Age-Dependent Decline of NAD+—Universal Truth or Confounded Consensus?
Abstract
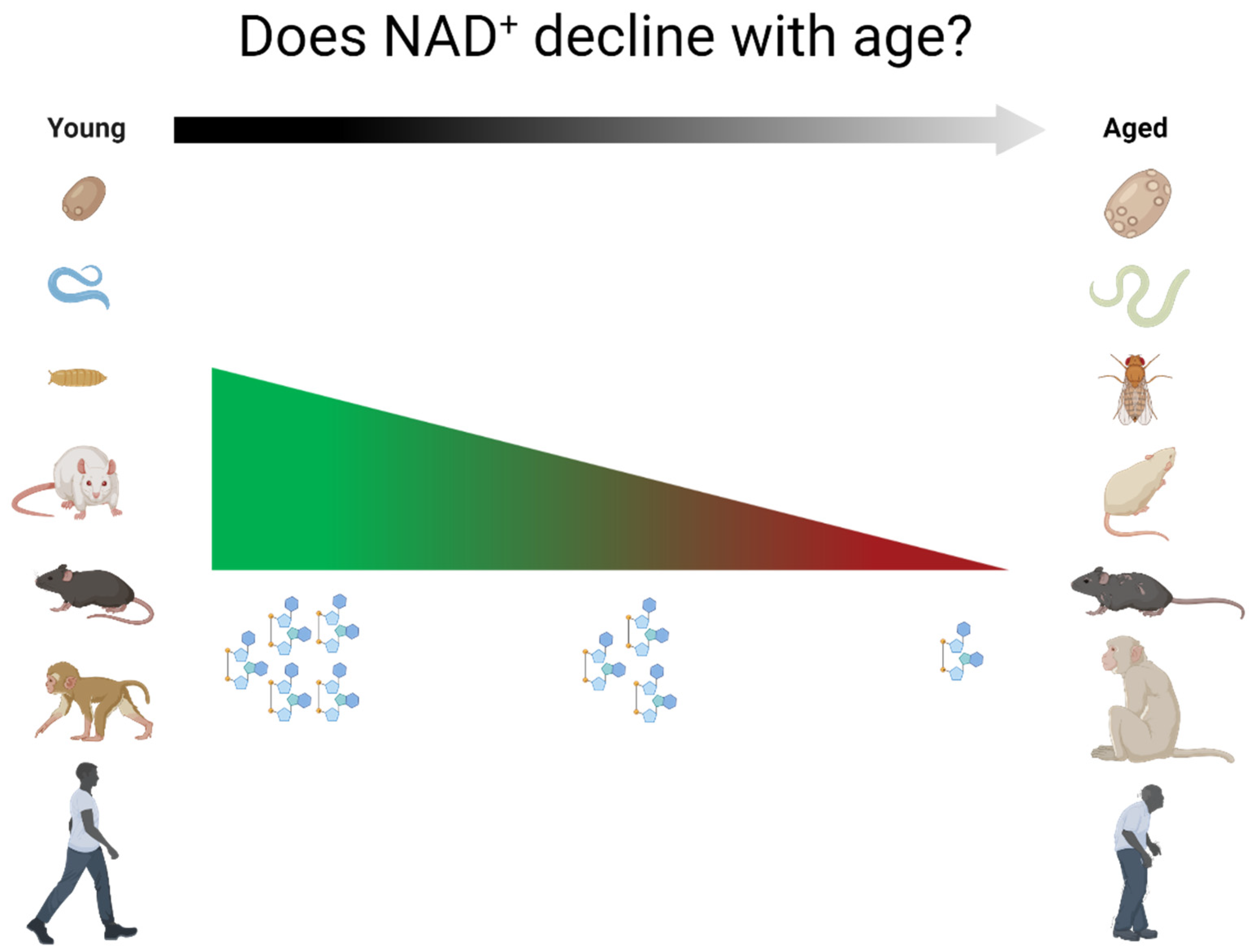
:1. Introduction
2. Relationship between NAD+ Levels and Aging across Species
2.1. Non-Mammalian Species
2.1.1. Yeast
2.1.2. Caenorhabditis Elegans
2.1.3. Drosophila Melanogaster
2.2. Rodents
2.2.1. Rats
2.2.2. Mice
2.3. Primates
2.3.1. Monkeys
2.3.2. Humans
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bogan, K.L.; Brenner, C. Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Annu. Rev. Nutr. 2008, 28, 115–130. [Google Scholar] [CrossRef] [Green Version]
- Wilk, A.; Hayat, F.; Cunningham, R.; Li, J.; Garavaglia, S.; Zamani, L.; Ferraris, D.M.; Sykora, P.; Andrews, J.; Clark, J.; et al. Extracellular NAD+ Enhances PARP-Dependent DNA Repair Capacity Independently of CD73 Activity. Sci. Rep. 2020, 10, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, M.B.; Sinclair, D.A. Why NAD+ Declines during Aging: It’s Destroyed. Cell Metab. 2016, 23, 965–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lautrup, S.; Sinclair, D.A.; Mattson, M.P.; Fang, E.F. NAD+ in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019, 30, 630–655. [Google Scholar] [CrossRef]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD+ Repletion Improves Mitochondrial and Stem Cell Function and Enhances Life Span in Mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strømland, Ø.; Niere, M.; Nikiforov, A.A.; VanLinden, M.R.; Heiland, I.; Ziegler, M. Keeping the Balance in NAD Metabolism. Biochem. Soc. Trans. 2019, 47, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badawy, A.A.-B. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Su, X.; Quinn, W.J.; Hui, S.; Krukenberg, K.; Frederick, D.W.; Redpath, P.; Zhan, L.; Chellappa, K.; White, E.; et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018, 27, 1067–1080.e5. [Google Scholar] [CrossRef] [Green Version]
- Minhas, P.S.; Liu, L.; Moon, P.K.; Joshi, A.U.; Dove, C.; Mhatre, S.; Contrepois, K.; Wang, Q.; Lee, B.A.; Coronado, M.; et al. Macrophage de Novo NAD+ Synthesis Specifies Immune Function in Aging and Inflammation. Nat. Immunol. 2019, 20, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, V.; Shabalin, K.; Nerinovski, K.; Yakimov, A.; Svetlova, M.; Solovjeva, L.; Kropotov, A.; Khodorkovskiy, M.; Migaud, M.E.; Ziegler, M.; et al. Degradation of Extracellular NAD+ Intermediates in Cultures of Human HEK293 Cells. Metabolites 2019, 9, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, C.; Shen, G.; Zou, B. NAD+ Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct. Target. Ther. 2020, 5, 227. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef] [Green Version]
- Okabe, K.; Yaku, K.; Tobe, K.; Nakagawa, T. Implications of Altered NAD Metabolism in Metabolic Disorders. J. Biomed. Sci. 2019, 26, 34. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhang, Z.; Zhang, N.; Li, H.; Zhang, L.; Baines, C.P.; Ding, S. Subcellular NAMPT-Mediated NAD+ Salvage Pathways and Their Roles in Bioenergetics and Neuronal Protection after Ischemic Injury. J. Neurochem. 2019, 151, 732–748. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD Biosynthesis Pathway Mediated by Nicotinamide Phosphoribosyltransferase Regulates Sir2 Activity in Mammalian Cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [Green Version]
- Bieganowski, P.; Brenner, C. Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD+ in Fungi and Humans. Cell 2004, 117, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ Metabolism and Its Roles in Cellular Processes during Ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Navas, L.E.; Carnero, A. NAD+ Metabolism, Stemness, the Immune Response, and Cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin Functions in Health and Disease. Mol. Endocrinol. 2007, 21, 1745–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, S.; Guarente, L. NAD+ and Sirtuins in Aging and Disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.; Cantó, C.; Oudart, H.; Brunyánszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 Inhibition Increases Mitochondrial Metabolism through SIRT1 Activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Krishnakumar, R.; Kraus, W.L. The PARP Side of the Nucleus: Molecular Actions, Physiological Outcomes, and Clinical Targets. Mol. Cell 2010, 39, 8–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munnur, D.; Ahel, I. Reversible Mono-ADP-Ribosylation of DNA Breaks. FEBS J. 2017, 284, 4002–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, M.T.P.; Soares, S.M.; Novak, C.M.; Sinclair, D.; Levine, J.A.; Aksoy, P.; Chini, E.N. The Enzyme CD38 (a NAD Glycohydrolase, EC 3.2.2.5) Is Necessary for the Development of Diet-Induced Obesity. FASEB J. 2007, 21, 3629–3639. [Google Scholar] [CrossRef]
- Wang, L.; Miao, L.; Wang, X.; Huang, C.; Qian, Y.; Huang, X.; Wang, X.; Jin, W.; Ji, G.; Fu, M.; et al. CD38 Deficiency Suppresses Adipogenesis and Lipogenesis in Adipose Tissues through Activating Sirt1/PPARγ Signaling Pathway. J. Cell. Mol. Med. 2018, 22, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Chini, E.N. CD38 as a Regulator of Cellular NAD: A Novel Potential Pharmacological Target for Metabolic Conditions. Curr. Pharm. Des. 2009, 15, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chini, E.N.; Chini, C.C.S.; Espindola Netto, J.M.; de Oliveira, G.C.; van Schooten, W. The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging. Trends Pharmacol. Sci. 2018, 39, 424–436. [Google Scholar] [CrossRef]
- Musso, T.; Deaglio, S.; Franco, L.; Calosso, L.; Badolato, R.; Garbarino, G.; Dianzani, U.; Malavasi, F. CD38 Expression and Functional Activities Are Up-Regulated by IFN-Gamma on Human Monocytes and Monocytic Cell Lines. J. Leukoc. Biol. 2001, 69, 605–612. [Google Scholar]
- Arthur-Farraj, P.; Coleman, M.P. Lessons from Injury: How Nerve Injury Studies Reveal Basic Biological Mechanisms and Therapeutic Opportunities for Peripheral Nerve Diseases. Neurotherapeutics 2021. [Google Scholar] [CrossRef] [PubMed]
- Figley, M.D.; Gu, W.; Nanson, J.D.; Shi, Y.; Sasaki, Y.; Cunnea, K.; Malde, A.K.; Jia, X.; Luo, Z.; Saikot, F.K.; et al. SARM1 Is a Metabolic Sensor Activated by an Increased NMN/NAD+ Ratio to Trigger Axon Degeneration. Neuron 2021, 109, 1118–1136.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Y.; Xie, X.J.; Li, W.H.; Liu, J.; Chen, Z.; Zhang, B.; Li, T.; Li, S.L.; Lu, J.G.; Zhang, L.; et al. A Cell-Permeant Mimetic of NMN Activates SARM1 to Produce Cyclic ADP-Ribose and Induce Non-Apoptotic Cell Death. iScience 2019, 15, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Jin, Z.; Zheng, H.; Yan, L.-J. Sources and Implications of NADH/NAD(+) Redox Imbalance in Diabetes and Its Complications. Diabetes Metab. Syndr. Obes. 2016, 9, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Cacicedo, J.M.; Ido, Y. Impaired Nicotinamide Adenine Dinucleotide (NAD+) Metabolism in Diabetes and Diabetic Tissues: Implications for Nicotinamide-related Compound Treatment. J. Diabetes Investig. 2020, 11, 1403–1419. [Google Scholar] [CrossRef]
- Long, A.N.; Owens, K.; Schlappal, A.E.; Kristian, T.; Fishman, P.S.; Schuh, R.A. Effect of Nicotinamide Mononucleotide on Brain Mitochondrial Respiratory Deficits in an Alzheimer’s Disease-Relevant Murine Model. BMC Neurol. 2015, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Wang, K.; Stock, A.J.; Gong, Y.; Demarest, T.G.; Yang, B.; Giri, N.; Harrington, L.; Alter, B.P.; Savage, S.A.; et al. Re-Equilibration of Imbalanced NAD Metabolism Ameliorates the Impact of Telomere Dysfunction. EMBO J. 2020, 39, e103420. [Google Scholar] [CrossRef]
- Strømland, Ø.; Diab, J.; Ferrario, E.; Sverkeli, L.J.; Ziegler, M. The Balance between NAD+ Biosynthesis and Consumption in Ageing. Mech. Ageing Dev. 2021, 199, 111569. [Google Scholar] [CrossRef]
- Mehmel, M.; Jovanović, N.; Spitz, U. Nicotinamide Riboside—The Current State of Research and Therapeutic Uses. Nutrients 2020, 12, 1616. [Google Scholar] [CrossRef]
- Trammell, S.A.J.; Weidemann, B.J.; Chadda, A.; Yorek, M.S.; Holmes, A.; Coppey, L.J.; Obrosov, A.; Kardon, R.H.; Yorek, M.A.; Brenner, C. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci. Rep. 2016, 6, 26933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide Mononucleotide, a Key NAD(+) Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revollo, J.R.; Körner, A.; Mills, K.F.; Satoh, A.; Wang, T.; Garten, A.; Dasgupta, B.; Sasaki, Y.; Wolberger, C.; Townsend, R.R.; et al. Nampt/PBEF/Visfatin Regulates Insulin Secretion in Beta Cells as a Systemic NAD Biosynthetic Enzyme. Cell Metab. 2007, 6, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.Y.; Oosterveer, M.H.; Cen, Y.; Fernandez-Marcos, P.J.; Yamamoto, H.; Andreux, P.A.; Cettour-Rose, P.; et al. The NAD+ Precursor Nicotinamide Riboside Enhances Oxidative Metabolism and Protects against High-Fat Diet Induced Obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Lautrup, S.; Cordonnier, S.; Wang, Y.; Croteau, D.L.; Zavala, E.; Zhang, Y.; Moritoh, K.; O’Connell, J.F.; Baptiste, B.A.; et al. NAD+ Supplementation Normalizes Key Alzheimer’s Features and DNA Damage Responses in a New AD Mouse Model with Introduced DNA Repair Deficiency. Proc. Natl. Acad. Sci. USA 2018, 115, E1876–E1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Hackett, A.R.; Kim, S.; Strickland, A.; Milbrandt, J. Dysregulation of NAD+ Metabolism Induces a Schwann Cell Dedifferentiation Program. J. Neurosci. 2018, 38, 6546–6562. [Google Scholar] [CrossRef] [Green Version]
- Xing, S.; Hu, Y.; Huang, X.; Shen, D.; Chen, C. Nicotinamide Phosphoribosyltransferase-related Signaling Pathway in Early Alzheimer’s Disease Mouse Models. Mol. Med. Rep. 2019, 20, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Yaku, K.; Okabe, K.; Hikosaka, K.; Nakagawa, T. NAD Metabolism in Cancer Therapeutics. Front. Oncol. 2018, 8, 622. [Google Scholar] [CrossRef]
- Denoth Lippuner, A.; Julou, T.; Barral, Y. Budding Yeast as a Model Organism to Study the Effects of Age. FEMS Microbiol. Rev. 2014, 38, 300–325. [Google Scholar] [CrossRef] [Green Version]
- Longo, V.D.; Shadel, G.S.; Kaeberlein, M.; Kennedy, B. Replicative and Chronological Aging in Saccharomyces Cerevisiae. Cell Metab. 2012, 16, 18–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, R.M.; Bitterman, K.J.; Wood, J.G.; Medvedik, O.; Cohen, H.; Lin, S.S.; Manchester, J.K.; Gordon, J.I.; Sinclair, D.A. Manipulation of a Nuclear NAD+ Salvage Pathway Delays Aging without Altering Steady-State NAD+ Levels. J. Biol. Chem. 2002, 277, 18881–18890. [Google Scholar] [CrossRef] [Green Version]
- Belenky, P.; Racette, F.G.; Bogan, K.L.; McClure, J.M.; Smith, J.S.; Brenner, C. Nicotinamide Riboside Promotes Sir2 Silencing and Extends Lifespan via Nrk and Urh1/Pnp1/Meu1 Pathways to NAD+. Cell 2007, 129, 473–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-J.; Ford, E.; Haigis, M.; Liszt, G.; Guarente, L. Calorie Restriction Extends Yeast Life Span by Lowering the Level of NADH. Genes Dev. 2004, 18, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.-P.; Lin, S.-J. Regulation of Yeast Sirtuins by NAD(+) Metabolism and Calorie Restriction. Biochim. Biophys. Acta 2010, 1804, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandmeier, J.J.; Celic, I.; Boeke, J.D.; Smith, J.S. Telomeric and RDNA Silencing in Saccharomyces Cerevisiae Are Dependent on a Nuclear NAD(+) Salvage Pathway. Genetics 2002, 160, 877–889. [Google Scholar] [CrossRef]
- Wierman, M.B.; Smith, J.S. Yeast Sirtuins and the Regulation of Aging. FEMS Yeast Res. 2014, 14, 73–88. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.S.; Manchester, J.K.; Gordon, J.I. Enhanced Gluconeogenesis and Increased Energy Storage as Hallmarks of Aging in Saccharomyces Cerevisiae. J. Biol. Chem. 2001, 276, 36000–36007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beas, A.O.; Gordon, P.B.; Prentiss, C.L.; Olsen, C.P.; Kukurugya, M.A.; Bennett, B.D.; Parkhurst, S.M.; Gottschling, D.E. Independent Regulation of Age Associated Fat Accumulation and Longevity. Nat. Commun. 2020, 11, 2790. [Google Scholar] [CrossRef]
- Orlandi, I.; Stamerra, G.; Vai, M. Altered Expression of Mitochondrial NAD+ Carriers Influences Yeast Chronological Lifespan by Modulating Cytosolic and Mitochondrial Metabolism. Front. Genet. 2018, 9, 676. [Google Scholar] [CrossRef] [Green Version]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.-S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD(+)/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, E.F.; Scheibye-Knudsen, M.; Brace, L.E.; Kassahun, H.; SenGupta, T.; Nilsen, H.; Mitchell, J.R.; Croteau, D.L.; Bohr, V.A. Defective Mitophagy in XPA via PARP-1 Hyperactivation and NAD(+)/SIRT1 Reduction. Cell 2014, 157, 882–896. [Google Scholar] [CrossRef] [Green Version]
- Fang, E.F.; Kassahun, H.; Croteau, D.L.; Scheibye-Knudsen, M.; Marosi, K.; Lu, H.; Shamanna, R.A.; Kalyanasundaram, S.; Bollineni, R.C.; Wilson, M.A.; et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016, 24, 566–581. [Google Scholar] [CrossRef] [Green Version]
- Ryu, D.; Zhang, H.; Ropelle, E.R.; Sorrentino, V.; Mázala, D.A.G.; Mouchiroud, L.; Marshall, P.L.; Campbell, M.D.; Ali, A.S.; Knowels, G.M.; et al. NAD+ Repletion Improves Muscle Function in Muscular Dystrophy and Counters Global PARylation. Sci. Transl. Med. 2016, 8, 361ra139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, M.D.W.; Partridge, L. Drosophila as a Model for Ageing. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2707–2717. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox Signalling and Ageing: Insights from Drosophila. Biochem. Soc. Trans. 2020, 48, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, M.; Tang, C.; Xiang, Z.; Li, Q.; Ruan, X.; Xiong, K.; Zheng, L. Overexpression of Nmnat Improves the Adaption of Health Span in Aging Drosophila. Exp. Gerontol. 2018, 108, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Hou, Y.; Lautrup, S.; Jensen, M.B.; Yang, B.; SenGupta, T.; Caponio, D.; Khezri, R.; Demarest, T.G.; Aman, Y.; et al. NAD+ Augmentation Restores Mitophagy and Limits Accelerated Aging in Werner Syndrome. Nat. Commun. 2019, 10, 5284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braidy, N.; Guillemin, G.J.; Mansour, H.; Chan-Ling, T.; Poljak, A.; Grant, R. Age Related Changes in NAD+ Metabolism Oxidative Stress and Sirt1 Activity in Wistar Rats. PLoS ONE 2011, 6, e19194. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Poljak, A.; Grant, R.; Jayasena, T.; Mansour, H.; Chan-Ling, T.; Guillemin, G.J.; Smythe, G.; Sachdev, P. Mapping NAD(+) Metabolism in the Brain of Ageing Wistar Rats: Potential Targets for Influencing Brain Senescence. Biogerontology 2014, 15, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Pi, C.; Yang, Y.; Lin, L.; Shi, Y.; Li, Y.; Li, Y.; He, X. Nampt Expression Decreases Age-Related Senescence in Rat Bone Marrow Mesenchymal Stem Cells by Targeting Sirt1. PLoS ONE 2017, 12, e0170930. [Google Scholar] [CrossRef] [Green Version]
- Pi, C.; Yang, Y.; Sun, Y.; Wang, H.; Sun, H.; Ma, M.; Lin, L.; Shi, Y.; Li, Y.; Li, Y.; et al. Nicotinamide Phosphoribosyltransferase Postpones Rat Bone Marrow Mesenchymal Stem Cell Senescence by Mediating NAD+-Sirt1 Signaling. Aging 2019, 11, 3505–3522. [Google Scholar] [CrossRef]
- Dall, M.; Penke, M.; Sulek, K.; Matz-Soja, M.; Holst, B.; Garten, A.; Kiess, W.; Treebak, J.T. Hepatic NAD+ Levels and NAMPT Abundance Are Unaffected during Prolonged High-Fat Diet Consumption in C57BL/6JBomTac Mice. Mol. Cell. Endocrinol. 2018, 473, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Dall, M.; Trammell, S.A.J.; Asping, M.; Hassing, A.S.; Agerholm, M.; Vienberg, S.G.; Gillum, M.P.; Larsen, S.; Treebak, J.T. Mitochondrial Function in Liver Cells Is Resistant to Perturbations in NAD+ Salvage Capacity. J. Biol. Chem. 2019, 294, 13304–13326. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD(+) Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederick, D.W.; Loro, E.; Liu, L.; Davila, A.; Chellappa, K.; Silverman, I.M.; Quinn, W.J.; Gosai, S.J.; Tichy, E.D.; Davis, J.G.; et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016, 24, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, L.R.; Imai, S. Specific Ablation of Nampt in Adult Neural Stem Cells Recapitulates Their Functional Defects during Aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.; Wozniak, D.F.; Imai, S. CA1 Nampt Knockdown Recapitulates Hippocampal Cognitive Phenotypes in Old Mice Which Nicotinamide Mononucleotide Improves. NPJ Aging Mech. Dis. 2018, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.A.; DeMuth, T.M.; Miller, K.N.; Pugh, T.D.; Polewski, M.A.; Colman, R.J.; Eliceiri, K.W.; Beasley, T.M.; Johnson, S.C.; Anderson, R.M. Regional Metabolic Heterogeneity of the Hippocampus Is Nonuniformly Impacted by Age and Caloric Restriction. Aging Cell 2016, 15, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Blacher, E.; Dadali, T.; Bespalko, A.; Haupenthal, V.J.; Grimm, M.O.W.; Hartmann, T.; Lund, F.E.; Stein, R.; Levy, A. Alzheimer’s Disease Pathology Is Attenuated in a CD38-Deficient Mouse Model. Ann. Neurol. 2015, 78, 88–103. [Google Scholar] [CrossRef]
- Scheibye-Knudsen, M.; Mitchell, S.J.; Fang, E.F.; Iyama, T.; Ward, T.; Wang, J.; Dunn, C.A.; Singh, N.; Veith, S.; Hasan-Olive, M.M.; et al. A High-Fat Diet and NAD(+) Activate Sirt1 to Rescue Premature Aging in Cockayne Syndrome. Cell Metab. 2014, 20, 840–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, M.J.; Kwon, Y.; Son, T.; Cho, Y.S. Restoration of Mitochondrial NAD+ Levels Delays Stem Cell Senescence and Facilitates Reprogramming of Aged Somatic Cells. Stem Cells 2016, 34, 2840–2851. [Google Scholar] [CrossRef]
- McReynolds, M.R.; Chellappa, K.; Chiles, E.; Jankowski, C.; Shen, Y.; Chen, L.; Descamps, H.C.; Mukherjee, S.; Bhat, Y.R.; Lingala, S.R.; et al. NAD+ Flux Is Maintained in Aged Mice despite Lower Tissue Concentrations. Cell Syst. 2021. [Google Scholar] [CrossRef] [PubMed]
- Chiou, K.L.; Montague, M.J.; Goldman, E.A.; Watowich, M.M.; Sams, S.N.; Song, J.; Horvath, J.E.; Sterner, K.N.; Ruiz-Lambides, A.V.; Martínez, M.I.; et al. Rhesus Macaques as a Tractable Physiological Model of Human Ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190612. [Google Scholar] [CrossRef]
- Pugh, T.D.; Conklin, M.W.; Evans, T.D.; Polewski, M.A.; Barbian, H.J.; Pass, R.; Anderson, B.D.; Colman, R.J.; Eliceiri, K.W.; Keely, P.J.; et al. A Shift in Energy Metabolism Anticipates the Onset of Sarcopenia in Rhesus Monkeys. Aging Cell 2013, 12, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.M.; Tran, V.; Wachtman, L.M.; Green, C.L.; Jones, D.P.; Promislow, D.E.L. A Longitudinal Analysis of the Effects of Age on the Blood Plasma Metabolome in the Common Marmoset, Callithrix Jacchus. Exp. Gerontol. 2016, 76, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-Associated Changes in Oxidative Stress and NAD+ Metabolism in Human Tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Ummarino, S.; Mozzon, M.; Zamporlini, F.; Amici, A.; Mazzola, F.; Orsomando, G.; Ruggieri, S.; Raffaelli, N. Simultaneous Quantitation of Nicotinamide Riboside, Nicotinamide Mononucleotide and Nicotinamide Adenine Dinucleotide in Milk by a Novel Enzyme-Coupled Assay. Food Chem. 2017, 221, 161–168. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic Genome Assessment of B-Vitamin Biosynthesis Suggests Co-Operation among Gut Microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [Green Version]
- Gazzaniga, F.; Stebbins, R.; Chang, S.Z.; McPeek, M.A.; Brenner, C. Microbial NAD Metabolism: Lessons from Comparative Genomics. Microbiol. Mol. Biol. Rev. 2009, 73, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Ear, P.H.; Chadda, A.; Gumusoglu, S.B.; Schmidt, M.S.; Vogeler, S.; Malicoat, J.; Kadel, J.; Moore, M.M.; Migaud, M.E.; Stevens, H.E.; et al. Maternal Nicotinamide Riboside Enhances Postpartum Weight Loss, Juvenile Offspring Development, and Neurogenesis of Adult Offspring. Cell Rep. 2019, 26, 969–983.e4. [Google Scholar] [CrossRef] [Green Version]
- Guest, J.; Grant, R.; Mori, T.A.; Croft, K.D. Changes in Oxidative Damage, Inflammation and [NAD(H)] with Age in Cerebrospinal Fluid. PLoS ONE 2014, 9, e85335. [Google Scholar] [CrossRef] [Green Version]
- Clement, J.; Wong, M.; Poljak, A.; Sachdev, P.; Braidy, N. The Plasma NAD+ Metabolome Is Dysregulated in “Normal” Aging. Rejuvenation Res 2019, 22, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Seyedsadjadi, N.; Berg, J.; Bilgin, A.A.; Braidy, N.; Salonikas, C.; Grant, R. High Protein Intake Is Associated with Low Plasma NAD+ Levels in a Healthy Human Cohort. PLoS ONE 2018, 13, e0201968. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Berg, J.; Mestayer, R.; Braidy, N.; Bennett, J.; Broom, S.; Watson, J. A Pilot Study Investigating Changes in the Human Plasma and Urine NAD+ Metabolome During a 6 Hour Intravenous Infusion of NAD. Front. Aging Neurosci. 2019, 11, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.-H.; Lu, M.; Lee, B.-Y.; Ugurbil, K.; Chen, W. In Vivo NAD Assay Reveals the Intracellular NAD Contents and Redox State in Healthy Human Brain and Their Age Dependences. Proc. Natl. Acad. Sci. USA 2015, 112, 2876–2881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagga, P.; Hariharan, H.; Wilson, N.E.; Beer, J.C.; Shinohara, R.T.; Elliott, M.A.; Baur, J.A.; Marincola, F.M.; Witschey, W.R.; Haris, M.; et al. Single-Voxel 1 H MR Spectroscopy of Cerebral Nicotinamide Adenine Dinucleotide (NAD+ ) in Humans at 7T Using a 32-Channel Volume Coil. Magn. Reson. Med. 2020, 83, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-Inflammatory Signatures in Aged Subjects: A Placebo-Controlled, Randomized Trial. bioRxiv 2019. [Google Scholar] [CrossRef] [Green Version]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD+ Metabolome and Induces Transcriptomic and Anti-Inflammatory Signatures. Cell Rep. 2019, 28, 1717–1728.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaleckis, R.; Murakami, I.; Takada, J.; Kondoh, H.; Yanagida, M. Individual Variability in Human Blood Metabolites Identifies Age-Related Differences. Proc. Natl. Acad. Sci. USA 2016, 113, 4252–4259. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.-C.; Yang, X.; Hua, X.; Liu, J.; Fan, M.-B.; Li, G.-Q.; Song, J.; Xu, T.-Y.; Li, Z.-Y.; Guan, Y.-F.; et al. Hepatic NAD(+) Deficiency as a Therapeutic Target for Non-Alcoholic Fatty Liver Disease in Ageing. Br. J. Pharmacol. 2016, 173, 2352–2368. [Google Scholar] [CrossRef] [Green Version]
- de Guia, R.M.; Agerholm, M.; Nielsen, T.S.; Consitt, L.A.; Søgaard, D.; Helge, J.W.; Larsen, S.; Brandauer, J.; Houmard, J.A.; Treebak, J.T. Aerobic and Resistance Exercise Training Reverses Age-Dependent Decline in NAD+ Salvage Capacity in Human Skeletal Muscle. Physiol. Rep. 2019, 7, e14139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cambronne, X.A.; Kraus, W.L. Location, Location, Location: Compartmentalization of NAD+ Synthesis and Functions in Mammalian Cells. Trends Biochem. Sci. 2020, 45, 858–873. [Google Scholar] [CrossRef]
- Viña, J.; Saez, G.T.; Gambini, J.; Gomez-Cabrera, M.C.; Borrás, C. Role of NAD(+)/NADH Redox Ratio in Cell Metabolism: A Tribute to Helmut Sies and Theodor Bücher and Hans A. Krebs. Arch. Biochem. Biophys. 2016, 595, 176–180. [Google Scholar] [CrossRef]

| Species | NAD+ Decline with Age | Tissue | Age Comparison | Gender/Sex | Reference # in Manuscript |
|---|---|---|---|---|---|
| Yeast | No | n/a | Replicative ages of 0–1 vs. 7–8 | [55] | |
| Yeast | No | n/a | Replicative ages of 0 vs. 16 | [56] | |
| Yeast | Yes | n/a | Switch to senescent stage | [57] | |
| Yeast | Yes | n/a | Cells grown under increasing optical densities | [50] | |
| C. elegans | Yes | Whole worm | Day 1 vs. 8 | [58] | |
| C. elegans | Yes | Whole worm | Day 1 vs. 17 | [59] | |
| Rat | Yes | Heart, lung, liver and kidney | 3- vs. 12- vs. 24-month-old female Wistar | Female | [66] |
| Rat | Yes | Hippocampus, cortex, cerebellum and brainstem | 3- vs. 12- vs. 24-month-old female Wistar | Female | [67] |
| Rat | Yes | Bone Marrow Mesenchymal Stem Cells | Isolated cells from 1–2 vs. 15–18 months-old male Wistar | Male | [68] |
| Rat | Yes | Bone Marrow Mesenchymal Stem Cells | Senescence-induced cells from 1–2-month-old vs. controls | Male | [69] |
| Mouse | Yes | Pancreas, adipose tissue, skeletal muscle | 3–6 vs. 25–31-month-old | Both sexes | [40] |
| Mouse | No | Liver | 3–6 vs. 25–31-month-old | Both sexes | [40] |
| Mouse | No | Liver | 6- vs. 12- vs. 24- vs. 48-week-old C57BL/6J on HFD or chow | Male | [70] |
| Mouse | No | Liver | 8-,14-,27- vs.55-,110-week-old | Female | [71] |
| Mouse | Yes | Liver, adipose tissue, spleen, skeletal muscle | 5-, 12-, 18-, 24- vs. 32-month-old | Male | [14] |
| Mouse | Yes | Liver, skeletal muscle | 6- vs. 24-month-old | Male | [58] |
| Mouse | Yes | Skeletal muscle (gastrocnemius) | 4- vs. 24-month-old | Male | [73] |
| Mouse | Yes | Gastrocnemius | 6- vs. 22- and 30-month-old | Not specified | [72] |
| Mouse | Yes | Isolated muscle stem cells | Cells from 1- vs. 22–24-month-old | Male | [5] |
| Mouse | Yes | Hippocampus | 1 vs. 3–4 or 3–4 vs. 6-month-old. No differences: 6-vs. 10–12-month-old | Not specified | [74] |
| Mouse | Yes | Hippocampus | 2-vs.19-month-old. No differences: 2- vs. 7- or 7- vs. 19-month-old | Both sexes | [75] |
| Mouse | Yes | Dentate gyrus | 10- vs. 20–30-week-old | Male | [76] |
| Mouse | No | whole-brain tissue | 2- vs. 8-month-old | Male | [77] |
| Mouse | No | Cerebellum | 4- vs. 16-month-old | Male | [78] |
| Mouse | Yes | Tail-tip fibroblast | 2-vs. 22-month-old | Not specified | [79] |
| Mouse | Yes | primary peritoneal macrophages | 3- vs. 16–20-month-old | Not specified | [9] |
| Mouse | Yes | BAT, rWAT, iWAT, jejunum, quadriceps, gastrocnemius, soleus, liver, kidney, and descending colon | 3- vs. 25-month-old | Male | [80] |
| Mouse | No | Heart, brain, spleen, pancreas, lungs, proximal colon, duodenum, ileum, gWAT, cecum, stomach | 3- vs. 25-month-old | Male | [80] |
| Human | Yes | Human mesenchymal stem cells | Cells were aged in vitro | [79] | |
| Human | Yes | Pelvic skin sample | Spanning 0–77 years of age | Both sexes; children were male | [84] |
| Human | Yes | Cerebrospinal fluid | Young (<45 years) vs. elderly groups (>45 years) | Both sexes | [89] |
| Human | No | Plasma | Young (<45 years) vs. elderly groups (>45 years) | Both sexes | [89] |
| Human | Yes | Brain | Spanning 21–68 years of age | Both sexes | [93] |
| Human | Yes | Liver | Young (<45 years) vs. old patients (>60 years) undergoing hepatectomy | Both sexes | [98] |
| Human | Yes | Whole blood, plasma | Young (<32 years) vs. elderly (>75 years) groups | Both sexes | [97] |
| Human | No | Red blood cells | Young (<32 years) vs. elderly (>75 years) groups | Both sexes | [97] |
| Human | No | Brain, calf muscle | Young (21 ± 4 years) vs. Elderly 69 ± 4 years) groups | Both sexes | [95] |
| Human | Yes | Brain | Spanning 26–78 years of age | Both sexes | [94] |
| Human | Yes | Plasma | Spanning 20–87 years of age | Both sexes | [90] |
| Human | Yes | Monocyte-derived macrophages | Cells from young (≤35 years) vs. elderly (≥65 years) people | Not specified | [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peluso, A.; Damgaard, M.V.; Mori, M.A.S.; Treebak, J.T. Age-Dependent Decline of NAD+—Universal Truth or Confounded Consensus? Nutrients 2022, 14, 101. https://doi.org/10.3390/nu14010101
Peluso A, Damgaard MV, Mori MAS, Treebak JT. Age-Dependent Decline of NAD+—Universal Truth or Confounded Consensus? Nutrients. 2022; 14(1):101. https://doi.org/10.3390/nu14010101
Chicago/Turabian StylePeluso, Augusto, Mads V. Damgaard, Marcelo A. S. Mori, and Jonas T. Treebak. 2022. "Age-Dependent Decline of NAD+—Universal Truth or Confounded Consensus?" Nutrients 14, no. 1: 101. https://doi.org/10.3390/nu14010101
APA StylePeluso, A., Damgaard, M. V., Mori, M. A. S., & Treebak, J. T. (2022). Age-Dependent Decline of NAD+—Universal Truth or Confounded Consensus? Nutrients, 14(1), 101. https://doi.org/10.3390/nu14010101






