Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation
Abstract
:1. Introduction
2. Results
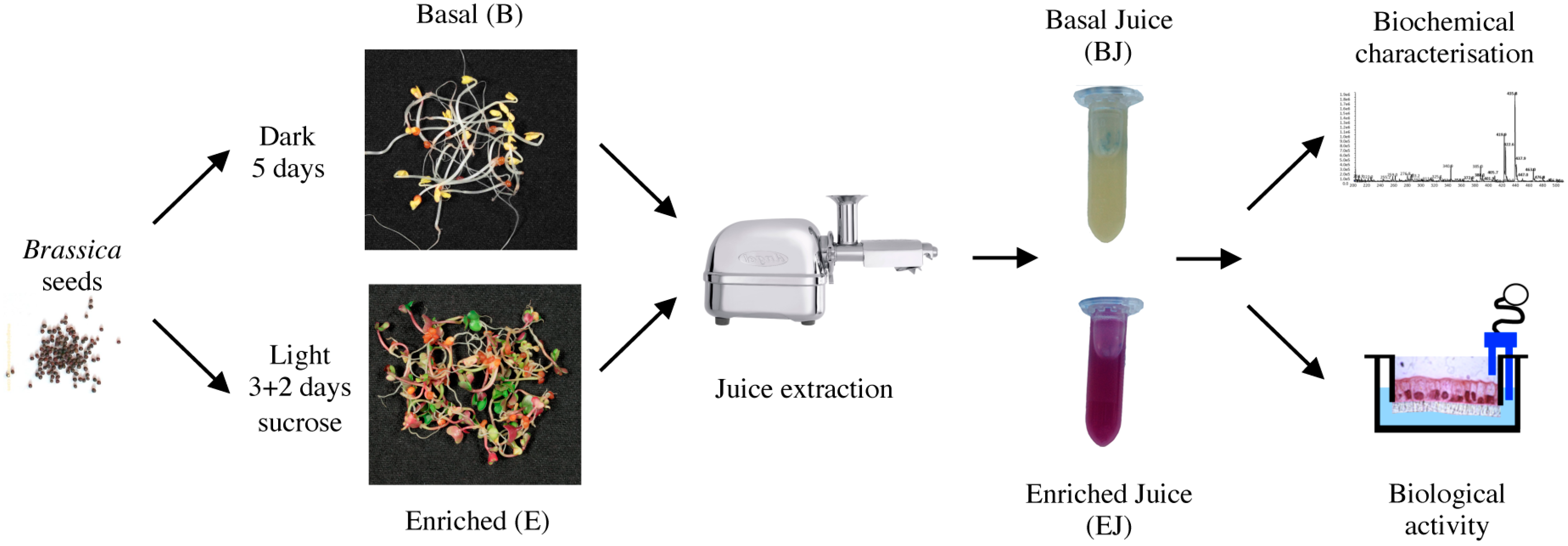
2.1. Preparation of Basal and Enriched Broccoli Sprout Juices
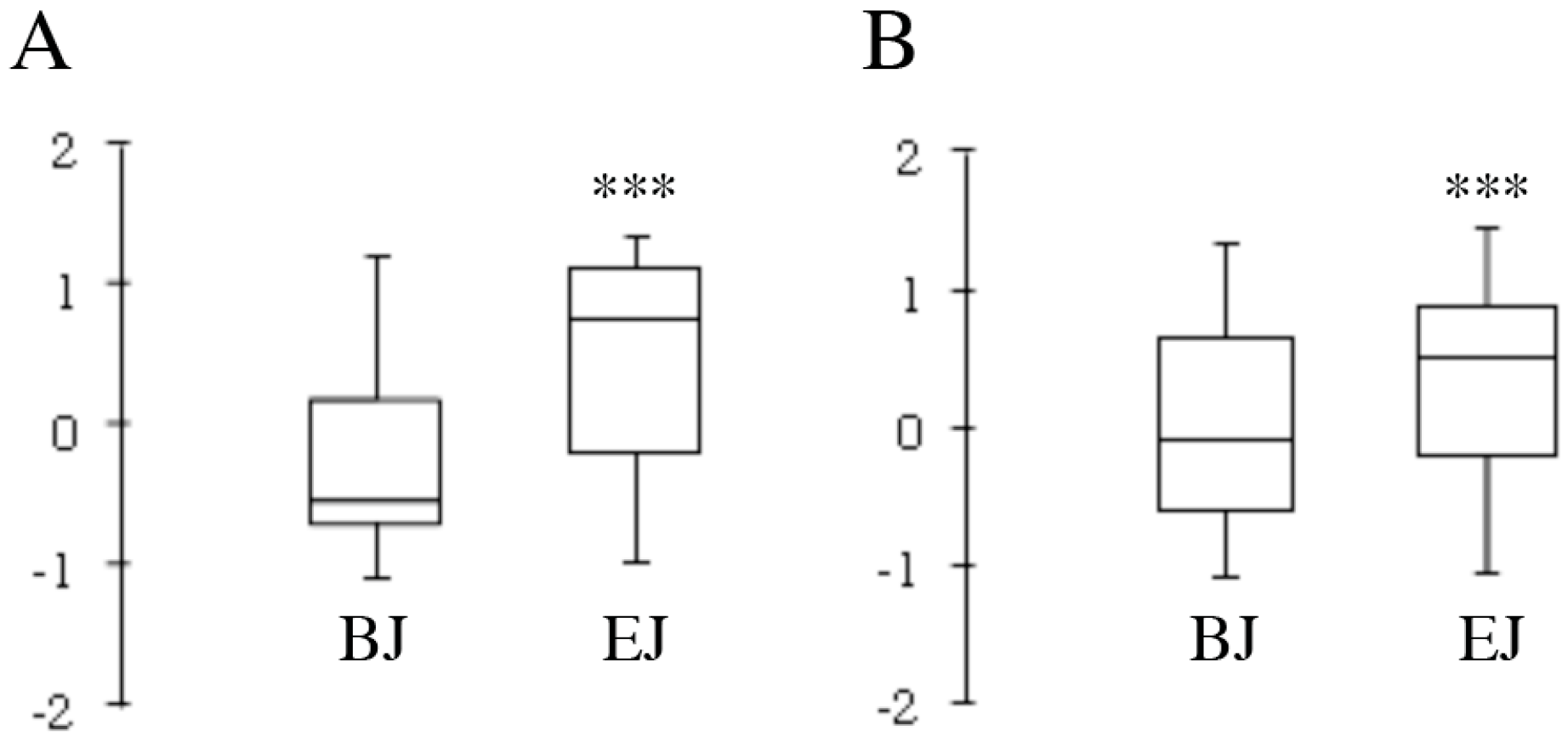
2.2. Compositional Analysis
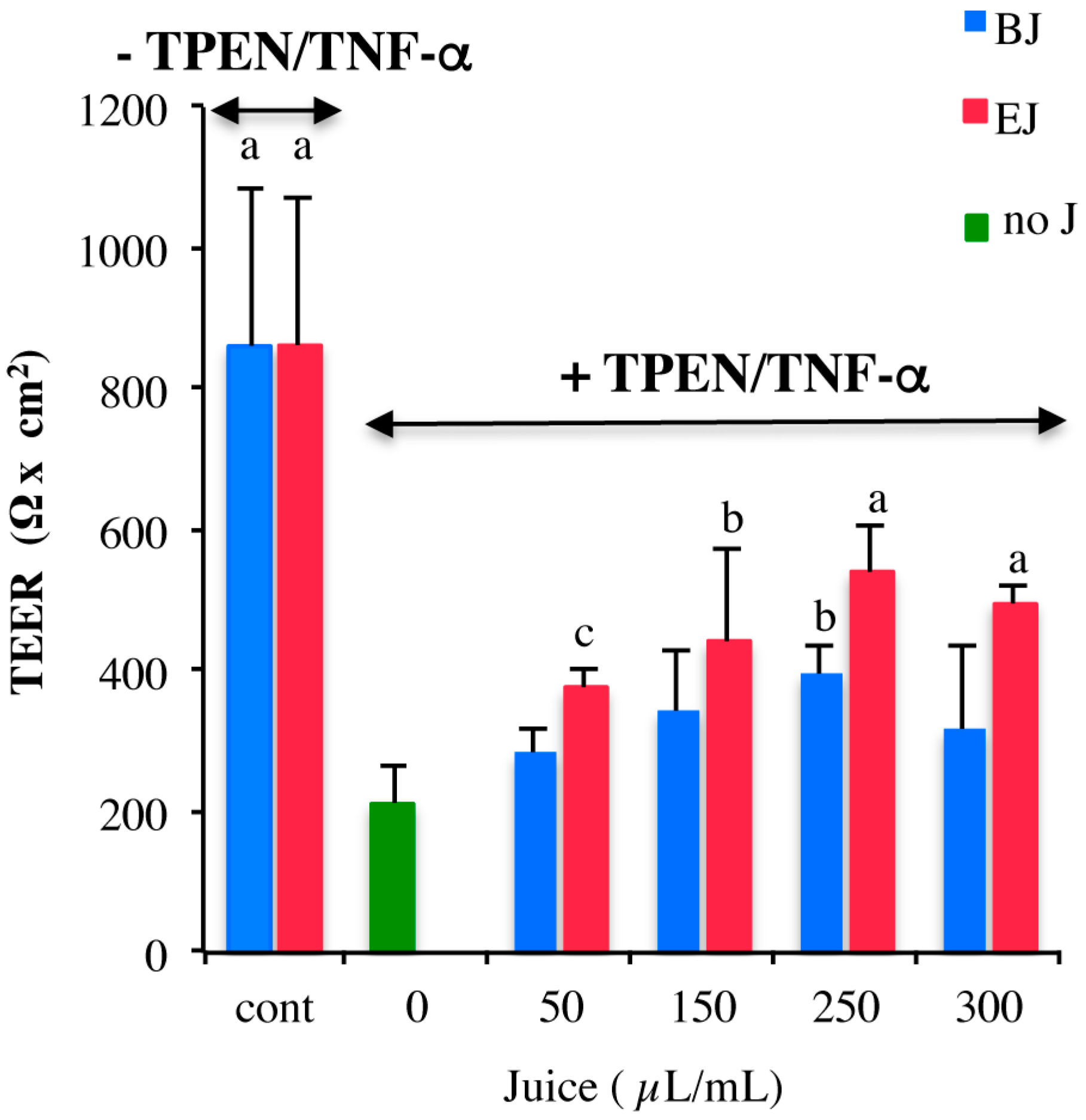
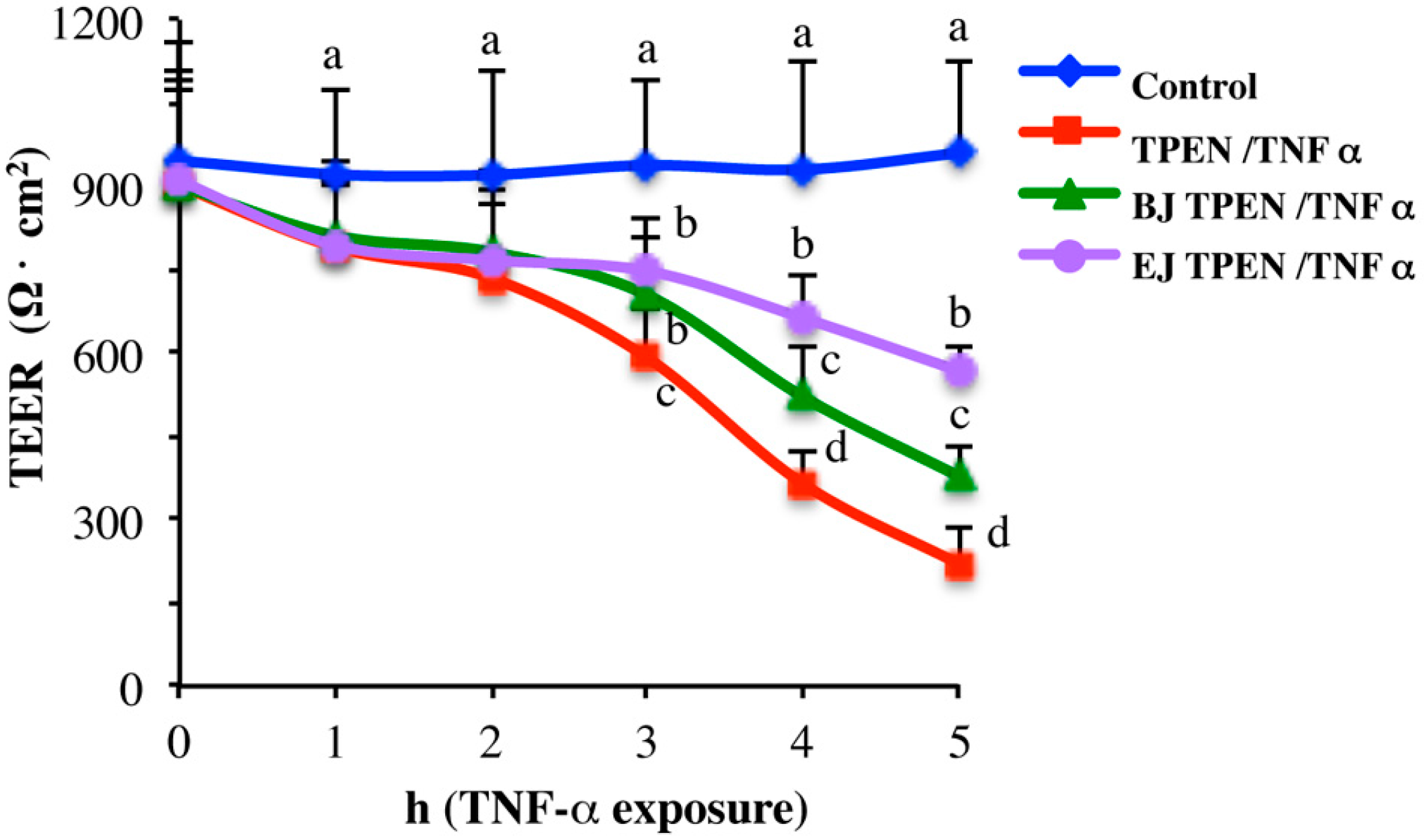
2.3. In Vitro Testing of Biological Effects
2.4. Multivariate Analysis of Composition and Correlation with the Biological Effect
3. Discussion
4. Materials and Methods
4.1. Broccoli Sprouts’ Growth and Juice Preparation
4.2. Chemicals
4.3. High-Resolution Untargeted Analysis
4.4. Total Polyphenol, Flavonoid, and Anthocyanin Content
4.5. Phenolics Profiling
4.6. Glucosinolate, Sulforaphane and Sulforaphane Nitrile Determination
4.7. Cell Culture
4.8. Measure of Monolayer Integrity
4.9. Experimental Intestinal Cell Model
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar]
- Leenders, M.; Sluijs, I.; Ros, M.M.; Boshuizen, H.C.; Siersema, P.D.; Ferrari, P.; Weikert, C.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.C. Fruit and Vegetable Consumption and Mortality European Prospective Investigation Into Cancer and Nutrition. Am. J. Epidemiol. 2013, 178, 590–602. [Google Scholar] [PubMed]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014, 349, g4490. [Google Scholar]
- Hu, D.; Huang, J.; Wang, Y.; Zhang, D.; Qu, Y. Fruits and Vegetables Consumption and Risk of Stroke: A Meta-Analysis of Prospective Cohort Studies. Stroke 2014, 45, 1613–1619. [Google Scholar]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [PubMed]
- Rodrigo, R.; Libuy, M.; Feliu, F.; Hasson, D. Polyphenols in disease: From diet to supplements. Curr. Pharm. Biotechnol. 2014, 15, 304–317. [Google Scholar] [PubMed]
- Finley, J.W. Proposed criteria for assessing the efficacy of cancer reduction by plant foods enriched in carotenoids, glucosinolates, polyphenols and selenocompounds. Ann. Bot. 2005, 95, 1075–1096. [Google Scholar]
- Fahey, J.W.; Talalay, P.; Kensler, T.W. Notes from the field: “Green” chemoprevention as frugal medicine. Cancer Prev. Res. 2012, 5, 179–188. [Google Scholar]
- Hall, R.D. Plant metabolomics: From holistic hope, to hype, to hot topic. New Phytol. 2006, 169, 453–468. [Google Scholar]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, andecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Biotic elicitors effectively increase the glucosinolates content in Brassicaceae sprouts. J. Agric. Food Chem. 2014, 62, 1881–1889. [Google Scholar] [PubMed]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [PubMed]
- Vasanthi, H.R.; Mukherjee, S.; Das, D.K. Potential health benefits of broccoli- a chemico-biological overview. Mini Rev. Med. Chem. 2009, 9, 749–759. [Google Scholar]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health promoting effects of brassica-derived phytochemicals: From chemopreventive and anti-inflammatory activities to epigenetic regulation. Oxid. Med. Cell. Longev. 2013, 2013, 964539. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane: Translational research from laboratory bench to clinic. Nutr. Rev. 2013, 71, 709–726. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [PubMed]
- Bhandari, S.; Jo, J.; Lee, J. Comparison of Glucosinolate Profiles in Different Tissues of Nine Brassica Crops. Molecules 2015, 20, 15827–15841. [Google Scholar]
- Hanlon, P.R.; Barnes, D.M. Phytochemical Composition and Biological Activity of 8 Varieties of Radish (Raphanus sativus L.) Sprouts and Mature Taproots. J. Food Sci. 2011, 76, C185–C192. [Google Scholar] [PubMed]
- Traka, M.H.; Mithen, R.F. Plant science and human nutrition: Challenges in assessing health-promoting properties of phytochemicals. Plant Cell 2011, 23, 2483–2497. [Google Scholar] [PubMed]
- Jiang, Y.; Wu, S.H.; Shu, X.O.; Xiang, Y.B.; Ji, B.T.; Milne, G.L.; Cai, Q.; Zhang, X.; Gao, Y.T.; Zheng, W.; et al. Cruciferous vegetable intake is inversely correlated with circulating levels of proinflammatory markers in women. J. Acad. Nutr. Diet. 2014, 114, 700–708.e2. [Google Scholar] [CrossRef]
- Medina, S.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C.; Ferreres, F.; Gil, J.I.; Gil-Izquierdo, Á. The intake of broccoli sprouts modulates the inflammatory and vascular prostanoids but not the oxidative stress-related isoprostanes in healthy humans. Food Chem. 2015, 173, 1187–1194. [Google Scholar]
- Lippmann, D.; Lehmann, C.; Florian, S.; Barknowitz, G.; Haack, M.; Mewis, I.; Wiesner, M.; Schreiner, M.; Glatt, H.; Brigelius-Flohé, R.; et al. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014, 5, 1073–1081. [Google Scholar]
- Ranaldi, G.; Ferruzza, S.; Canali, R.; Leoni, G.; Zalewski, P.D.; Sambuy, Y.; Perozzi, G.; Murgia, C. Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNF? J. Nutr. Biochem. 2013, 24, 967–976. [Google Scholar]
- Lee, S.G.; Kim, J.; Son, M.; Lee, E.; Park, W.; Kim, J.; Lee, S.; Lee, I. Influence of Extraction Method on Quality and Functionality of Broccoli Juice. Prev. Nutr. Food Sci. 2013, 18, 133–138. [Google Scholar] [PubMed]
- Maldini, M.; Baima, S.; Morelli, G.; Scaccini, C.; Natella, F. A liquid chromatography-mass spectrometry approach to study “glucosinoloma” in broccoli sprouts. J. Mass Spectrom. 2012, 47, 1198–1206. [Google Scholar]
- Delie, F.; Rubas, W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: Advantages and limitations of the Caco-2 model. Crit. Rev. Ther. Drug Carr. Syst. 1997, 14, 221–286. [Google Scholar]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar]
- Ferruzza, S.; Rossi, C.; Scarino, M.L.; Sambuy, Y. A protocol for differentiation of human intestinal Caco-2 cells in asymmetric serum-containing medium. Toxicol. Vitr. 2012, 26, 8–11. [Google Scholar]
- Natoli, M.; Leoni, B.D.; D’Agnano, I.; D’Onofrio, M.; Brandi, R.; Arisi, I.; Zucco, F.; Felsani, A. Cell growing density affects the structural and functional properties of Caco-2 differentiated monolayer. J. Cell. Physiol. 2011, 226, 1531–1543. [Google Scholar] [PubMed]
- Denis, M.C.; Furtos, A.; Dudonné, S.; Montoudis, A.; Garofalo, C.; Desjardins, Y.; Delvin, E.; Levy, E. Apple Peel Polyphenols and Their Beneficial Actions on Oxidative Stress and Inflammation. PLoS ONE 2013, 8, e53725. [Google Scholar]
- Denis, M.; Desjardins, Y.; Furtos, A.; Marcil, V.; Dudonné, S.; Montoudis, A.; Garofalo, C.; Delvin, E.; Marette, A.; Levy, E. Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin. Sci. 2015, 128, 197–212. [Google Scholar] [PubMed]
- Ferruzza, S.; Scarino, M.L.; Rotilio, G.; Ciriolo, M.R.; Santaroni, P.; Muda, A.O.; Sambuy, Y. Copper treatment alters the permeability of tight junctions in cultured human intestinal Caco-2 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G1138–G1148. [Google Scholar]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [PubMed]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [PubMed]
- James, D.; Devaraj, S.; Bellur, P.; Lakkanna, S.; Vicini, J.; Boddupalli, S. Novel concepts of broccoli sulforaphanes and disease: Induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli. Nutr. Rev. 2012, 70, 654–665. [Google Scholar] [PubMed]
- Jung, H.A.; Karki, S.; Ehom, N.; Yoon, M.; Kim, E.J.; Choi, J.S. Anti-Diabetic and Anti-Inflammatory Effects of Green and Red Kohlrabi Cultivars (Brassica oleracea var. gongylodes). Prev. Nutr. Food Sci. 2014, 19, 281–290. [Google Scholar]
- Vale, A.P.; Santos, J.; Brito, N.V.; Peixoto, V.; Carvalho, R.; Rosa, E.; Oliveira, M.B.P.P. Light influence in the nutritional composition of Brassica oleracea sprouts. Food Chem. 2015, 178, 292–300. [Google Scholar]
- Vale, A.P.; Cidade, H.; Pinto, M.; Oliveira, M.B.P.P. Effect of sprouting and light cycle on antioxidant activity of Brassica oleracea varieties. Food Chem. 2014, 165, 379–387. [Google Scholar] [PubMed]
- Jahangir, M.; Abdel-Farid, I.B.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 2009, 67, 23–33. [Google Scholar]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar]
- Baenas, N.; García-Viguera, C.; Moreno, D. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541–13563. [Google Scholar]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. (Amst.) 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Tapsell, L.C. Food, not nutrients, is the fundamental unit in nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Perles, R.; Mena, P.; García-Viguera, C.; Moreno, D.A. Brassica foods as a dietary source of vitamin C: A review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1076–1091. [Google Scholar] [CrossRef] [PubMed]
- Di Gesso, J.L.; Kerr, J.S.; Zhang, Q.; Raheem, S.; Yalamanchili, S.K.; O’Hagan, D.; Kay, C.D.; O’Connell, M.A. Flavonoid metabolites reduce tumor necrosis factor-α secretion to a greater extent than their precursor compounds in human THP-1 monocytes. Mol. Nutr. Food Res. 2015, 59, 1143–1154. [Google Scholar] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Milkowski, C.; Strack, D. Sinapate esters in brassicaceous plants: Biochemistry, molecular biology, evolution and metabolic engineering. Planta 2010, 232, 19–35. [Google Scholar] [PubMed]
- Moreno, D.A.; Pérez-Balibrea, S.; Ferreres, F.; Gil-Izquierdo, Á.; García-Viguera, C. Acylated anthocyanins in broccoli sprouts. Food Chem. 2010, 123, 358–363. [Google Scholar]
- Baenas, N.; Ferreres, F.; García-Viguera, C.; Moreno, D.A. Radish sprouts—Characterization and elicitation of novel varieties rich in anthocyanins. Food Res. Int. 2015, 69, 305–312. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Sanchez-Ballesta, M.T. Anthocyanins: From plant to health. Phytochem. Rev. 2007, 7, 281–299. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Matsui, T.; Ueda, T.; Oki, T.; Sugita, K.; Terahara, N.; Matsumoto, K. α-Glucosidase Inhibitory Action of Natural Acylated Anthocyanins. α-Glucosidase Inhibition by Isolated Acylated Anthocyanins. J. Agric. Food Chem. 2001, 49, 1952–1956. [Google Scholar] [PubMed]
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Risitano, R.; Currò, M.; Cirmi, S.; Ferlazzo, N.; Campiglia, P.; Caccamo, D.; Ientile, R.; Navarra, M. Flavonoid fraction of Bergamot juice reduces LPS-induced inflammatory response through SIRT1-mediated NF-κB inhibition in THP-1 monocytes. PLoS ONE 2014, 9, e107431. [Google Scholar]
- Ma, M.-M.; Li, Y.; Liu, X.-Y.; Zhu, W.-W.; Ren, X.; Kong, G.-Q.; Huang, X.; Wang, L.-P.; Luo, L.-Q.; Wang, X.-Z. Cyanidin-3-O-Glucoside Ameliorates Lipopolysaccharide-Induced Injury Both In Vivo and In Vitro Suppression of NF-κB and MAPK Pathways. Inflammation 2015, 38, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mohri, S.; Hirai, S.; Lin, S.; Goto, T.; Ohyane, C.; Sakamoto, T.; Takahashi, H.; Shibata, D.; Takahashi, N.; et al. Tomato extract suppresses the production of proinflammatory mediators induced by interaction between adipocytes and macrophages. Biosci. Biotechnol. Biochem. 2015, 79, 82–87. [Google Scholar]
- Parada, J.; Aguilera, J.M. Food microstructure affects the bioavailability of several nutrients. J. Food Sci. 2007, 72, 21–32. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Toydemir, G.; Boyacioglu, D.; Capanoglu, E.; Van Der Meer, I.M.; Tomassen, M.M.M.; Hall, R.D.; Mes, J.J.; Beekwilder, J. Investigating the transport dynamics of anthocyanins from unprocessed fruit and processed fruit juice from sour cherry (Prunus cerasus L.) across intestinal epithelial cells. J. Agric. Food Chem. 2013, 61, 11434–11441. [Google Scholar] [PubMed]
- Melillo De Magalhães, P.; Dupont, I.; Hendrickx, A.; Joly, A.; Raas, T.; Dessy, S.; Sergent, T.; Schneider, Y.J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chem. 2012, 134, 864–871. [Google Scholar] [PubMed]
- Sergent, T.; Piront, N.; Meurice, J.; Toussaint, O.; Schneider, Y.J. Anti-inflammatory effects of dietary phenolic compounds in an in vitro model of inflamed human intestinal epithelium. Chem. Biol. Interact. 2010, 188, 659–667. [Google Scholar] [CrossRef]
- Masci, A.; Mattioli, R.; Costantino, P.; Baima, S.; Morelli, G.; Punzi, P.; Giordano, C.; Pinto, A.; Donini, L.M.; Erme, M.; et al. Neuroprotective Effect of Brassica oleracea Sprouts Crude Juice in a Cellular Model of Alzheimer’ s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 781938. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Di Castro, S.; Cotugno, M.; Bianchi, F.; Mattioli, R.; Baima, S.; Stanzione, R.; Madonna, M.; Bozzao, C.; Marchitti, S.; et al. Protective effects of Brassica oleracea sprouts extract toward renal damage in high-salt-fed SHRSP. J. Hypertens. 2015, 33, 1465–1479. [Google Scholar] [PubMed]
- Berrueta, L.A.; Alonso-Salces, R.M.; Héberger, K. Supervised pattern recognition in food analysis. J. Chromatogr. A 2007, 1158, 196–214. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, A.F.; Haminiuk, C.W.I.; Nunes, C.A.; Schnitzler, E.; Van Ruth, S.M.; Granato, D. Chemical Composition, Sensory Properties, Provenance, and Bioactivity of Fruit Juices as Assessed by Chemometrics: A Critical Review and Guideline. Compr. Rev. Food Sci. Food Saf. 2014, 13, 300–316. [Google Scholar]
- Cassidy, A.; Rogers, G.; Peterson, J.J.; Dwyer, J.T.; Lin, H.; Jacques, P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015, 102, 3–5. [Google Scholar]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2015. [Google Scholar] [CrossRef]
- Hadjipavlou-Litina, D.; Pontiki, E. Aryl-acetic and cinnamic acids as lipoxygenase inhibitors with antioxidant, anti-inflammatory, and anticancer activity. Methods Mol. Biol. 2015, 1208, 361–377. [Google Scholar]
- Das, N.; Berhow, M.A.; Angelino, D.; Jeffery, E.H. Camelina sativa Defatted Seed Meal Contains Both Alkyl Sulfinyl Glucosinolates and Quercetin That Synergize Bioactivity. J. Agric. Food Chem. 2014, 62, 8385–8391. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Hebbar, V.; Shen, G.; Gopalakrishnan, A.; Khor, T.O.; Yu, S.; Xu, C.; Kong, A.-N. Synergistic effects of a combination of dietary factors sulforaphane and (−)epigallocatechin-3-gallate in HT-29 AP-1 human colon carcinoma cells. Pharm. Res. 2008, 25, 387–399. [Google Scholar] [PubMed]
- Appari, M.; Babu, K.R.; Kaczorowski, A.; Gross, W.; Herr, I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int. J. Oncol. 2014, 45, 1391–1400. [Google Scholar] [PubMed]
- Pradhan, S.J.; Mishra, R.; Sharma, P.; Kundu, G.C. Quercetin and sulforaphane in combination suppress the progression of melanoma through the down-regulation of matrix metalloproteinase. Exp. Ther. Med. 2010, 1, 915–920. [Google Scholar] [PubMed]
- Rochfort, S.J.; Trenerry, V.C.; Imsic, M.; Panozzo, J.; Jones, R. Class targeted metabolomics: ESI ion trap screening methods for glucosinolates based on MSn fragmentation. Phytochemistry 2008, 69, 1671–1679. [Google Scholar]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar]
- Rapisarda, P.; Fanella, F.; Maccarone, E. Reliability of Analytical Methods for Determining Anthocyanins in Blood Orange Juices. J. Agric. Food Chem. 2000, 48, 2249–2252. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Gasperotti, M.; Franceschi, P.; Caputi, L.; Viola, R.; Mattivi, F. A versatile targeted metabolomics method for the rapid quantification of multiple classes of phenolics in fruits and beverages. J. Agric. Food Chem. 2012, 60, 8831–8840. [Google Scholar] [PubMed]
- Arapitsas, P.; Perenzoni, D.; Nicolini, G.; Mattivi, F. Study of Sangiovese Wines Pigment Profile by UHPLC-MS/MS. J. Agric. Food Chem. 2012, 60, 10461–10471. [Google Scholar] [PubMed]
- Ferruzza, S.; Scarino, M.L.; Gambling, L.; Natella, F.; Sambuy, Y. Biphasic effect of iron on human intestinal Caco-2 cells: Early effect on tight junction permeability with delayed onset of oxidative cytotoxic damage. Cell Mol. Biol. 2003, 49, 89–99. [Google Scholar] [PubMed]
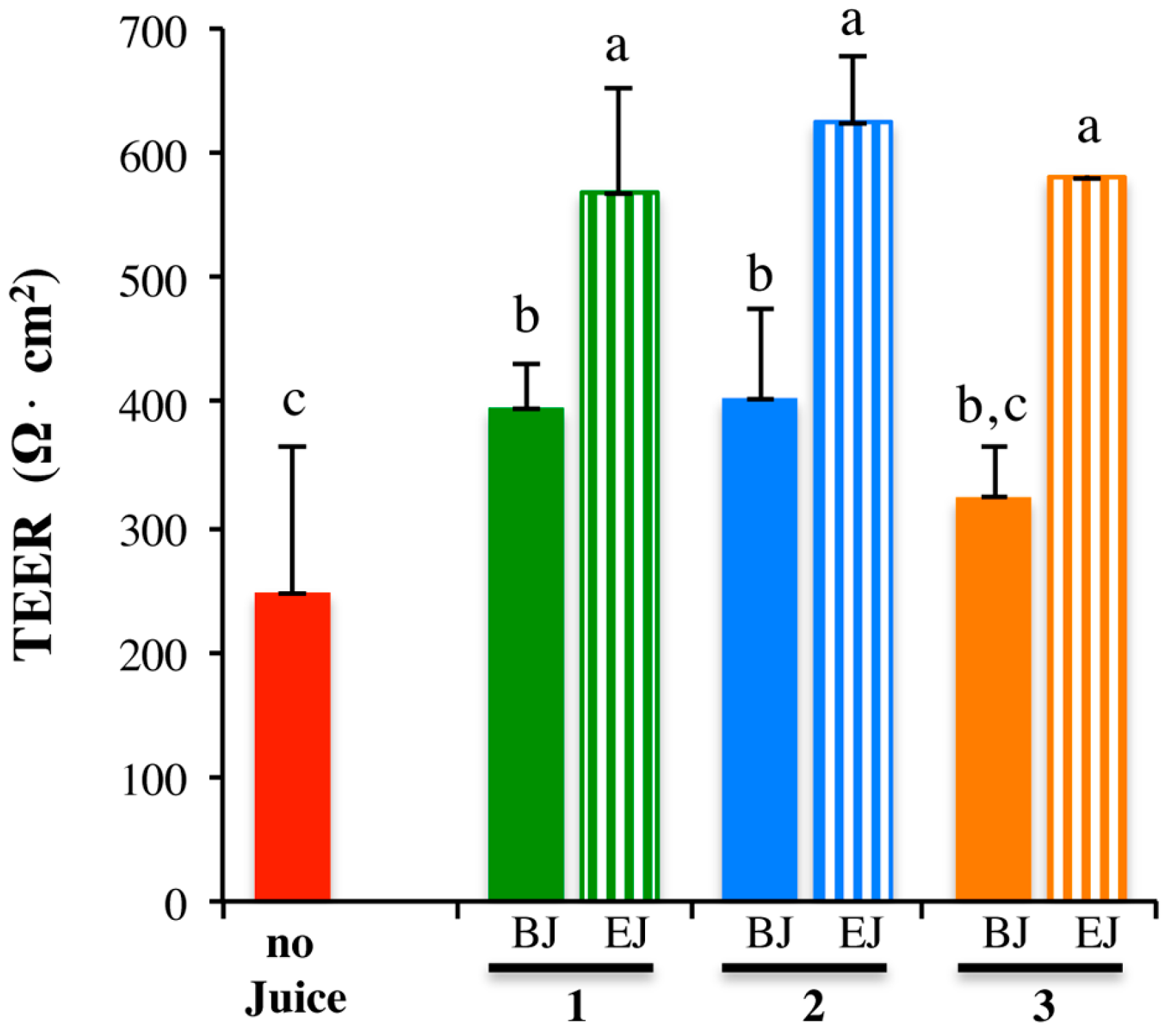
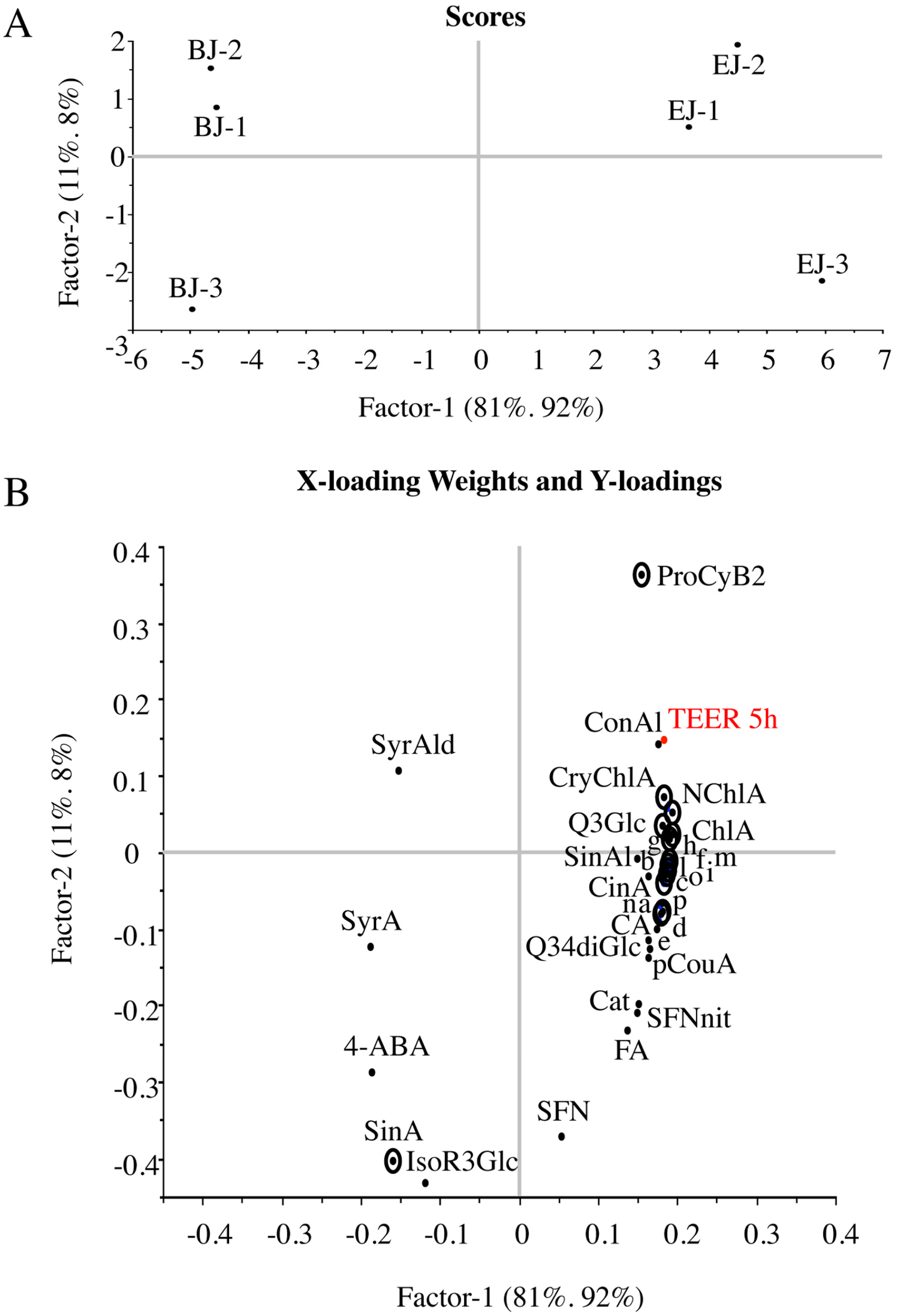
| Basal Juice | Enriched Juice | Fold | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (BJ) | (EJ) | (EJ/BJ) | |||||||
| TP | Total Phenols (mg GAE/mL) | 1.86 | ± | 0.24 | 3.01 | ± | 0.34 | 1.6 | ** |
| TF | Total Flavonoids (mg CE/mL) | 0.50 | ± | 0.08 | 1.29 | ± | 0.30 | 2.6 | * |
| TA | Total Anthocyanins (μg Cy_3_GlcE/mL) | 4.29 | ± | 0.48 | 40.15 | ± | 15.28 | 9.4 | * |
| SFN | Sulforaphane (μg/mL) | 19.37 | ± | 2.65 | 21.90 | ± | 1.57 | 1.1 | |
| SFNnit | Sulforaphane nitrile (μg/mL) | 3.47 | ± | 0.31 | 4.50 | ± | 0.44 | 1.3 | * |
| Polyphenols (μg/mL) | |||||||||
| 4-ABA | 4-Aminobenzoic acid | 0.08 | ± | 0.02 | 0.04 | ± | 0.01 | 0.6 | |
| CA | Caffeic acid | 0.01 | ± | 0.01 | 0.19 | ± | 0.06 | 13.5 | * |
| Cat | Catechin | 2.37 | ± | 0.24 | 3.06 | ± | 0.20 | 1.3 | * |
| ChlA | Chlorogenic acid | 0.08 | ± | 0.02 | 1.11 | ± | 0.37 | 13.5 | * |
| CinA | Cinnamic acid | 0.01 | ± | 0.00 | 0.04 | ± | 0.01 | 3.5 | ** |
| ConAl | Coniferyl alcohol | 0.17 | ± | 0.01 | 0.39 | ± | 0.13 | 2.4 | * |
| pCouA | p-Coumaric acid | 0.29 | ± | 0.06 | 1.54 | ± | 0.47 | 5.2 | * |
| CryChlA | Cryptochlorogenic acid | 0.02 | ± | 0.01 | 0.15 | ± | 0.03 | 7.1 | ** |
| FA | Ferulic acid | 0.38 | ± | 0.03 | 0.64 | ± | 0.17 | 1.7 | |
| IsoR3Glc | Isorhamnetin-3-Glc | 0.05 | ± | 0.03 | 0.03 | ± | 0.01 | 0.7 | |
| NChlA | Neochlorogenic acid | 0.24 | ± | 0.02 | 12.03 | ± | 1.96 | 49.5 | *** |
| ProCyB2 | Procyanidin B2 | 0.02 | ± | 0.01 | 0.04 | ± | 0.01 | 1.7 | |
| Q3Glc | Quercetin-3-Glc | n.d. | 0.21 | ± | 0.01 | *** | |||
| Q34diGlc | Quercetin-3.4-diGlc | 0.15 | ± | 0.05 | 0.88 | ± | 0.34 | 5.9 | * |
| SinA | Sinapic acid | 38.01 | ± | 12.02 | 25.59 | ± | 7.12 | 0.7 | |
| SinAl | Sinapyl alcohol | 0.15 | ± | 0.07 | 0.33 | ± | 0.04 | 2.2 | * |
| SyrAld | Syringaldehyde | 0.05 | ± | 0.01 | 0.02 | ± | 0.01 | 0.4 | * |
| SyrA | Syringic acid | 0.41 | ± | 0.02 | 0.28 | ± | 0.06 | 0.7 | * |
| Anthocyanins (μg/mL) 1 | |||||||||
| Cy3_a | Cy_3_sinapoyl_sinapoyl_diGlc_5_malonyl_Glc | n.d. | 0.46 | ± | 0.11 | ** | |||
| Cy3_b | Cy_3_sinapoyl_feruloyl_diGlc_5_malonyl_Glc | n.d. | 0.76 | ± | 0.27 | * | |||
| Cy3_c | Cy_3_coumaryl_synapoyl_diGlc_5_malonyl_Glc | n.d. | 1.66 | ± | 0.27 | *** | |||
| Cy3_d | Cy_3_sinapoyl_synapoyl_diGlc_5_Glc | n.d. | 1.08 | ± | 0.34 | ** | |||
| Cy3_e | Cy_3_coumaryl_feruoyl_diGlc_5_malonyl_Glc | n.d. | 1.11 | ± | 0.38 | * | |||
| Cy3_f | Cy_3_sinapoyl_feruloyl_diGlc_5_Glc | n.d. | 1.68 | ± | 0.30 | ** | |||
| Cy3_g | Cy_3_sinapoyl_diGlc_5_malonyl_Glc | n.d. | 0.37 | ± | 0.08 | ** | |||
| Cy3_h | Cy_3_feruloyl_diGlc_5_malonyl_Glc | n.d. | 0.29 | ± | 0.01 | *** | |||
| Cy3_i | Cy_3_caffeyl_diGlc_5_malonyl_Glc | n.d. | 0.07 | ± | 0.06 | ||||
| Cy3_l | Cy_3_coumaryl_diGlc_5_malonyl_Glc | n.d. | 0.55 | ± | 0.07 | *** | |||
| Cy3_m | Cy_3_sinapoyl_diGlc_5_Glc | n.d. | 0.94 | ± | 0.11 | *** | |||
| Cy3_n | Cy_3_feruloyl_diGlc_5_Glc | n.d. | 0.85 | ± | 0.20 | ** | |||
| Cy3_o | Cy_3_coumaroyl_diGlc_5_Glc | n.d. | 0.84 | ± | 0.08 | *** | |||
| Cy3_p | Cy_3_diGlc_5_Glc | 0.58 | ± | 0.05 | 1.61 | ± | 0.25 | 2.8 | ** |
| Attributes | % Explained Variance | No. of Factors | Correlation a | Validation a | BW b | ||||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | R2 | RMSE | R2 | RMSE | Positive | Negative | ||
| TEER | 92% | 100% | 2 | 0.99 | 3.59 | 0.96 | 25.31 | Cinnamic acid; Cryptochlorogenic acid; Neochlorogenic acid; Procyanidin B2; Quercetin_3_Glc; Cy_3_diGlc_5_Glc; Cy_3_sinapoyl_feruloyl_diGlc_5_Glc; Cy_3_feruloyl_diGlc_5_malonyl_Glc; Cy_3_coumaroyl_diGlc_5_Glc; Cy_3_coumaryl_diGlc_5_malonyl_Glc; Cy_3_coumaryl_synapoyl_diGlc_5_ malonyl_Glc; | Synapic acid |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trošt, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation. Pharmaceuticals 2016, 9, 48. https://doi.org/10.3390/ph9030048
Ferruzza S, Natella F, Ranaldi G, Murgia C, Rossi C, Trošt K, Mattivi F, Nardini M, Maldini M, Giusti AM, et al. Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation. Pharmaceuticals. 2016; 9(3):48. https://doi.org/10.3390/ph9030048
Chicago/Turabian StyleFerruzza, Simonetta, Fausta Natella, Giulia Ranaldi, Chiara Murgia, Carlotta Rossi, Kajetan Trošt, Fulvio Mattivi, Mirella Nardini, Mariateresa Maldini, Anna Maria Giusti, and et al. 2016. "Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation" Pharmaceuticals 9, no. 3: 48. https://doi.org/10.3390/ph9030048
APA StyleFerruzza, S., Natella, F., Ranaldi, G., Murgia, C., Rossi, C., Trošt, K., Mattivi, F., Nardini, M., Maldini, M., Giusti, A. M., Moneta, E., Scaccini, C., Sambuy, Y., Morelli, G., & Baima, S. (2016). Nutraceutical Improvement Increases the Protective Activity of Broccoli Sprout Juice in a Human Intestinal Cell Model of Gut Inflammation. Pharmaceuticals, 9(3), 48. https://doi.org/10.3390/ph9030048











