2013, 37, 482–497. [Google Scholar] [CrossRef]
- Bell, J.J.; Young, A.; Hill, J.; Banks, M.; Comans, T.; Barnes, R.; Keller, H.H. Rationale and developmental methodology for the SIMPLE approach: A Systematised, Interdisciplinary Malnutrition Pathway for impLementation and Evaluation in hospitals. Nutr. Diet. 2018, 75, 226–234. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 10, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Elia, M.; Zellipour, L.; Stratton, R. To screen or not to screen for adult malnutrition? Clin. Nutr. 2005, 24, 867–884. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Capra, S.; Ferguson, M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur. J. Clin. Nutr. 2002, 56, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Bohringer, E.; Brown, L. Nutrition Screening and Referrals in Two Rural Australian Oncology Clinics. Food Nutr. Sci. 2016, 7, 1070–1081. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.S. Electronic Health Records: Then, Now, and in the Future. Yearb. Med. Inform. 2016, 25, S48–S61. [Google Scholar] [CrossRef]
- Litzelman, D.; Dittus, R.S.; Miller, M.E.; Tierney, W.M. Requiring physicians to respond to computerized reminders improves their compliance with preventive care protocols. J. Gen. Intern. Med. 1993, 8, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Woodward, T.; Josephson, C.; Ross, L.; Hill, J.; Hosking, B.; Naumann, F.; Stoney, R.; Palmer, M. A retrospective study of the incidence and characteristics of long-stay adult inpatients with hospital-acquired malnutrition across five Australian public hospitals. Eur. J. Clin. Nutr. 2020, 74, 1668–1676. [Google Scholar] [CrossRef]
- Larsen, B.M.; Luchak, M.; Prenoslo, L.; Wood, K.B.; Mazurak, V. Indicators of Pediatric Malnutrition in a Tertiary Care Hospital. Can. J. Diet. Pract. Res. 2014, 75, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Alston, L.; Green, M.; Versace, V.L.; Bolton, K.A.; Widdicombe, K.; Buccheri, A.; Imran, D.; Allender, S.; Orellana, L.; Nichols, M. Profiling Malnutrition Prevalence among Australian Rural In-Patients Using a Retrospective Census of Electronic Medical Files over a 12-Month Period. Int. J. Environ. Res. Public Health 2020, 17, 5909. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Rural & Remote Health Canberra, Australia: Australian Institute of Health and Welfare. 2018. Available online: https://www.aihw.gov.au/reports/australias-health/rural-and-remote-health (accessed on 3 April 2020).
- Bourke, L.; Humphreys, J.S.; Wakerman, J.; Taylor, J. Understanding drivers of rural and remote health outcomes: A conceptual framework in action. Aust. J. Rural Health 2012, 20, 318–323. [Google Scholar] [CrossRef]
- Alston, L.; Allender, S.; Peterson, K.; Jacobs, J.; Nichols, M. Rural Inequalities in the Australian Burden of Ischaemic Heart Disease: A Systematic Review. Hear. Lung Circ. 2017, 26, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Alston, L.; Partridge, S.R. Limited dietary interventions in rural Australian communities: A systematic review. Nutr. Diet. 2020, 78, 57–68. [Google Scholar] [CrossRef]
- MacLeod, M.L.P.; Stewart, N.J.; Kulig, J.C.; Anguish, P.; Andrews, M.E.; Banner, D.; Garraway, L.; Hanlon, N.; Karunanayake, C.; Kilpatrick, K.; et al. Nurses who work in rural and remote communities in Canada: A national survey. Hum. Resour. Heal. 2017, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pt Global. Patient-Generated Subjective Global Assessment (PG-SGA©) Pt Global. 2014. Available online: https://pt-global.org/pt-global (accessed on 11 March 2021).
- Ottery, F.D. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition 1996, 12, S15–S19. [Google Scholar] [CrossRef]
- StataCorp. STATA Software 2019. Available online: https://www.stata.com/ (accessed on 12 December 2021).
- Gong, Y.; Zhou, H.; Zhang, Y.; Zhu, X.; Wang, X.; Shen, B.; Xian, J.; Ding, Y. Validation of the 7-item Generalized Anxiety Disorder scale (GAD-7) as a screening tool for anxiety among pregnant Chinese women. J. Affect. Disord. 2021, 282, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Australian Digital Health Agency. Australia’s National Digital Health Strategy Australia: Australian Federal Government. 2021. Available online: https://www.digitalhealth.gov.au/sites/default/files/2020-11/Australia%27s%20National%20Digital%20Health%20Strategy%20-%20Safe%2C%20seamless%20and%20secure.pdf (accessed on 24 November 2021).
- Nature. The Future of Electronic Health Records: Nature. 2019. Available online: https://www.nature.com/articles/d41586-019-02876-y (accessed on 24 November 2021).
- Vivanti, A.; Lee, H.-C.; Palmer, M. Capitalising on opportunities: Malnutrition coding in hospital before and after the introduction of electronic health records with an embedded malnutrition screening tool. Clin. Nutr. ESPEN 2021, 41, 193–197. [Google Scholar] [CrossRef]
- McCamley, J.; Vivanti, A.; Edirippulige, S. Dietetics in the digital age: The impact of an electronic medical record on a tertiary hospital dietetic department. Nutr. Diet. 2019, 76, 480–485. [Google Scholar] [CrossRef]
| Patient Characteristic | Sample n = 50 |
|---|---|
| Mean Age (SD) (years) | 70.6 (14.9) |
| Median LoS (range) (days) | 5.0 (3–19) |
| Female N (%) | 31 (62%) |
| Proportion residing in the main rural township N (%) | 39 (78%) |
| Nutrition symptoms documented in record N (%) | |
| Presence of malnutrition risk conditions (cancer, AIDs, pulmonary cachexia, cardiac cachexia, open wound or trauma) | 16 (32%) |
| Loss of appetite | 14 (28%) |
| Early satiety | 1 (2%) |
| Poor taste | 1 (2%) |
| Mouth sores | 1 (2%) |
| Nausea | 8 (16%) |
| Constipation | 16 (32%) |
| Vomiting | 6 (12%) |
| Swallowing difficulties | 1 (2%) |
| Malnutrition screening results | |
| Mean bedside score (range) | 8.0 (1–29) |
| Mean EMR score (range) | 7.9 (0–22) |
| At risk of malnutrition based on bedside score (95% CI) (PG SGA score >4) | 74% (59–84%) |
| At risk based on EMR score (95% CI) (PG SGA score >4) | 80% (66–89%) |
| Cutpoint (Ideal > 4) | Sensitivity | Specificity | Correctly Classified | Youden Index |
|---|---|---|---|---|
| (≥0) | 100.0% | 0.0% | 74.0% | 0.0 |
| (≥1) | 100.0% | 7.7% | 76.0% | 0.08 |
| (≥2) | 100.0% | 15.4% | 78.0% | 0.15 |
| (≥3) | 94.6% | 23.1% | 76.0% | 0.18 |
| (≥4) | 86.5% | 38.5% | 74.0% | 0.25 |
| (≥5) | 83.8% | 46.2% | 74.0% | 0.30 |
| (≥6) | 78.4% | 61.5% | 74.0% | 0.40 |
| (≥7) | 67.6% | 76.9% | 70.0% | 0.45 |
| (≥8) | 51.4% | 76.9% | 58.0% | 0.28 |
| (≥9) | 51.4% | 84.6% | 60.0% | 0.36 |
| (≥10) | 40.5% | 92.3% | 54.0% | 0.33 |
| (≥11) | 35.1% | 100.0% | 52.0% | 0.35 |
| (≥12) | 27.0% | 100.0% | 46.0% | 0.27 |
| (≥14) | 18.9% | 100.0% | 40.0% | 0.19 |
| (≥15) | 13.5% | 100.0% | 36.0% | 0.14 |
| (≥16) | 10.8% | 100.0% | 34.0% | 0.11 |
| (≥18) | 5.4% | 100.0% | 30.0% | 0.05 |
| (≥22) | 2.7% | 100.0% | 28.0% | 0.03 |
| (>22) | 0.0% | 100.0% | 26.0% | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alston, L.; Green, M.; Nichols, M.; Partridge, S.R.; Buccheri, A.; Bolton, K.A.; Versace, V.L.; Field, M.; Launder, A.J.; Lily, A.; et al. Testing the Accuracy of a Bedside Screening Tool Framework to Clinical Records for Identification of Patients at Risk of Malnutrition in a Rural Setting: An Exploratory Study. Nutrients 2022, 14, 205. https://doi.org/10.3390/nu14010205
Alston L, Green M, Nichols M, Partridge SR, Buccheri A, Bolton KA, Versace VL, Field M, Launder AJ, Lily A, et al. Testing the Accuracy of a Bedside Screening Tool Framework to Clinical Records for Identification of Patients at Risk of Malnutrition in a Rural Setting: An Exploratory Study. Nutrients. 2022; 14(1):205. https://doi.org/10.3390/nu14010205
Chicago/Turabian StyleAlston, Laura, Megan Green, Melanie Nichols, Stephanie R. Partridge, Alison Buccheri, Kristy A. Bolton, Vincent L. Versace, Michael Field, Ambrose J. Launder, Amy Lily, and et al. 2022. "Testing the Accuracy of a Bedside Screening Tool Framework to Clinical Records for Identification of Patients at Risk of Malnutrition in a Rural Setting: An Exploratory Study" Nutrients 14, no. 1: 205. https://doi.org/10.3390/nu14010205






