Odanacatib, a Cathepsin K Cysteine Protease Inhibitor, Kills Hookworm In Vivo
Abstract
:1. Introduction
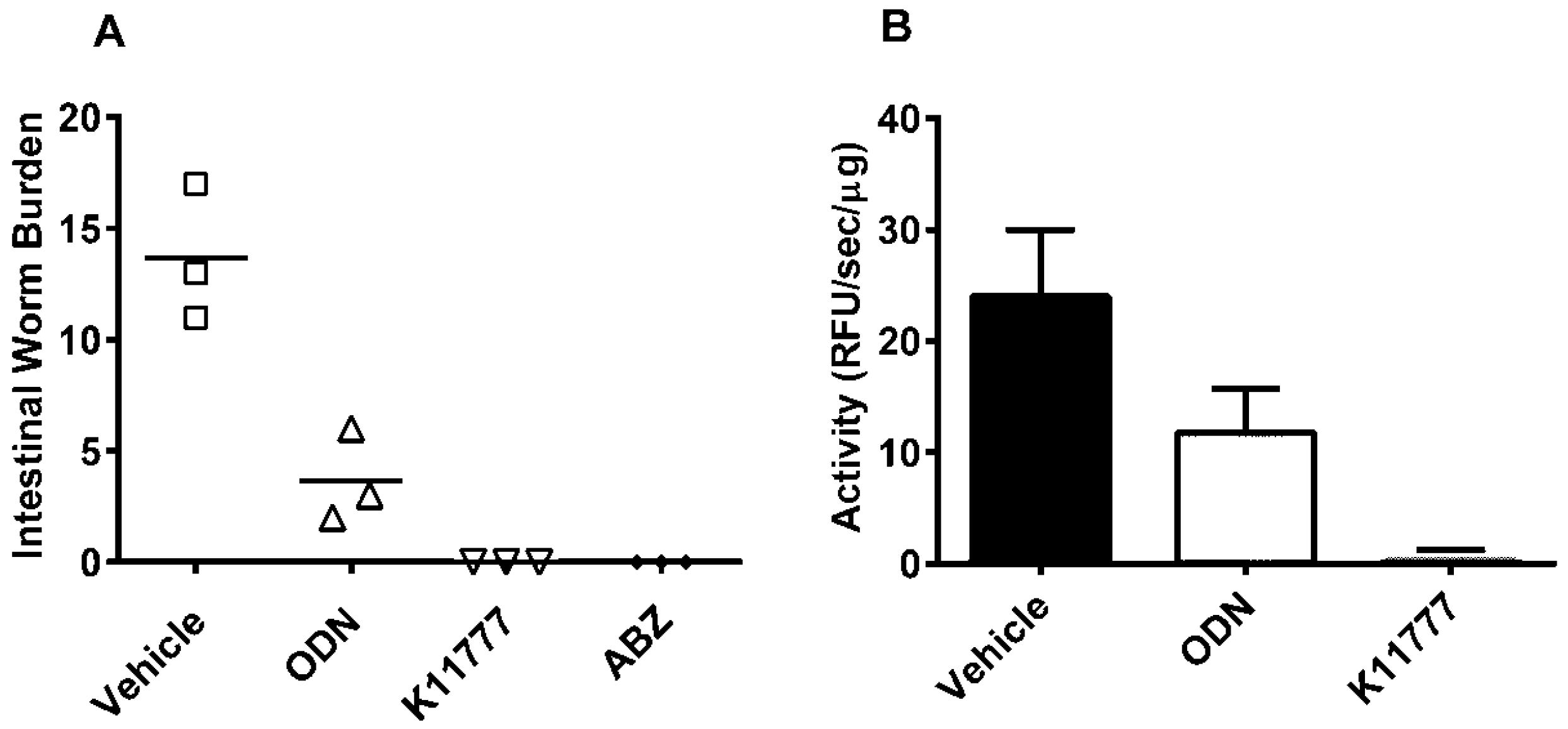
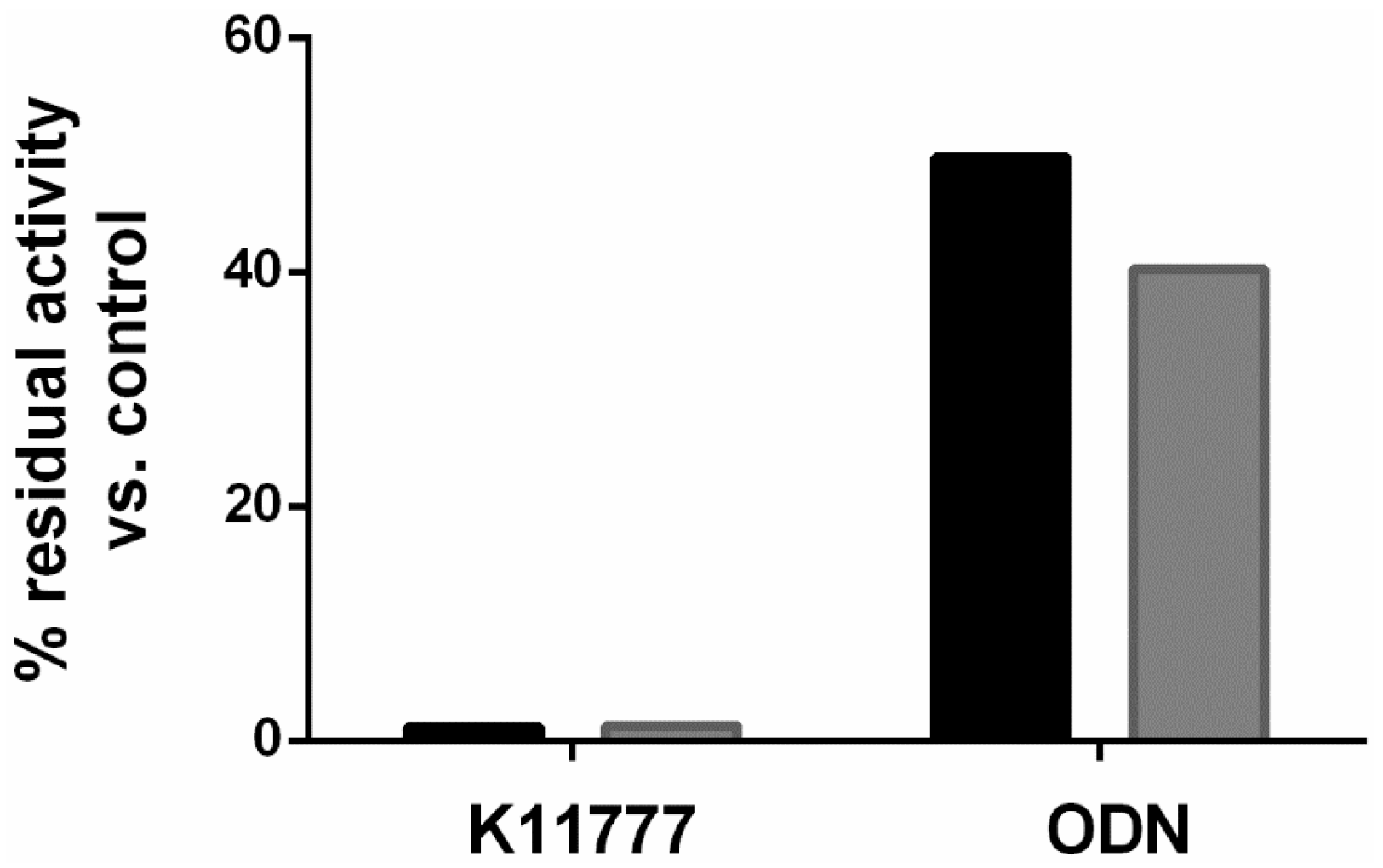
2. Results and Discussion
3. Experimental Section
3.1. Animals and Compounds
3.2. Treatment Regimens and Cysteine Protease Activity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stoll, N.R. This wormy world. J. Parasitol. 1947, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology 2010, 137, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 386, 743–800. [Google Scholar]
- Sen, H.G. Man and his hookworm parasites. BIOS 1974, 45, 68–73. [Google Scholar]
- Jardim-Botelho, A.; Raff, S.; Rodrigues Rde, A.; Hoffman, H.J.; Diemert, D.J.; Correa-Oliveira, R.; Bethony, J.M.; Gazzinelli, M.F. Hookworm, Ascaris lumbricoides infection and polyparasitism associated with poor cognitive performance in brazilian schoolchildren. Trop. Med. Int. Health 2008, 13, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Sakti, H.; Nokes, C.; Hertanto, W.S.; Hendratno, S.; Hall, A.; Bundy, D.A.; Satoto. Evidence for an association between hookworm infection and cognitive function in indonesian school children. Trop. Med. Int. Health 1999, 4, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Hotez, P.J.; Bundy, D.A. Hookworm-related anaemia among pregnant women: A systematic review. PLoS Negl. Trop. Dis. 2008, 2, e291. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Magnussen, P.; Ouma, J.H.; Andreassen, J.; Friis, H. The contribution of hookworm and other parasitic infections to haemoglobin and iron status among children and adults in western Kenya. Trans. R. Soc. Trop. Med. Hyg. 1998, 92, 643–649. [Google Scholar] [CrossRef]
- Melku, M.; Addis, Z.; Alem, M.; Enawgaw, B. Prevalence and predictors of maternal anemia during pregnancy in Gondar, northwest Ethiopia: An institutional based cross-sectional study. Anemia 2014, 2014, 108593. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.G.; Woo, K.; McCarthy, J.S.; Mackenzie, C.D.; Horton, J.; Prichard, R.K.; de Silva, N.R.; Olliaro, P.L.; Lazdins-Helds, J.K.; Engels, D.A.; et al. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int. J. Parasitol. 2010, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.L. Prospects for the control of neglected tropical diseases by mass drug administration. Expert Rev. Anti-Infect. Therapy 2009, 7, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Van den Enden, E. Pharmacotherapy of helminth infection. Expert Opin. Pharmacother. 2009, 10, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Boatin, B.A.; Basanez, M.G.; Prichard, R.K.; Awadzi, K.; Barakat, R.M.; Garcia, H.H.; Gazzinelli, A.; Grant, W.N.; McCarthy, J.S.; N’Goran, E.K.; et al. A research agenda for helminth diseases of humans: Towards control and elimination. PLoS Negl. Trop. Dis. 2012, 6, e1547. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, D.C.; Maayan, N.; Soares-Weiser, K.; Donegan, S.; Garner, P. Deworming drugs for soil-transmitted intestinal worms in children: Effects on nutritional indicators, haemoglobin, and school performance. Cochrane Database Syst. Rev. 2015, 7, CD000371. [Google Scholar] [PubMed]
- Nontasut, P.; Singhasivanon, V.; Prarinyanuparp, V.; Chiamratana, B.; Sanguankiat, S.; Dekumyoy, P.; Setasuban, P. Effect of single-dose albendazole and single-dose mebendazole on Necator americanus. South. Asian J. Trop. Med. Public Health 1989, 20, 237–242. [Google Scholar]
- Horton, J. Albendazole: A broad spectrum anthelminthic for treatment of individuals and populations. Curr. Opin. Infect. Dis. 2002, 15, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Keiser, J.; Utzinger, J. Efficacy of current drugs against soil-transmitted helminth infections: Systematic review and meta-analysis. JAMA 2008, 299, 1937–1948. [Google Scholar] [CrossRef] [PubMed]
- Flohr, C.; Tuyen, L.N.; Lewis, S.; Minh, T.T.; Campbell, J.; Britton, J.; Williams, H.; Hien, T.T.; Farrar, J.; Quinnell, R.J. Low efficacy of mebendazole against hookworm in vietnam: two randomized controlled trials. Am. J. Trop. Med. Hyg. 2007, 76, 732–736. [Google Scholar] [PubMed]
- Levecke, B.; Montresor, A.; Albonico, M.; Ame, S.M.; Behnke, J.M.; Bethony, J.M.; Noumedem, C.D.; Engels, D.; Guillard, B.; Kotze, A.C.; et al. Assessment of anthelmintic efficacy of mebendazole in school children in six countries where soil-transmitted helminths are endemic. PLoS Negl. Trop. Dis. 2014, 8, e3204. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.; Seiler, J.; Kuesel, A.; Horton, J.; Clark, J.N.; Don, R.; Keiser, J. Potential drug development candidates for human soil-transmitted helminthiases. PLoS Negl. Trop. Dis. 2011, 5, e1138. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Research Priorities for Helminth Infections: Technical Report of the TDR Disease Reference Group on Helminth Infections; WHO Technical Report Series; WHO: Geneva, Switzerland, 2012; p. 972. [Google Scholar]
- World Health Organization. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases—A Roadmap for Implementation 2012. Available online: http://www.emro.who.int/neglected-tropical-diseases/ntd-infocus/ntd-roadmap.html (accessed on 10 May 2016).
- Uniting to Combat Neglected Tropical Diseases. Available online: http://unitingtocombatntds.org/ (accessed on 28 June 2016).
- Humphries, D.; Mosites, E.; Otchere, J.; Twum, W.A.; Woo, L.; Jones-Sanpei, H.; Harrison, L.M.; Bungiro, R.D.; Benham-Pyle, B.; Bimi, L.; et al. Epidemiology of hookworm infection in Kintampo north municipality, Ghana: Patterns of malaria coinfection, anemia, and albendazole treatment failure. Am. J. Trop. Med. Hyg. 2011, 84, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, A.U.; Sjoberg, M.K.; Allangba, A.; Traore, M.; Lohourignon, L.K.; Tschannen, A.B.; N’Goran, E.K.; Utzinger, J. Sequential analysis of helminth egg output in human stool samples following albendazole and praziquantel administration. Acta Trop. 2009, 109, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Bungiro, R.; Cappello, M. Twenty-first century progress toward the global control of human hookworm infection. Curr. Infect. Dis. Rep. 2011, 13, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Albonico, M.; Behnke, J.M.; Kotze, A.C.; Prichard, R.K.; McCarthy, J.S.; Montresor, A.; Levecke, B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int. J. Parasitol. Drugs Drug Resist. 2011, 1, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.H.; Hui-Ming, W.; Tanner, M.; Utzinger, J.; Chong, W. Tribendimidine: A promising, safe and broad-spectrum anthelmintic agent from china. Acta Trop. 2005, 94, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cappello, M.; Bungiro, R.D.; Harrison, L.M.; Bischof, L.J.; Griffitts, J.S.; Barrows, B.D.; Aroian, R.V. A purified bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc. Natl. Acad. Sci. USA 2006, 103, 15154–15159. [Google Scholar] [CrossRef] [PubMed]
- McKerrow, J.H.; Caffrey, C.; Kelly, B.; Loke, P.; Sajid, M. Proteases in parasitic diseases. Annu. Rev. Pathol. 2006, 1, 497–536. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, C.R.; Lima, A.P.; Steverding, D. Cysteine peptidases of kinetoplastid parasites. Adv. Exp. Med. Biol. 2011, 712, 84–99. [Google Scholar] [PubMed]
- Abdulla, M.H.; Lim, K.C.; Sajid, M.; McKerrow, J.H.; Caffrey, C.R. Schistosomiasis mansoni: Novel chemotherapy using a cysteine protease inhibitor. PLoS Med. 2007, 4, e14. [Google Scholar] [CrossRef] [PubMed]
- Dalton, J.P.; Caffrey, C.R.; Sajid, M.; Stack, C.; Donnelly, S.; Loukas, A.; Don, T.; McKerrow, J.; Halton, D.W.; Brindley, P.J. Proteases in trematode biology. In Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology; Maule, A.G., Marks, N.J., Eds.; CAB International Wallingford: Oxfordshire, UK, 2006. [Google Scholar]
- Caffrey, C.R.; Britton, C.; McKerrow, J.H. Helminth cysteine proteases. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Elsevier: Oxford, UK, 2011. [Google Scholar]
- Vermeire, J.J.; Lantz, L.D.; Caffrey, C.R. Cure of hookworm infection with a cysteine protease inhibitor. PLoS Negl. Trop. Dis. 2012, 6, e1680. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.T.; Rasnick, D.; Klaus, J.L.; Bromme, D. Vinyl sulfones as mechanism-based cysteine protease inhibitors. J. Med. Chem. 1995, 38, 3193–3196. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, J.Y.; Chauret, N.; Cromlish, W.; Desmarais, S.; Duong le, T.; Falgueyret, J.P.; Kimmel, D.B.; Lamontagne, S.; Leger, S.; LeRiche, T.; et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorg. Med. Chem. Lett. 2008, 18, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, K.; Black, W.C.; Nicoll-Griffith, D.; McIntosh, I.; Chauret, N.; Day, S.; Rosenberg, E.; Koeplinger, K. Pharmacokinetics and metabolism in rats, dogs, and monkeys of the cathepsin K inhibitor odanacatib: Demethylation of a methylsulfonyl moiety as a major metabolic pathway. Drug Metab. Dispos. 2011, 39, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Kassahun, K.; McIntosh, I.; Koeplinger, K.; Sun, L.; Talaty, J.E.; Miller, D.L.; Dixon, R.; Zajic, S.; Stoch, S.A. Disposition and metabolism of the cathepsin k inhibitor odanacatib in humans. Drug Metab. Dispos. 2014, 42, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.J.; Kirschke, H.; Cathepsin, B.; Cathepsin, H.; Cathepsin, L. Methods in Enzymol; Elsevier: Amsterdam, The Netherlands, 1981; Volume 80, pp. 535–561. [Google Scholar]
- Caffrey, C.R.; Ruppel, A. Cathepsin B-like activity predominates over cathepsin L-like activity in adult Schistosoma mansoni and S. japonicum. Parasitol. Res. 1997, 83, 632–635. [Google Scholar] [CrossRef] [PubMed]
- Ranjit, N.; Jones, M.K.; Stenzel, D.J.; Gasser, R.B.; Loukas, A. A survey of the intestinal transcriptomes of the hookworms, Necator americanus and Ancylostoma caninum, using tissues isolated by laser microdissection microscopy. Int. J. Parasitol. 2006, 36, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.C.; Asgian, J.L.; Ekici, O.D.; James, K.E. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 2002, 102, 4639–4750. [Google Scholar] [CrossRef] [PubMed]
- Ndao, M.; Beaulieu, C.; Black, W.C.; Isabel, E.; Vasquez-Camargo, F.; Nath-Chowdhury, M.; Masse, F.; Mellon, C.; Methot, N.; Nicoll-Griffith, D.A. Reversible cysteine protease inhibitors show promise for a Chagas disease cure. Antimicrob. Agents Chemother. 2014, 58, 1167–1178. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Robertson, S.A.; Brinen, L.S.; McKerrow, J.H. Cruzain: The path from target validation to the clinic. Adv. Exp. Med. Biol. 2011, 712, 100–115. [Google Scholar] [PubMed]
- Ray, D.K.; Bhopale, K.K.; Shrivastava, V.B. Migration and growth of Ancylostoma ceylanicum in golden hamsters Mesocricetus auratus. J. Helminthol. 1972, 46, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Bhopale, K.K. Complete development of Ancylostoma ceylanicum (Looss, 1911) in golden hamsters, Mesocricetus auratus. Experientia 1972, 28, 359–361. [Google Scholar] [CrossRef] [PubMed]
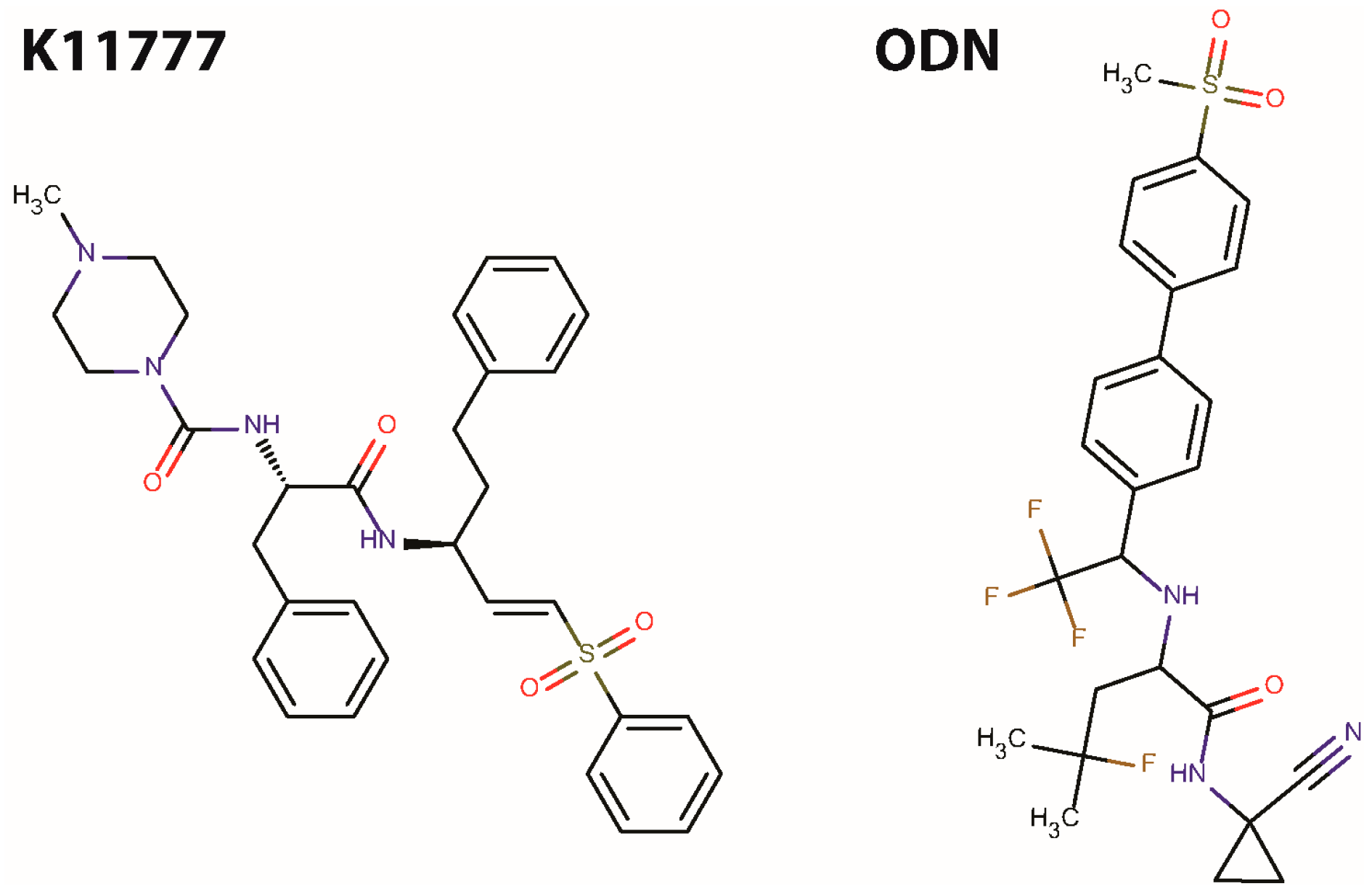
| Inhibitor | Target Cathepsin and IC50 Value (nM) | |||||
|---|---|---|---|---|---|---|
| CatB | CatF | CatK | CatL | CatS | Cruzain | |
| K11777 | 9 | 3 | 1.8 | <0.2 | <0.2 | 3.5 |
| ODN | 1034 | n.t. | 0.2 | 2995 | 60 | n.t. |
| Compound | Vehicle | Dose | Cmax | Tmax | AUC0–∞ | T1/2 | F |
|---|---|---|---|---|---|---|---|
| (mg/kg) | (μM) | (h) | (μM·h) | (h) | % | ||
| ODN (rat) | 100% PEG400 | 5 | 2.2 ± 0.4 | 1.8 ± 1.5 | 36 ± 10 | 5.8 ± 0.8 | 43 ± 12 |
| ODN (dog) | 60% PEG400 | 1 | 3.6 | 8 | 318 | 64 | 122 |
| ODN (monkey) | Imwitor-Tween 80 (1:1) | 5 | 0.3 ± 0.1 | 6 ± 2.3 | 4.8 ± 1.8 | 18 ± 4.3 | 18 ± 3.8 |
| ODN (man) | capsule | 25 1 | 0.24 ± 0.052 | 14.2 ± 8.1 | 19.9 ± 4.1 | 96.7 ± 18.3 | 34 |
| K11777 (mouse) | water | 92 | 2.6 | 0.3 | 3.9 | 0.8 | n.d. |
| K11777 (rat) | water | 100 | 3.1 | 4 | 10.5 | 1.9 | 22 |
| K11777 (dog) | water | 50 | 1.4 | 0.34 | 1.0 | 0.5 | 15 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vermeire, J.J.; Suzuki, B.M.; Caffrey, C.R. Odanacatib, a Cathepsin K Cysteine Protease Inhibitor, Kills Hookworm In Vivo. Pharmaceuticals 2016, 9, 39. https://doi.org/10.3390/ph9030039
Vermeire JJ, Suzuki BM, Caffrey CR. Odanacatib, a Cathepsin K Cysteine Protease Inhibitor, Kills Hookworm In Vivo. Pharmaceuticals. 2016; 9(3):39. https://doi.org/10.3390/ph9030039
Chicago/Turabian StyleVermeire, Jon J., Brian M. Suzuki, and Conor R. Caffrey. 2016. "Odanacatib, a Cathepsin K Cysteine Protease Inhibitor, Kills Hookworm In Vivo" Pharmaceuticals 9, no. 3: 39. https://doi.org/10.3390/ph9030039
APA StyleVermeire, J. J., Suzuki, B. M., & Caffrey, C. R. (2016). Odanacatib, a Cathepsin K Cysteine Protease Inhibitor, Kills Hookworm In Vivo. Pharmaceuticals, 9(3), 39. https://doi.org/10.3390/ph9030039






