An Allosteric Modulator of the Adenosine A1 Receptor Improves Cardiac Function Following Ischaemia in Murine Isolated Hearts
Abstract
:1. Introduction
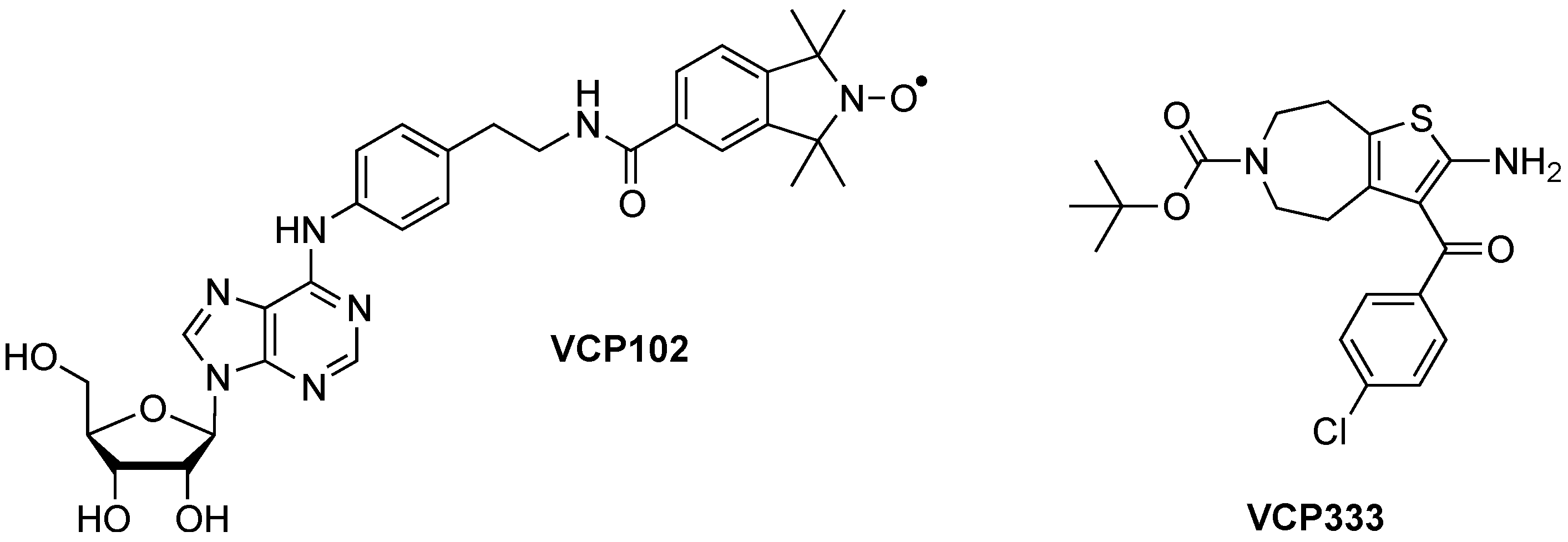
2. Experimental
2.1. Perfused Heart Preparation
2.2. Effluent Collection for Cardiac Troponin I Analysis
2.3. Statistical Analysis
3. Results and Discussion
3.1. Effect of Allosteric Modulator VCP333 Infusion during Early Reperfusion on Functional Recovery of Mouse Isolated Hearts
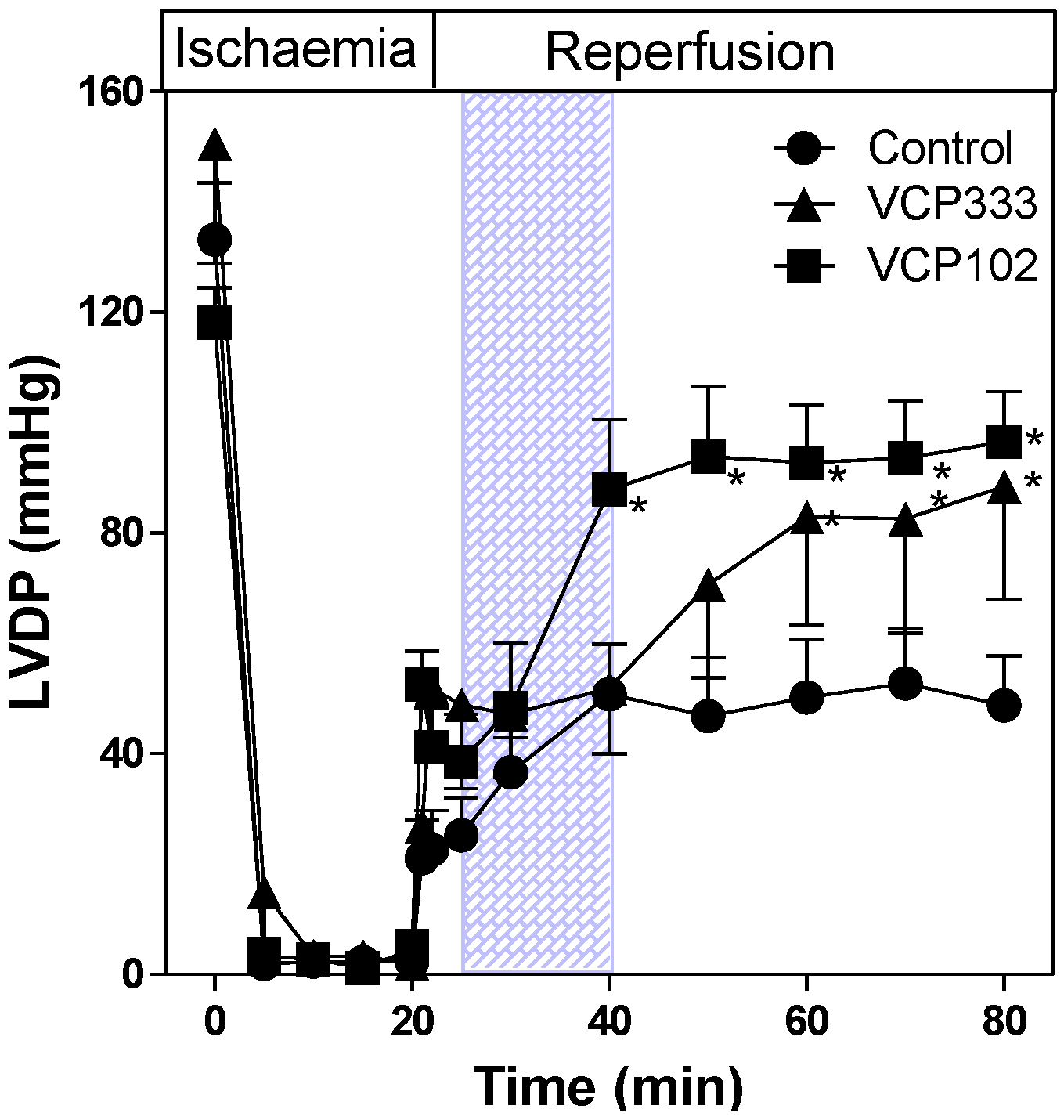
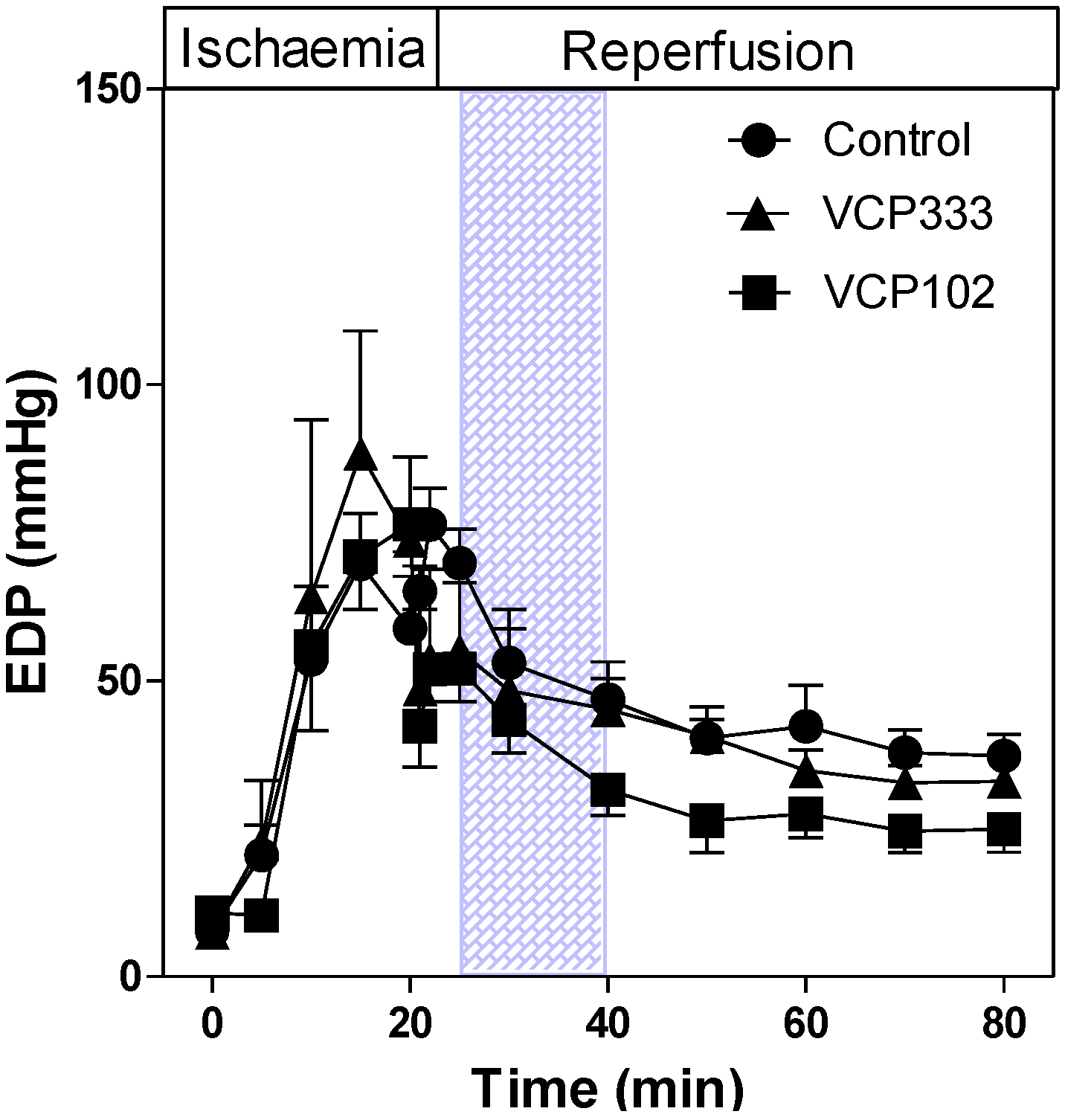
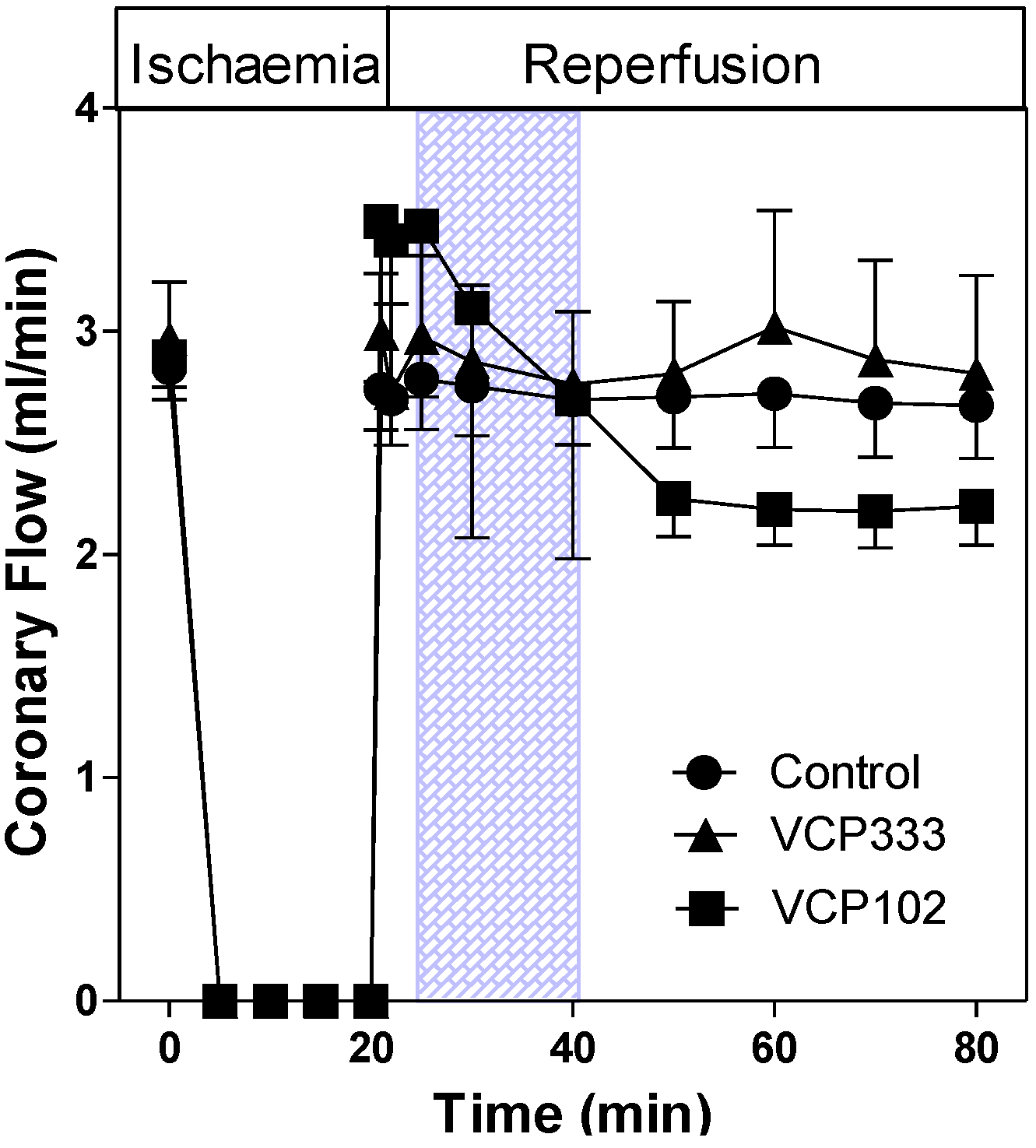
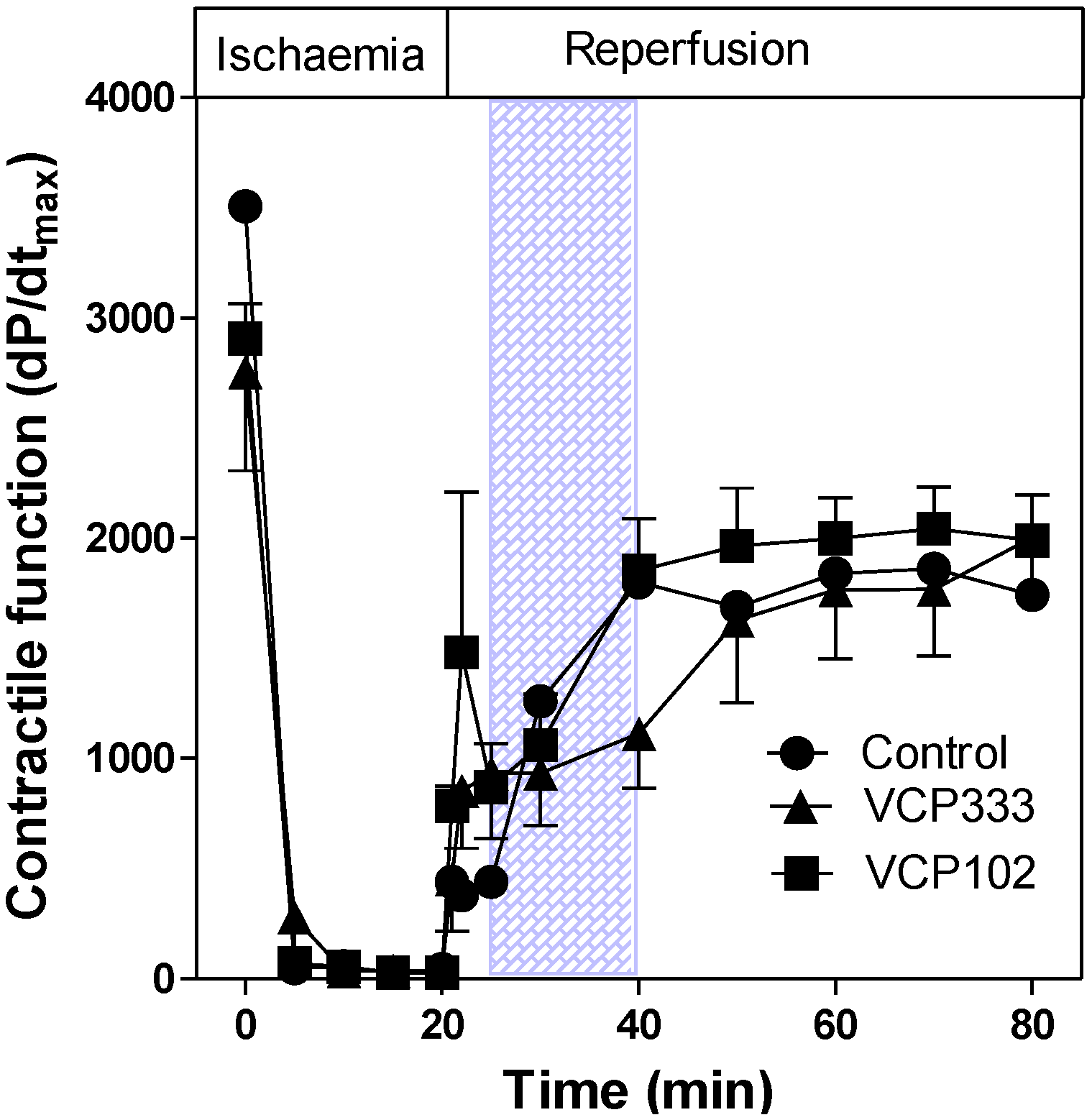
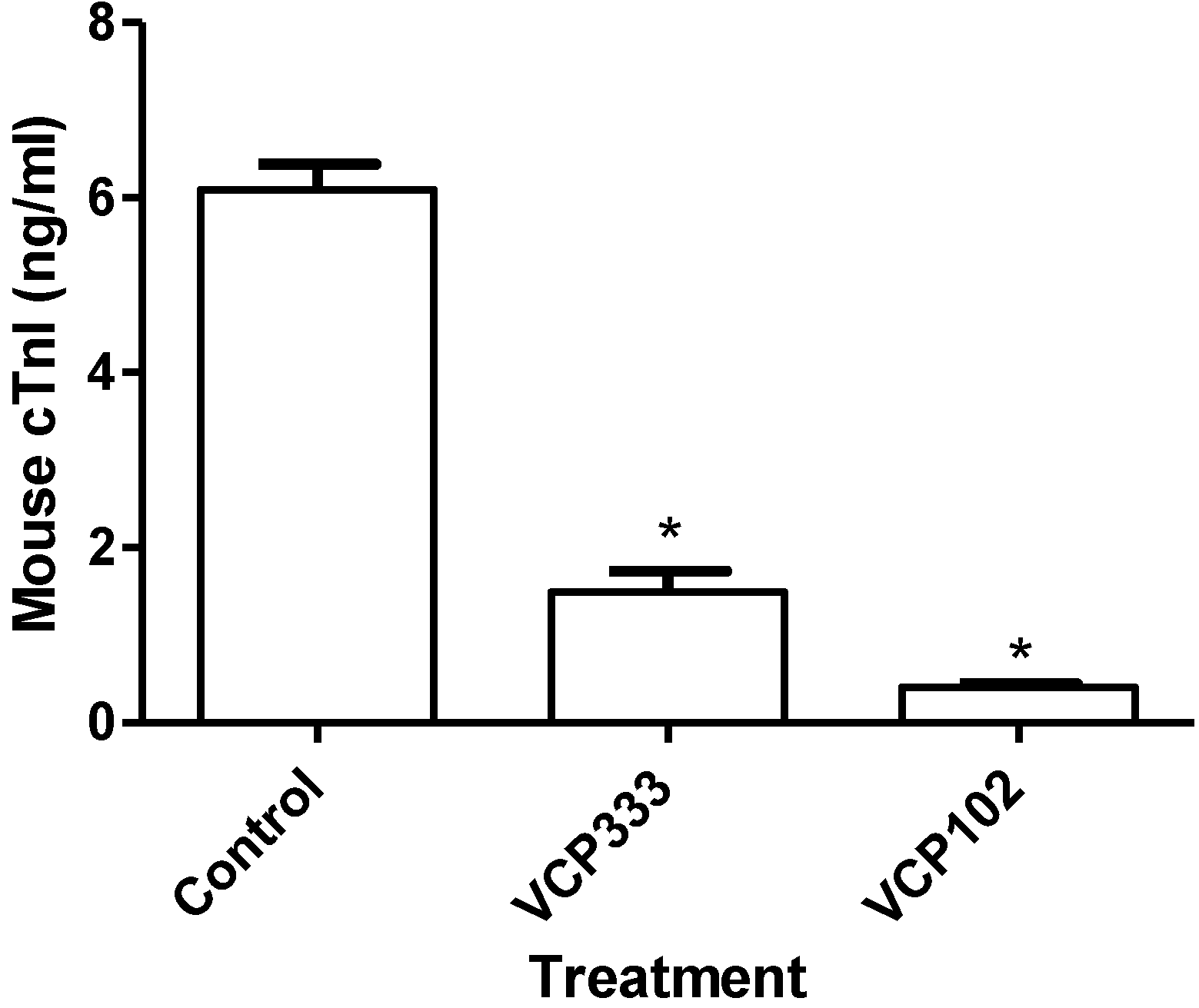
3.2. Effect of Allosteric Modulator VCP333 Infusion during Early Reperfusion on Cardiac Troponin I Production
4. Conclusions
Conflict of Interest
References
- Belardinelli, L.; Linden, J.; Berne, R.M. The cardiac effects of adenosine. Prog. Cardiovasc. Dis. 1989, 32, 73–97. [Google Scholar] [CrossRef]
- Braz, J.C.; Gregory, K.; Pathak, A.; Zhao, W.; Sahin, B.; Klevitsky, R.; Kimball, T.F.; Lorenz, J.N.; Nairn, A.C.; Liggett, S.B.; et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat. Med. 2004, 10, 248–254. [Google Scholar] [CrossRef]
- Nayeem, M.A.; Mustafa, S.J. Protein kinase C isoforms and A1 adenosine receptors in porcine coronary smooth muscle cells. Vascul. Pharmacol. 2002, 39, 47–54. [Google Scholar] [CrossRef]
- Ho, M.F.; Rose’meyer, R.B. Vascular adenosine receptors; Potential clinical applications. Curr. Vasc. Pharmacol. 2012, 10, 23140547. [Google Scholar]
- Ross, A.M.; Gibbons, R.J.; Stone, G.W.; Kloner, R.A.; Alexander, R.W. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II). J. Am. Coll. Cardiol. 2005, 45, 1775–1780. [Google Scholar] [CrossRef]
- Mubagwa, K.; Flameng, W. Adenosine, adenosine receptors and myocardial protection: An updated overview. Cardiovasc. Res. 2001, 52, 25–39. [Google Scholar] [CrossRef]
- Moser, G.H.; Schrader, J.; Deussen, A. Turnover of adenosine in plasma of human and dog blood. Am. J. physiol. 1989, 256, C799–C806. [Google Scholar]
- Auchampach, J.A.; Bolli, R. Adenosine receptor subtypes in the heart: Therapeutic opportunities and challenges. Am. J. physiol. 1999, 276, H1113–H1116. [Google Scholar]
- Matherne, G.P.; Linden, J.; Byford, A.M.; Gauthier, N.S.; Headrick, J.P. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc. Natl. Acad. Sci. USA 1997, 94, 6541–6546. [Google Scholar]
- Lohse, M.J.; Klotz, K.N.; Schwabe, U.; Cristalli, G.; Vittori, S.; Grifantini, M. 2-Chloro-N6-cyclopentyladenosine: A highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1988, 337, 687–689. [Google Scholar]
- Yan, L.; Burbiel, J.C.; Maass, A.; Muller, C.E. Adenosine receptor agonists: From basic medicinal chemistry to clinical development. Expert Opin. Emerg. Drugs 2003, 8, 537–576. [Google Scholar] [CrossRef]
- Peart, J.; Headrick, J.P. Intrinsic A(1) adenosine receptor activation during ischemia or reperfusion improves recovery in mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2166–H2175. [Google Scholar]
- Butcher, A.; Gregg, A.; Scammells, P.J.; Rose’Meyer, R.B. Effect of a novel partial adenosine A1 receptor agonist VCP102 in reducing ischemic damage in the mouse heart. Drug Dev. Res. 2007, 68, 529–537. [Google Scholar] [CrossRef]
- Aurelio, L.; Figler, H.; Flynn, B.L.; Linden, J.; Scammells, P.J. 5-Substituted 2-aminothiophenes as A1 adenosine receptor allosteric enhancers. Bioorg. Med. Chem. 2008, 16, 1319–1327. [Google Scholar] [CrossRef]
- Valant, C.; Aurelio, L.; Urmaliya, V.B.; White, P.; Scammells, P.J.; Sexton, P.M.; Christopoulos, A. Delineating the mode of action of adenosine A1 receptor allosteric modulators. Mol. Pharmacol. 2010, 78, 444–455. [Google Scholar]
- Christopoulos, A. Allosteric binding sites on cell-surface receptors: Novel targets for drug discovery. Nat. Rev. Drug Discov. 2002, 1, 198–210. [Google Scholar] [CrossRef]
- Leach, K.; Sexton, P.M.; Christopoulos, A. Allosteric GPCR modulators: Taking advantage of permissive receptor pharmacology. Trends Pharmacol. Sci. 2007, 28, 382–389. [Google Scholar] [CrossRef]
- Kiesman, W.F.; Elzein, E.; Zablocki, J. A1 adenosine receptor antagonists, agonists, and allosteric enhancers. Handb. Exp. Pharm. 2009, 193, 25–58. [Google Scholar] [CrossRef]
- Soudijn, W.; van Wijngaarden, I.; IJzerman, AP. Allosteric modulation of G protein-coupled receptors: Perspectives and recent developments. Drug Discov. Today 2004, 9, 752–758. [Google Scholar] [CrossRef]
- Mizumura, T.; Auchampach, J.A.; Linden, J.; Bruns, R.F.; Gross, G.J. PD 81,723, an allosteric enhancer of the A1 adenosine receptor, lowers the threshold for ischemic preconditioning in dogs. Circ. Res. 1996, 79, 415–423. [Google Scholar] [CrossRef]
- Gregg, A.; Bottle, S.E.; Devine, S.M.; Figler, H.; Linden, J.; White, P.; Pouton, C.W.; Urmaliya, V.; Scammells, P.J. Dual acting antioxidant A1 adenosine receptor agonists. Bioorg. Med. Chem. Lett. 2007, 17, 5437–5441. [Google Scholar] [CrossRef] [Green Version]
- Thornton, J.D.; Liu, G.S.; Olsson, R.A.; Downey, J.M. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation 1992, 85, 659–665. [Google Scholar] [CrossRef]
- Lasley, R.D.; Rhee, J.W.; Van Wylen, D.G.; Mentzer, R.M., Jr. Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. J. Mol. Cell. Cardiol. 1990, 22, 39–47. [Google Scholar]
- Zhao, Z.Q.; McGee, S.; Nakanishi, K.; Toombs, C.F.; Johnston, W.E.; Ashar, M.S.; Vinten-Johansen, J. Receptor-mediated cardioprotective effects of endogenous adenosine are exerted primarily during reperfusion after coronary occlusion in the rabbit. Circulation 1993, 88, 709–719. [Google Scholar] [CrossRef]
- Lagerstrom, M.C.; Schioth, H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008, 7, 339–357. [Google Scholar] [CrossRef]
- Elzein, E.; Zablocki, J. A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin. Investig. Drugs 2008, 17, 1901–1910. [Google Scholar] [CrossRef]
- May, L.T.; Leach, K.; Sexton, P.M.; Christopoulos, A. Allosteric modulation of G protein-coupled receptors. Ann. Rev. pharm. Toxicol. 2007, 47, 1–51. [Google Scholar] [CrossRef]
- Sanjani, M.S.; Teng, B.; Krahn, T.; Tilley, S.; Ledent, C.; Mustafa, S.J. Contributions of A2A and A2B adenosine receptors in coronary flow responses in relation to the KATP channel using A2B and A2A/2B double-knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2322–H2333. [Google Scholar] [CrossRef]
- Flood, A.; Headrick, J.P. Functional characterization of coronary vascular adenosine receptors in the mouse. Br. J. Pharmacol. 2001, 133, 1063–1072. [Google Scholar] [CrossRef]
- Amoah-Apraku, B.; Xu, J.; Lu, J.Y.; Pelleg, A.; Bruns, R.F.; Belardinelli, L. Selective potentiation by an A1 adenosine receptor enhancer of the negative dromotropic action of adenosine in the guinea pig heart. J. Pharmacol. Exp. Ther. 1993, 266, 611–617. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Butcher, A.; Scammells, P.J.; White, P.J.; Devine, S.M.; Rose'Meyer, R.B. An Allosteric Modulator of the Adenosine A1 Receptor Improves Cardiac Function Following Ischaemia in Murine Isolated Hearts. Pharmaceuticals 2013, 6, 546-556. https://doi.org/10.3390/ph6040546
Butcher A, Scammells PJ, White PJ, Devine SM, Rose'Meyer RB. An Allosteric Modulator of the Adenosine A1 Receptor Improves Cardiac Function Following Ischaemia in Murine Isolated Hearts. Pharmaceuticals. 2013; 6(4):546-556. https://doi.org/10.3390/ph6040546
Chicago/Turabian StyleButcher, Anna, Peter J. Scammells, Paul J. White, Shane M. Devine, and Roselyn B. Rose'Meyer. 2013. "An Allosteric Modulator of the Adenosine A1 Receptor Improves Cardiac Function Following Ischaemia in Murine Isolated Hearts" Pharmaceuticals 6, no. 4: 546-556. https://doi.org/10.3390/ph6040546
APA StyleButcher, A., Scammells, P. J., White, P. J., Devine, S. M., & Rose'Meyer, R. B. (2013). An Allosteric Modulator of the Adenosine A1 Receptor Improves Cardiac Function Following Ischaemia in Murine Isolated Hearts. Pharmaceuticals, 6(4), 546-556. https://doi.org/10.3390/ph6040546



