Removal of Cholera Toxin from Aqueous Solution by Probiotic Bacteria
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Cholera Toxin
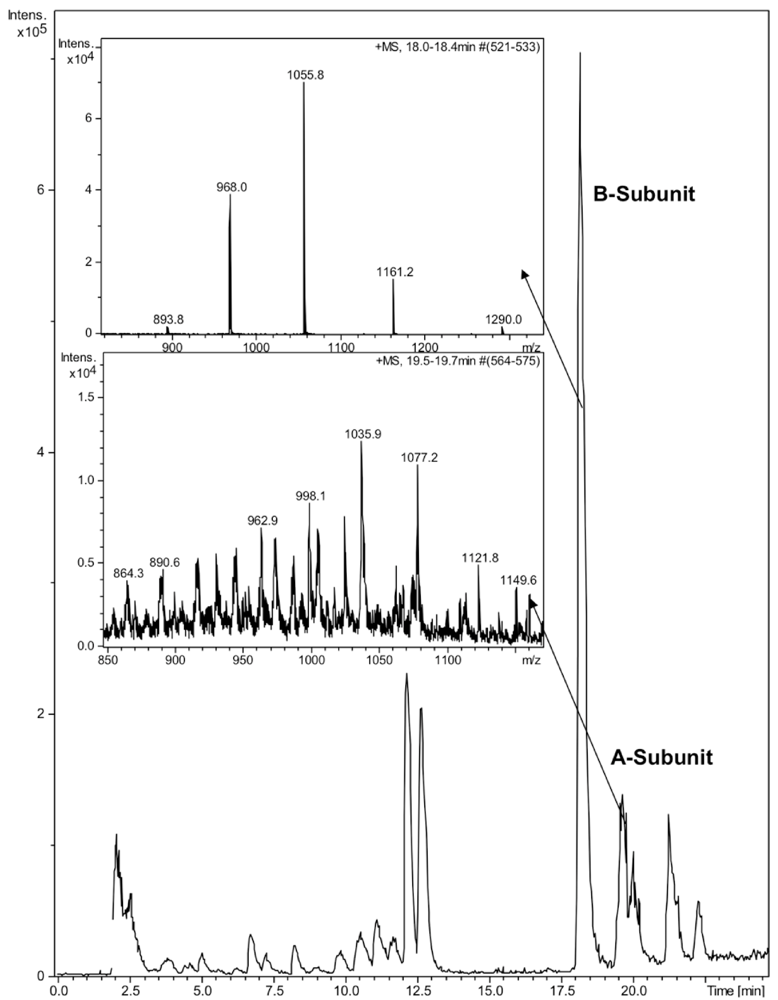
2.3. HPLC and LC-MS Analysis
2.4. Bacterial Strains and Cultivation
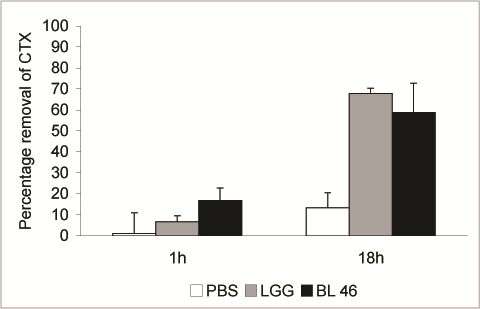
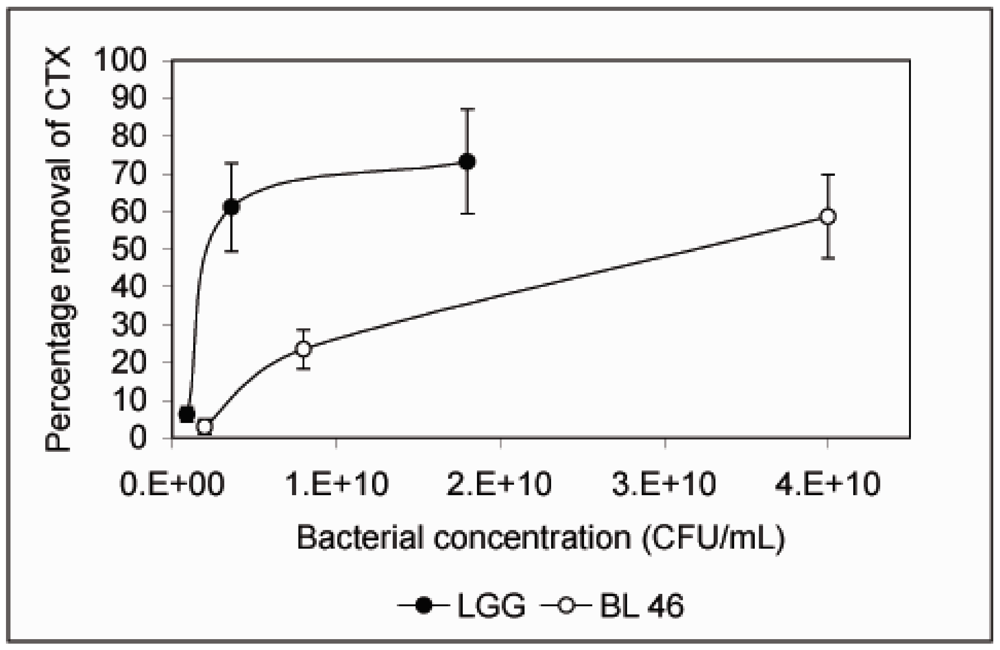
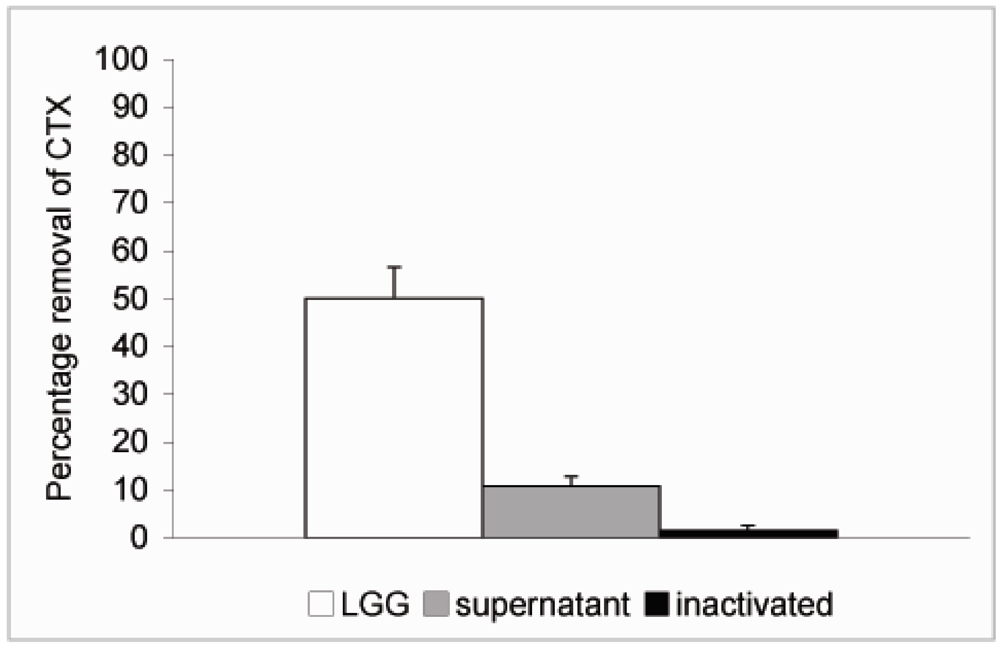
3. Results and Discussion

4. Conclusions
Acknowledgements
Conflict of Interests
References
- WHO. An old enemy returns? Bull. World Health Organ. 2009, 87, 85–86. [CrossRef]
- Deen, J.L.; von Seidlein, L.; Sur, D.; Agtini, M.; Lucas, M.E.; Lopez, A.L.; Kim, D.R.; Ali, M.; Clemens, J.D. The high burden of cholera in children: Comparison of incidence from endemic areas in Asia and Africa. PLoS Negl. Trop. Dis. 2008, 2, e173. [Google Scholar] [CrossRef]
- Clemens, J.D.; Sack, D.A.; Harris, J.R.; Chakraborty, J.; Khan, M.R.; Stanton, B.F.; Kay, B.A.; Khan, M.U.; Yunus, M.; Atkinson, W.; et al. Field trial of oral cholera vaccines in Bangladesh. Lancet 1986, 2, 124–127. [Google Scholar]
- Dutta, N.K.; Panse, M.V.; Kulkarni, D.R. Role of cholera a toxin in experimental cholera. J. Bacteriol. 1959, 78, 594–595. [Google Scholar]
- van Heyningen, S. The subunits of cholera toxin: Structure, stoichiometry, and function. J. Infect. Dis. 1976, 133, S5–S13. [Google Scholar] [CrossRef]
- Cassel, D.; Selinger, Z. Mechanism of adenylate cyclase activation by cholera toxin: Inhibition of GTP hydrolysis at the regulatory site. Proc. Natl. Acad. Sci. USA 1977, 74, 3307–3311. [Google Scholar] [CrossRef]
- Field, M.; Rao, M.C.; Chang, E.B. Intestinal electrolyte transport and diarrheal disease (1). N. Engl. J. Med. 1989, 321, 800–806. [Google Scholar] [CrossRef]
- FAO/WHO, Guidelines for the Evaluation of Probiotics in Food, Working Group Report; Food and Health Agricultural Organization of the United Nations and World Health Organization: Washington, DC, USA, 2002.
- Gill, H.S.; Guarner, F. Probiotics and human health: A clinical perspective. Postgrad. Med. J. 2004, 80, 516–526. [Google Scholar] [CrossRef]
- D’Souza, A.L.; Rajkumar, C.; Cooke, J.; Bulpitt, C.J. Probiotics in prevention of antibiotic associated diarrhoea: Meta-analysis. Br. Med. J. 2002, 324, 1361. [Google Scholar]
- Pedone, C.A.; Arnaud, C.C.; Postaire, E.R.; Bouley, C.F.; Reinert, P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int. J. Clin. Pract. 2000, 54, 568–571. [Google Scholar]
- Saavedra, J.M.; Bauman, N.A.; Oung, I.; Perman, J.A.; Yolken, R.H. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 1994, 344, 1046–1049. [Google Scholar]
- Marteau, P.R.; de Vrese, M.; Cellier, C.J.; Schrezenmeir, J. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 2001, 73, 430S–436S. [Google Scholar]
- Malin, M.; Suomalainen, H.; Saxelin, M.; Isolauri, E. Promotion of IgA immune response in patients with Crohn’s disease by oral bacteriotherapy with Lactobacillus GG. Ann. Nutr. Metab. 1996, 40, 137–145. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Turbic, A.; Ahokas, J.T.; Haskard, C.A. Selective in vitro binding of dietary mutagens, individually or in combination, by lactic acid bacteria. Food Addit. Contam. 2002, 19, 144–152. [Google Scholar]
- Asahara, T.; Shimizu, K.; Nomoto, K.; Hamabata, T.; Ozawa, A.; Takeda, Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 2004, 72, 2240–2247. [Google Scholar]
- Meriluoto, J.; Gueimonde, M.; Haskard, C.A.; Spoof, L.; Sjövall, O.; Salminen, S. Removal of the cyanobacterial toxin microcystin-LR by human probiotics. Toxicon 2005, 46, 111–114. [Google Scholar] [CrossRef]
- Nybom, S.M.K.; Salminen, S.J.; Meriluoto, J.A.O. Removal of microcystin-LR by strains of metabolically active probiotic bacteria. FEMS Microbiol. Lett. 2007, 270, 27–33. [Google Scholar] [CrossRef]
- Nybom, S.M.K.; Dziga, D.; Heikkilä, J.E.; Kull, T.P.; Salminen, S.J.; Meriluoto, J.A.O. Characterization of microcystin-LR removal process in the presence of probiotic bacteria. Toxicon 2012, 59, 171–181. [Google Scholar] [CrossRef]
- van Baar, B.L.; Hulst, A.G.; Wils, E.R. Characterisation of cholera toxin by liquid chromatography-electrospray mass spectrometry. Toxicon 1999, 37, 85–108. [Google Scholar] [CrossRef]
- Haskard, C.A.; El-Nezami, H.S.; Kankaanpää, P.E.; Salminen, S.; Ahokas, J.T. Surface binding of aflatoxin B(1) by lactic acid bacteria. Appl. Environ. Microbiol. 2001, 67, 3086–3091. [Google Scholar] [CrossRef]
- Kunji, E.R.; Mierau, I.; Hagting, A.; Poolman, B.; Konings, W.N. The proteolytic systems of lactic acid bacteria. Antonie Van Leeuwenhoek 1996, 70, 187–221. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Black, R.; Bourgeois, L.; Clemens, J.; Cravioto, A.; Deen, J.L.; Dougan, G.; Glass, R.; Grais, R.F.; Greco, M.; et al. Public health. The cholera crisis in Africa. Science 2009, 324, 885. [Google Scholar]
- Focareta, A.; Paton, J.C.; Morona, R.; Cook, J.; Paton, A.W. A recombinant probiotic for treatment and prevention of cholera. Gastroenterology 2006, 130, 1688–1695. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. Clinical indications for probiotics: An overview. Clin. Infect. Dis. 2008, 46, 596–600. [Google Scholar]
- Lindenbaum, J.; Greenough, W.B.; Islam, M.R. Antibiotic therapy of cholera. Bull. World Health Organ. 1967, 36, 871–883. [Google Scholar]
- Sack, D.A.; Islam, S.; Rabbani, H.; Islam, A. Single-dose doxycycline for cholera. Antimicrob. Agents Chemother. 1978, 14, 462–464. [Google Scholar]
- Saha, D.; Karim, M.M.; Khan, W.A.; Ahmed, S.; Salam, M.A.; Bennish, M.L. Single-dose azithromycin for the treatment of cholera in adults. N. Eng. J. Med. 2006, 354, 2452–2462. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Heikkilä, J.E.; Nybom, S.M.K.; Salminen, S.J.; Meriluoto, J.A.O. Removal of Cholera Toxin from Aqueous Solution by Probiotic Bacteria. Pharmaceuticals 2012, 5, 665-673. https://doi.org/10.3390/ph5060665
Heikkilä JE, Nybom SMK, Salminen SJ, Meriluoto JAO. Removal of Cholera Toxin from Aqueous Solution by Probiotic Bacteria. Pharmaceuticals. 2012; 5(6):665-673. https://doi.org/10.3390/ph5060665
Chicago/Turabian StyleHeikkilä, Jari E., Sonja M. K. Nybom, Seppo J. Salminen, and Jussi A. O. Meriluoto. 2012. "Removal of Cholera Toxin from Aqueous Solution by Probiotic Bacteria" Pharmaceuticals 5, no. 6: 665-673. https://doi.org/10.3390/ph5060665
APA StyleHeikkilä, J. E., Nybom, S. M. K., Salminen, S. J., & Meriluoto, J. A. O. (2012). Removal of Cholera Toxin from Aqueous Solution by Probiotic Bacteria. Pharmaceuticals, 5(6), 665-673. https://doi.org/10.3390/ph5060665