The Effects of Foliar Application of Phenoxy and Imidazoline Family Herbicides on the Limitation of Primary Photosynthetic Processes in Galega orientalis L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Cultivation
2.2. Application of Herbicides
2.3. Chlorophyll Fluorescence Measurements
2.4. Statistical Analysis
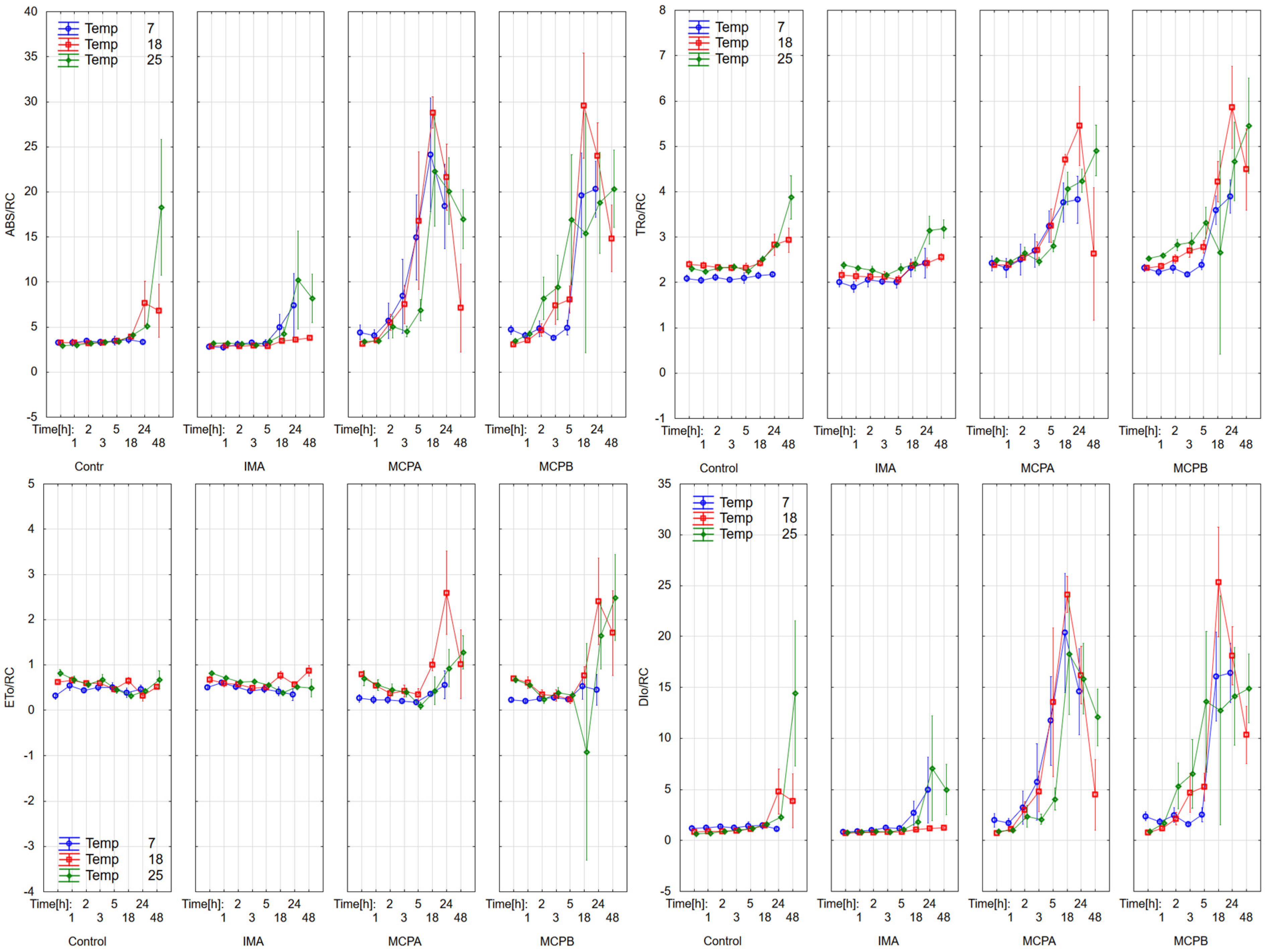
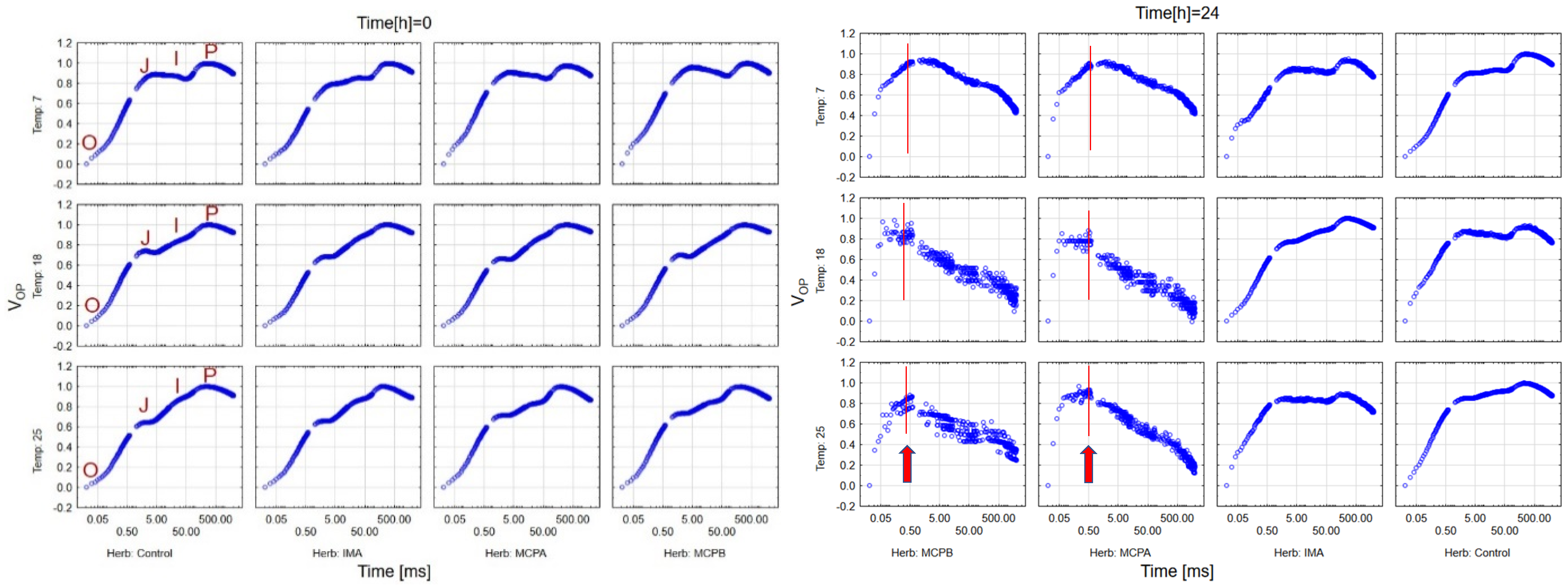
3. Results
3.1. Chlorophyll Fluorescence Parameters
3.2. Herbicide Effects on OJIP Shape
4. Discussion
4.1. Effects on OJIP Curve Shape
4.2. Effect on Chlorophyll Fluorescence Parameters
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Christensen, M.G.; Teicher, H.B.; Streibig, J.C. Linking fluorescence induction curve and biomass in herbicide screening. Pest Manag. Sci. 2003, 59, 1303–1310. [Google Scholar] [CrossRef]
- Dayan, F.E.; Zaccaro, M.L.M. Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pestic. Biochem. Physiol. 2012, 201, 189–197. [Google Scholar] [CrossRef]
- Jursík, M.; Soukup, J.; Holec, J.; Andr, J. Herbicide mode of actions and symptoms of plant injury by herbicides: Plant growth regulator herbicides (synthetic auxins). Listy Cukrov. Reparske 2011, 127, 88–92. [Google Scholar]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Ann. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, K.; Kwiatkowski, J.; Tresch, S. Auxin herbicides induce H2O2 overproduction and tissue damage in cleavers (Galium aparine L.). J. Exp. Bot. 2001, 52, 1811–1816. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, K. Mode of action of auxin herbicides: A new ending to a long, drawn out story. Trends Plant Sci. 2000, 5, 506–508. [Google Scholar] [CrossRef]
- Hansen, K.; Grossmann, K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000, 124, 1437–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žaltauskaitė, J.; Kišonaitė, G. The effects of phenoxy herbicide MCPA on non-target vegetation in spring wheat (Triticum aestivum L.) culture. Biologija 2014, 60, 148–154. [Google Scholar] [CrossRef]
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- Norsworthy, J.K.; Ward, S.; Shaw, D.; Llewellyn, R.; Nichols, R.; Webster, T.; Barrett, M. Reducing the risks of herbicide resistance: Best management practices and recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-tolerant crops: History, current status and future. Pest Manag. Sci. 2005, 61, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Asad, M.A.U.; Lavoie, M.; Song, H.; Jin, Y.; Fu, Z.; Qian, H. Interaction of chiral herbicides with soil microorganisms, algae and vascular plants. Sci. Total Environ. 2017, 580, 1287–1299, ISSN 0048-9697. [Google Scholar] [CrossRef]
- Fedtke, C.; Duke, S.O. Herbicides. In Plant Toxicology; Hock, B., Elstner, E.F., Eds.; Marcel Dekker: New York, NY, USA, 2005; p. 648. [Google Scholar]
- Wang, H. A Method of high throughput monitoring crop physiology using chlorophyll fluorescence and multispectral imaging. Front. Plant Sci. 2018, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early identification of herbicide modes of action by the use of chlorophyll fluorescence measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Dubis, B.; Jankowski, K.J.; Mikołaj Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass yield and energy balance of fodder galega in different production technologies: An 11-year field experiment in a large-area farm in Poland. Renew. Energy 2020, 154, 813–825. [Google Scholar] [CrossRef]
- Adamovich, A. Productivity and yield quality of fodder galega (Galega orientalis Lam.)—Grass mixed swards. Plant Nutrition. Dev. Plant Soil Sci. 2001, 92, 1008–1009. [Google Scholar]
- Żarczyński, P.J.; Sienkiewicz, S.; Wierzbowska, J.; Krzebietke, S.J. Fodder galega—A versatile plant. Agronomy 2021, 11, 1797. [Google Scholar] [CrossRef]
- Vergun, O.; Shymanska, O.; Rakhmetov, D.; Grygorieva, O.; Ivanišová, E.; Brindza, J. Parameters of antioxidant activity of Galega officinalis L. and Galega orientalis Lam. (Fabaceae Lindl.) plant raw material. Slovak J. Food Sci. 2020, 14, 125–134. [Google Scholar]
- Baležentienė, L. Šiuliauskienė, D. Chlorophyll fluor escence estimation of fodder galega (Galega orientalis Lam.) in situ and dependence on different leaf rank and cultivars. Agron. Res. 2007, 5, 3–12. [Google Scholar]
- Shymanska, O.; Vergun, O.; Rakhmetov, J.; Fishchenko, V. The content of photosynthetic pigments in the leaves of Galega officinalis L. and Galega orientalis Lam. cultivars. In Agrobiodiversity for Improving Nutrition, Health and Life Quality; Slovak University of Agriculture: Nitra, Slovakia, 2017; pp. 398–403. [Google Scholar]
- Eryashev, A.P.; Eryashev, P.A. influence of pesticides and albite on the photosynthetic activity and seed yield of eastern galega (Galega orientalis). J. Pharm. Sci. Res. 2018, 10, 3422–3425. [Google Scholar]
- Strasser, B.J.; Strasser, R.J. Measuring fast fluorescence transients to address environmental questions: The JIP test. In Photosynthesis: From Light to Biosphere; Mathis, P., Ed.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1995; Volume 5, pp. 977–980. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G., Govinjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.; Lazár, D.; Kromdijk, J.; Govindjee, G. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Matoušková, M.; Nauš, J.; Flašarová, M. A long-term response of chlorophyll fluorescence induction to one-shot application of cyanazine on barley plants and its relation to crop yield. Photosynthetica 1999, 37, 281–294. [Google Scholar] [CrossRef]
- Abbaspoor, M.; Streibig, J. Clodinafop changes the chlorophyll fluorescence induction curve. Weed Sci. 2005, 53, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Hu, H.; Song, X.; Ma, Y.; Yan, H. Novel ammonium dichloroacetates with enhanced herbicidal activity for weed control. RSC Adv. 2020, 10, 44512–44521. [Google Scholar] [CrossRef]
- Hess, F.D. Light-dependent herbicides: An overview. Weed Sci. 2000, 48, 160–170. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ohki, S.; Koizumi, K.; Tanaka, A.; Watanabe, H.; Kohno, H.; Van Rensen, J.J.S.; Böger, P.; Wakabayashi, K. Binding site of novel 2-benzylamino-4-methyl-6-trifluoromethyl-1,3,5-triazine herbicides in the D1 protein of Photosystem II. Photosynth. Res. 2003, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Battaglino, B.; Grinzato, A.; Pagliano, C. Binding properties of photosynthetic herbicides with the QB Site of the D1 protein in plant photosystem II: A combined functional and molecular docking study. Plants 2021, 10, 1501. [Google Scholar] [CrossRef]
- Stirbet, A.; Riznichenko, G.; Rubin, A.B. Govindjee. Modeling chlorophyll a fluorescence transient: Relation to photosynthesis. Biochemistry 2014, 79, 291–323. [Google Scholar]
- Porheidar, G.S.; Rahimian, M.H.; Alizadeh, H.; Hassannejad, S. Effect of different herbicides and salicylic acid treatment on the photosynthetic efficiency of corn cultivars using chlorophyll a fluorescence transient curve. J. Plant Physiol. Breed. 2017, 7, 21–40. [Google Scholar]
- Havaux, M. Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci. 1993, 94, 19–33. [Google Scholar] [CrossRef]
- Yamane, Y.; Shikanai, T.; Kashino, Y.; Koike, H.; Satoh, K. Reduction of QA in the dark: Another cause of fluorescence Fo increases by high temperatures in higher plants. Photosynth. Res. 2000, 63, 23–34. [Google Scholar] [CrossRef]
- Fufezan, C.; Rutherford, A.W.; Krieger-Liszkay, A. Singlet oxygen production in herbicide-treated photosystem II. FEBS Lett. 2002, 532, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.; Costa, A.; Pereira Alves, R.; Megguer, C. Chlorophyll fluorescence as an indicator of cellular damage by glyphosate herbicide in Raphanus sativus L. plants. Am. J. Plant Sci. 2014, 5, 2509–2519. [Google Scholar] [CrossRef] [Green Version]
- Hiraki, M.; Rensen, J.J.S.V.; Vredenberg, W.J.; Wakabayashi, K. Characterization of the alterations of the chlorophyll a fluorescence induction curve after addition of photosystem II inhibiting herbicides. Photosynth. Res. 2003, 78, 35–46. [Google Scholar] [CrossRef]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, Noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Kalkhoran, E.S.; Alebrahim, M.T.; Abad, H.R.M.C.; Streibig, J.C.; Ghavidel, A.; Tseng, T.-M.P. The joint action of some broadleaf herbicides on potato (Solanum tuberosum L.) weeds and photosynthetic performance of potato. Agriculture 2021, 11, 1103. [Google Scholar] [CrossRef]
- Zhang, C.J.; Lim, S.H.; Kim, J.W.; Nah, G.; Fischer, A.; Kim, D.S. Leaf chlorophyll fluorescence discriminates herbicide resistance in Echinochloa species. Weed Res. 2016, 56, 424–433. [Google Scholar] [CrossRef]
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haworth, M.; Marino, G.; Brunetti, C.; Killi, D.; De Carlo, A.; Centritto, M. The impact of heat stress and water deficit on the photosynthetic and stomatal physiology of olive (Olea europaea L.)—A case study of the heat wave. Plants 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednaříková, M.; Váczi, P.; Lazár, D.; Barták, M. Photosynthetic performance of Antarctic lichen Dermatocarpon polyphyllizum when affected by desiccation and low temperatures. Photosynth. Res. 2020, 145, 159–177. [Google Scholar] [CrossRef]
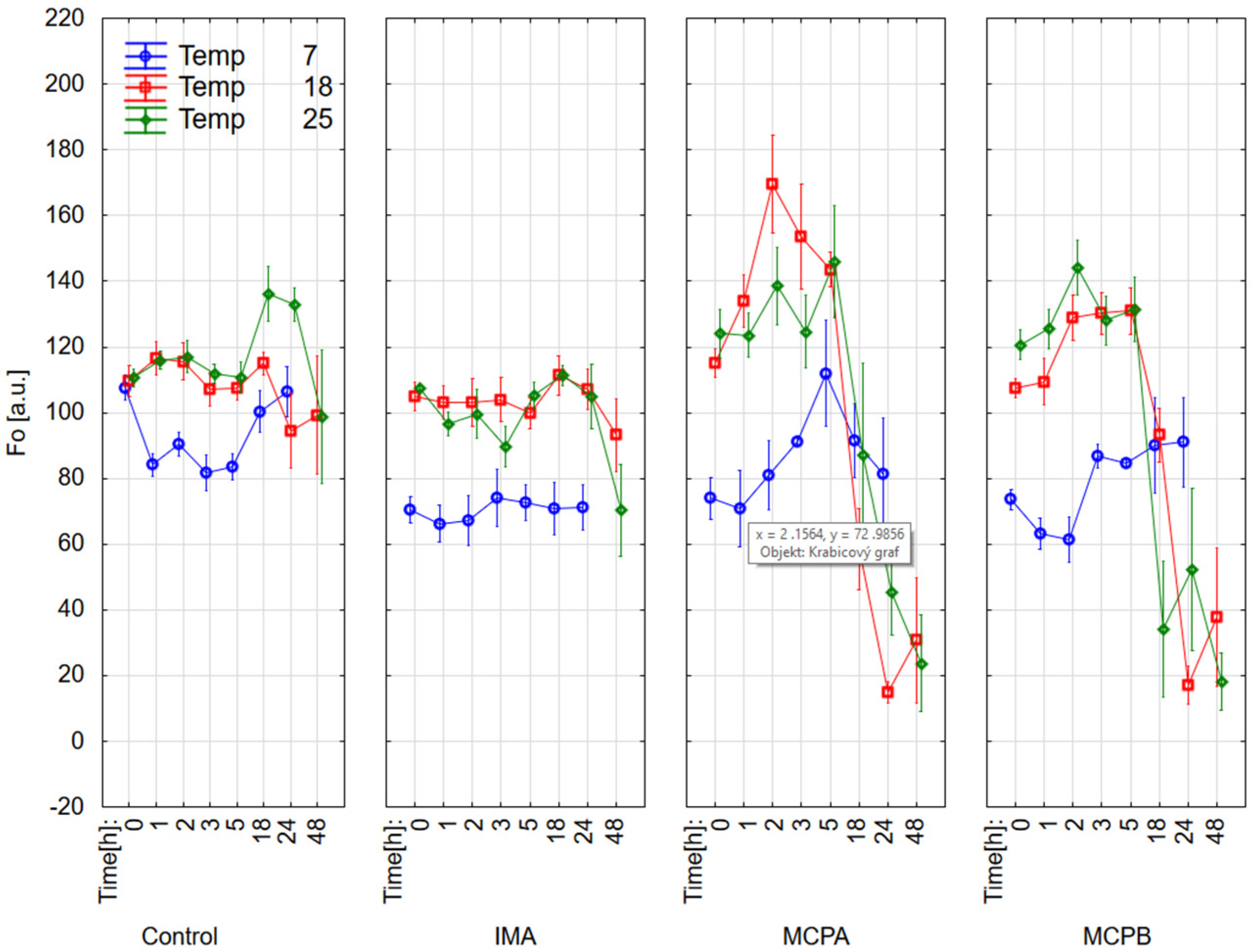
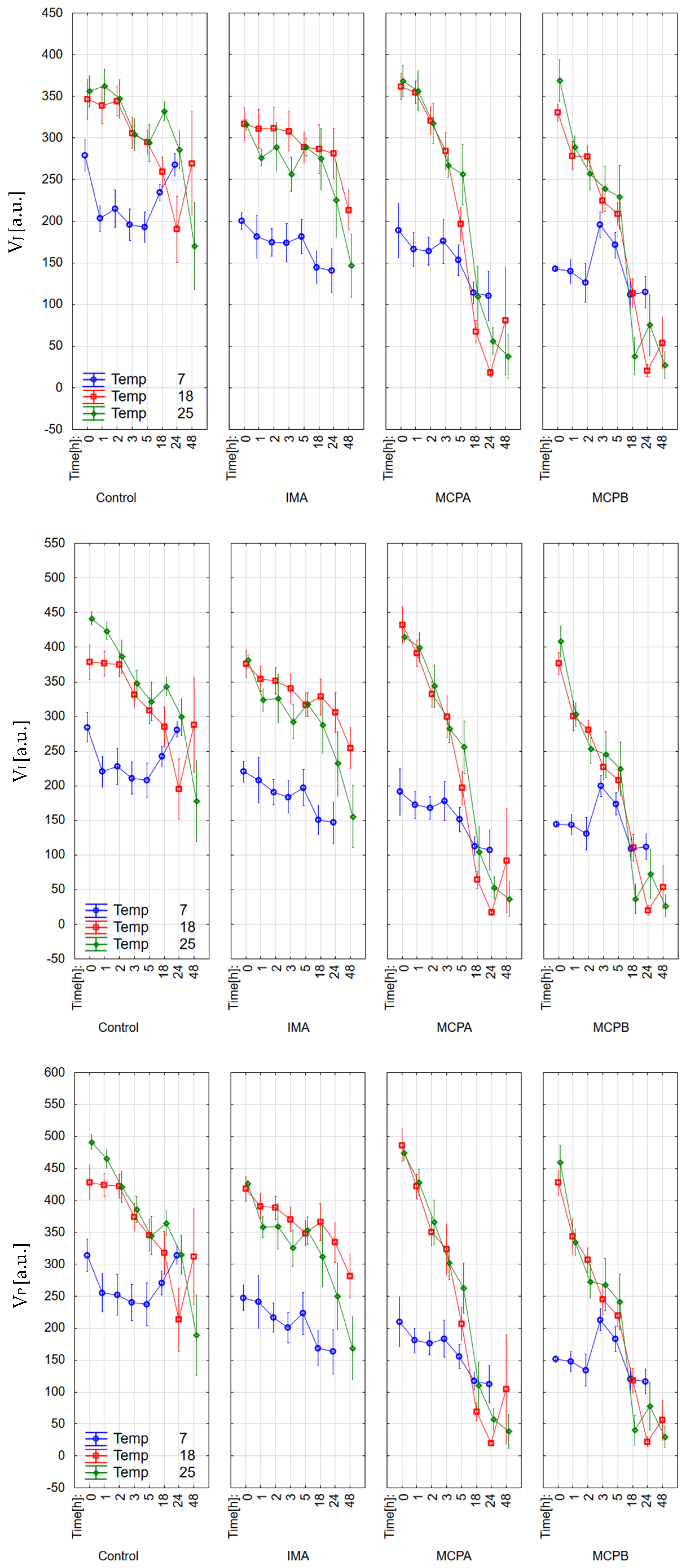
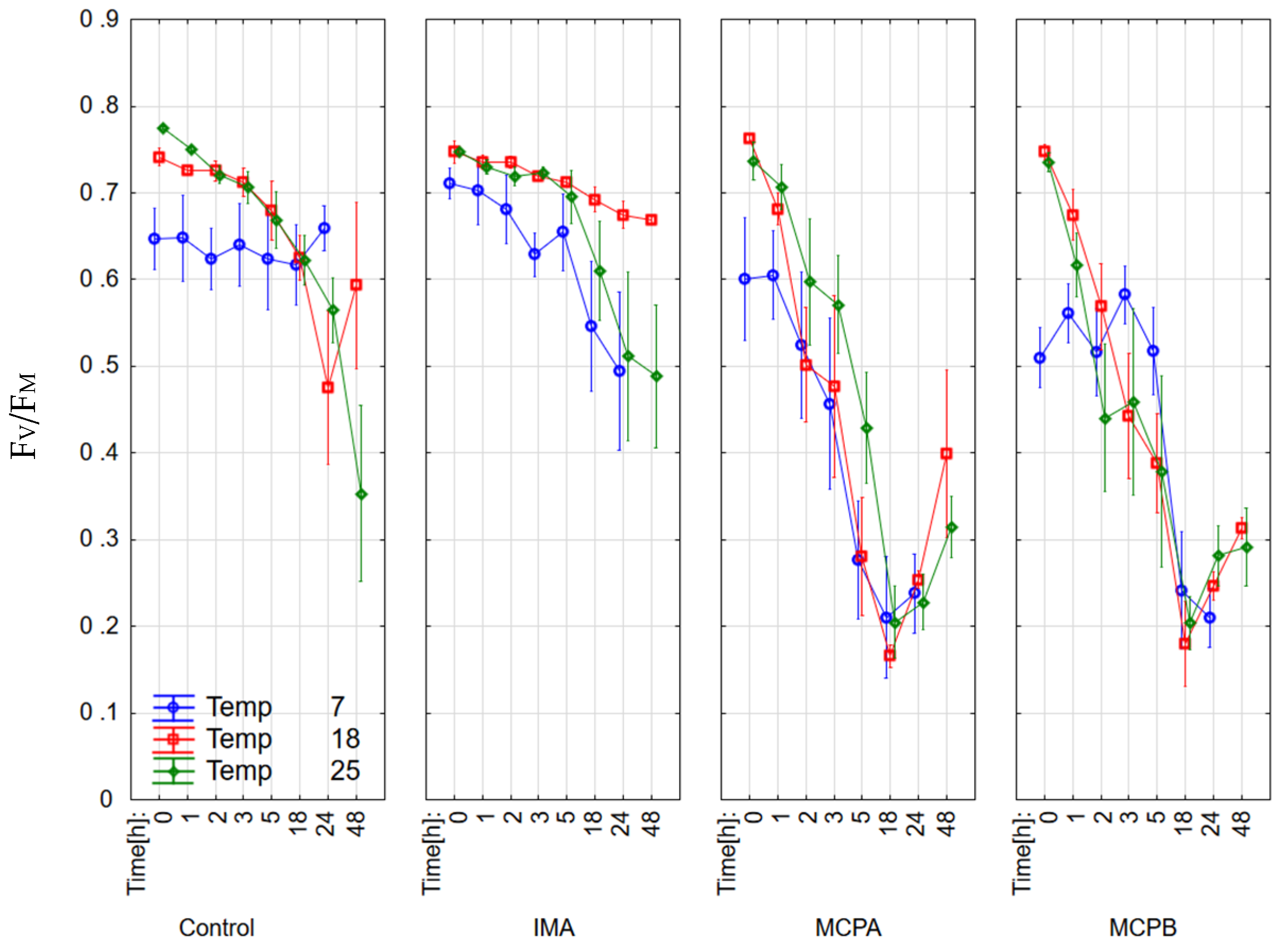

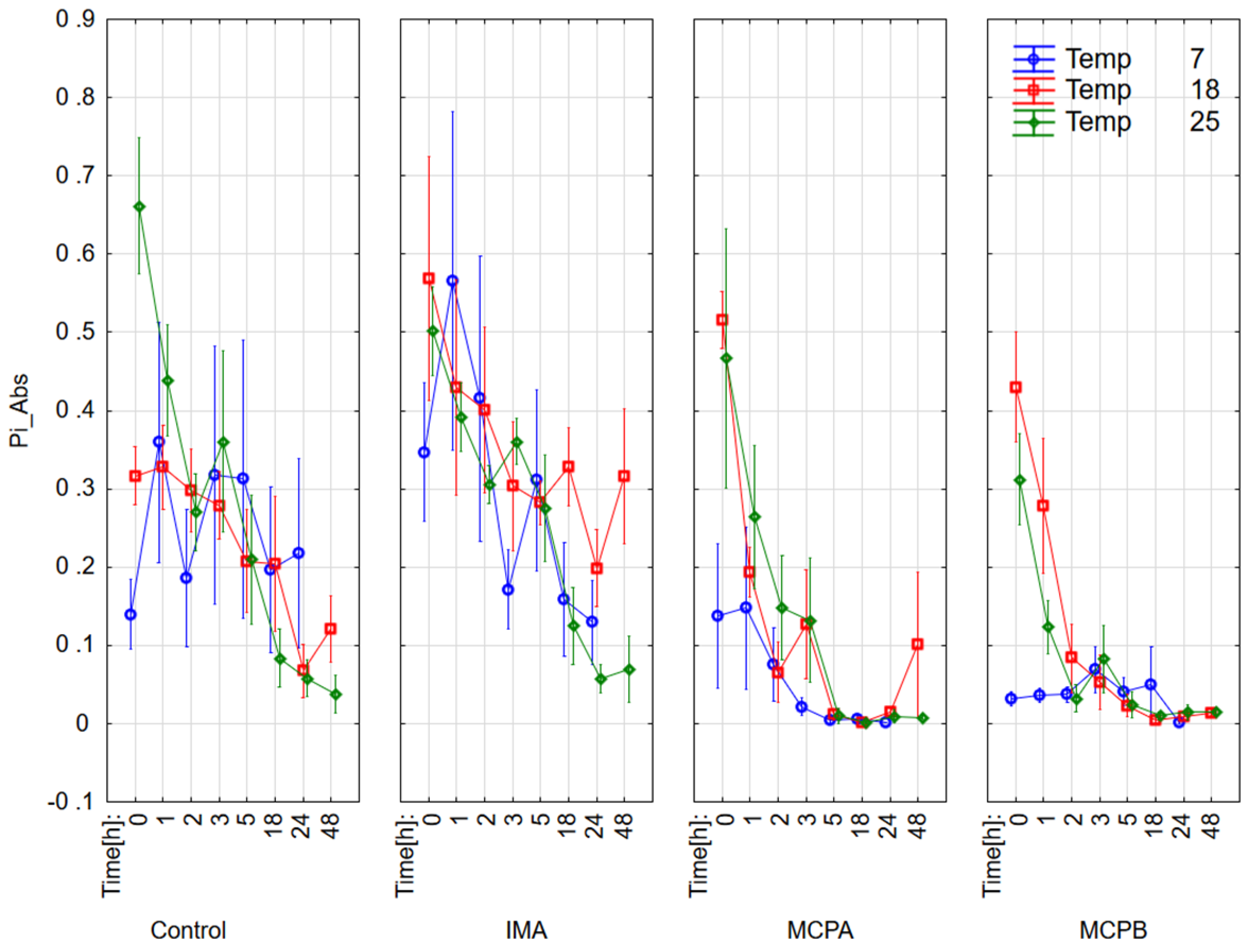


| Parameter | Meaning |
|---|---|
| F0 | Minimal ChlF (all PSII RCs are assumed to be open) |
| FV /FM | Maximal quantum yield of PSII photochemistry |
| VJ = FJ | ChlF in point J |
| VI = FI | ChlF in point I |
| VP = FP | ChlF in point P |
| ABS/RC = M0(1/VJ)(1/Phi_P0) | Absorption flux (of antenna chlorophylls) per RC |
| TR0/RC = M0(1/VJ) | Trapped energy flux (leading to QA reduction) per RC |
| ET0/ RC = M0(1/VJ)Psi_E0 | Electron transport flux (further than QA) per RC |
| DI0/RC = (ABS/RC) − (TR0/RC) | The flux of dissipated excitation energy |
| Phi_D0 = 1 − Phi_P0 = F0/FM | Quantum yield of energy dissipation |
| Phi_E0 = ET0/ABS = [1 − (F0/FM)]ΨE0 | Quantum yield for electron transport from QA to PQ pool (ET) |
| Phi_Pav | Effectivity of absorbed energy transfer in PS II |
| ΨE0 | Electron transport at the beginning of illumination |
| PI_abs | Performance index on absorption basis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, J.; Zikmundová, B.; Hájek, J.; Barták, M.; Váczi, P. The Effects of Foliar Application of Phenoxy and Imidazoline Family Herbicides on the Limitation of Primary Photosynthetic Processes in Galega orientalis L. Agronomy 2022, 12, 96. https://doi.org/10.3390/agronomy12010096
Lang J, Zikmundová B, Hájek J, Barták M, Váczi P. The Effects of Foliar Application of Phenoxy and Imidazoline Family Herbicides on the Limitation of Primary Photosynthetic Processes in Galega orientalis L. Agronomy. 2022; 12(1):96. https://doi.org/10.3390/agronomy12010096
Chicago/Turabian StyleLang, Jaroslav, Barbora Zikmundová, Josef Hájek, Miloš Barták, and Peter Váczi. 2022. "The Effects of Foliar Application of Phenoxy and Imidazoline Family Herbicides on the Limitation of Primary Photosynthetic Processes in Galega orientalis L." Agronomy 12, no. 1: 96. https://doi.org/10.3390/agronomy12010096





