Comparison of Intraocular Pressure, Blood Pressure, Ocular Perfusion Pressure and Blood Flow Fluctuations During Dorzolamide Versus Timolol Add-On Therapy in Prostaglandin Analogue Treated Glaucoma Subjects
Abstract
:1. Introduction
2. Objectives
3. Materials and Methods
4. Statistical Analysis
5. Results
| Mean (SD) | Number (percentage) | |
|---|---|---|
| Age [years] | 63.3 (8.9) | |
| Men (N (%)) | 4 (11.4%) | |
| Women (N (%)) | 31 (88.6%) | |
| Glaucoma treatment [years] | 6.1 (6.3) | |
| Patients receiving systemic medications: | 2.3 (1.9) | |
| Beta blockers (N (%)) | 12 (34.3%) | |
| ACE inhibitors (N (%)) | 7 (20.0%) | |
| Angiotensin II inhibitors (N (%)) | 6 (17.1%) | |
| Diuretics (N (%)) | 6 (17.1%) | |
| Other drugs (N (%)) | 18 (51.4%) | |
| Systolic BP-baseline [mmHg] | 139.5 (15.0) | |
| Diastolic BP-baseline [mmHg] | 82.8 (7.1) | |
| Pulse rate-baseline | 69.3 (13.8) | |
| Systolic BP-Dorzolamide add-on [mmHg] | 136.1 (14.0) | |
| Diastolic BP-Dorzolamide add-on [mmHg] | 81.2 (8.0) | |
| Pulse rate-Dorzolamide add-on | 71.8 (9.0) | |
| Systolic BP-Timolol add-on [mmHg] | 137.6 (14.0) | |
| Diastolic BP-Timolol add-on [mmHg] | 80.7 (8.7) | |
| Pulse rate-Timolol add-on | 68.2 (13.7) |
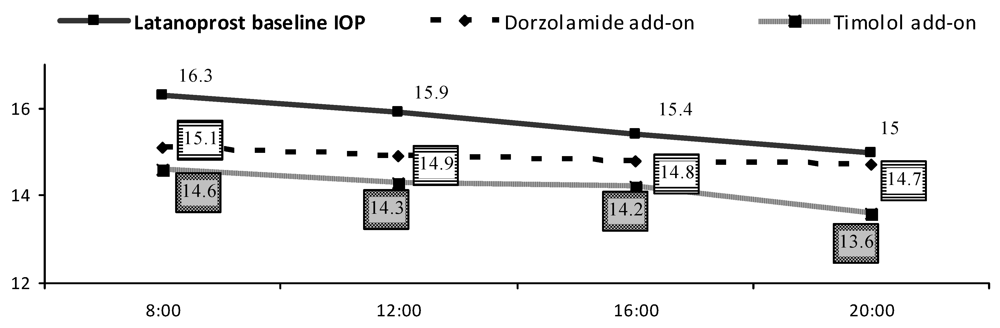
| IOP [mmHg] | Mean (SD) | ∆ | ||||
| Baseline | Dorzolamide | Timolol | Dorzolamide change from baseline | Timolol change from baseline | ||
| 15.7 ± 2.4 | 14.9 (2.2) * | 14.2 (1.9) * | 8 h | 1.2 (2.1) | 1.6 (2.1) | |
| 12 h | 0.9 (2.6) | 1.6 (2.3) | ||||
| 16 h | 0.6 (1.7) | 1.2 (1.7) | ||||
| 20 h | 0.2 (2.1) | 1.4 (2.4) ** | ||||
| Latanoprost baseline | Dorzolamide add-on | Timolol add-on | ||||
|---|---|---|---|---|---|---|
| Doppler parameters | Morning Mean (SD) | Evening Mean (SD) | Morning Mean (SD) | Evening Mean (SD) | Morning Mean (SD) | Evening Mean (SD) |
| OA PSV [cm/s] | 33.5 (6.7) | 33.6 (2.6) | 34.8 (7.7) | 35.1 (7.1) | 33.3 (6.9) | 33.5 (5.8) |
| OA EDV [cm/s] | 7.3 (2.6) | 7.5 (2.6) | 8.0 (3.2) | 7.7 (3.6) | 7.4 (2.8) | 7.0 (2.4) |
| CRA PSV [cm/s] | 11.7 (1.4) | 11.8 (1.8) | 11.4 (1.9) | 12.0 (2.0) | 11.6 (2.1) | 11.4 (1.9) |
| CRA EDV [cm/s] | 3.5 (0.7) | 3.6 (0.7) | 3.6 (1.1) | 3.5 (1.0) | 3.2 (0.9) | 3.5 (1.0) |
| CRA RI | 0.69 (0.07) | 0.69 (0.07) | 0.68 (0.08) | 0.70 (0.07) | 0.71 (0.08) | 0.69 (0.08) |
| nSPCA PSV [cm/s] | 8.4 (1.1) | 8.4 (1.2) | 8.7 (2.0) | 8.7 (1.6) | 8.5 (1.6) | 8.8 (2.0) |
| nSPCA EDV [cm/s] | 3.5 (0.6) | 3.5 (0.6) | 3.6 (0.9) | 3.8 (0.8) | 3.7 (1.0) | 3.7 (0.9) |
| nSPCA RI | 0.58 (0.07) | 0.58 (0.06) | 0.58 (0.09) | 0.56 (0.09) | 0.57 (0.08) | 0.58 (0.09) |
| tSPCA PSV [cm/s] | 8.2 (1.4) | 8.3 (1.3) | 8.0 (1.6) | 8.4 (1.4) | 8.1 (1.7) | 8.2 (1.8) |
| tSPCA EDV [cm/s] | 3.4 (0.6) | 3.6 (0.6) | 3.5 (0.7) | 3.5 (0.6) | 3.4 (0.6) | 3.4 (0.8) |
| tSPCA RI | 0.58 (0.08) | 0.56 (0.08) | 0.54 (0.11) | 0.58 (0.08) | 0.56 (0.09) | 0.57 (0.11) |
| Patient groups | CDI parameter | Latanoprost baseline Mean (SD) | Dorzolamide add-on Mean (SD) | Timolol add-on Mean (SD) |
|---|---|---|---|---|
| Age <65 years | OA RI | 0.79 (0.05) | 0.76 (0.09) | 0.77 (0.07) |
| CRA RI | 0.68 (0.07) | 0.67 (0.07) | 0.69 (0.07) | |
| tSPCA RI | 0.57 (0.07) | 0.56 (0.09) | 0.55 (0.08) | |
| nSPCA RI | 0.56 (0.08) | 0.55 (0.09) | 0.56 (0.09) | |
| Age ≥65 years | OA RI | 0.78 (0.04) | 0.79 (0.05) | 0.80 (0.04) |
| CRA RI | 0.72 (0.04) | 0.73 (0.06) | 0.71 (0.06) | |
| tSPCA RI | 0.59 (0.05) | 0.59 (0.05) | 0.61 (0.06) | |
| nSPCA RI | 0.58 (0.06) | 0.57 (0.08) | 0.58 (0.09) | |
| Glaucoma treatment <5 years | OA RI CRA RI | 0.77 (0.04) 0.67 (0.06) | 0.76 (0.07) 0.66 (0.06) | 0.77 (0.07) 0.68 (0.07) |
| tSPCA RI | 0.56 (0.05) | 0.54 (0.07) | 0.56 (0.08) | |
| nSPCA RI | 0.55 (0.05) | 0.52 (0.07) | 0.55 (0.08) | |
| Glaucoma treatment ≥5 years | OA RI | 0.79 (0.05) | 0.80 (0.07) | 0.81 (0.05) |
| CRA | ||||
| CRA RI | 0.73 (0.04) | 0.73 (0.05) | 0.73 (0.04) | |
| tSPCA RI | 0.60 (0.06) | 0.60 (0.06) | 0.59 (0.07) | |
| nSPCA RI | 0.59 (0.08) | 0.60 (0.08) | 0.59 (0.09) | |
| Taken systemic medication <2 | OA RI | 0.78 (0.05) | 0.76 (0.08) | 0.78 (0.07) |
| CRA RI | 0.69 (0.06) | 0.68 (0.06) | 0.69 (0.06) | |
| tSPCA RI | 0.57 (0.05) | 0.56 (0.06) | 0.58 (0.06) | |
| nSPCA RI | 0.56 (0.06) | 0.56 (0.08) | 0.57 (0.09) | |
| Taken systemic medication ≥2 | OA RI | 0.79 (0.04) | 0.79 (0.06) | 0.79 (0.06) |
| CRA RI | 0.71 (0.06) | 0.71 (0.07) | 0.72 (0.07) | |
| tSPCA RI | 0.59 (0.06) | 0.58 (0.09) | 0.58 (0.06) | |
| nSPCA RI | 0.58 (0.08) | 0.57 (0.09) | 0.57 (0.07) |
| Systolic BP | MAP | SPP | OPP | |||||
| Timolol | Dorzola-mide | Timolol | Dorzola-mide | Timolol | Dorzola-mide | Timolol | Dorzola-mide | |
| <65 year | 14.5(7.3) | 10.3(6.9) | 8.52(5.3) | 6.92(4.5) | 13.8(6.6) | 10.6(6.7) | 6.09(3.3) | 4.96(3.0) |
| ≥65 year | 17.0(10.6) | 16.5(9.3) | 12.8(6.8) | 10.9(5.6) | 17.2(9.8) | 15.8(8.2) | 7.28(4.4) | 7.98(4.9) |
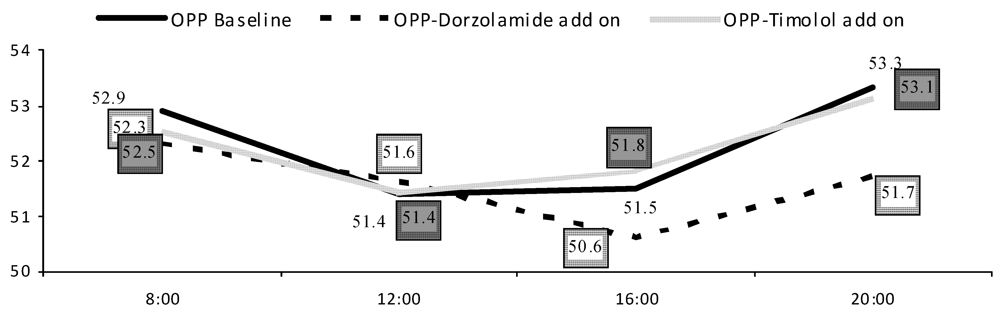
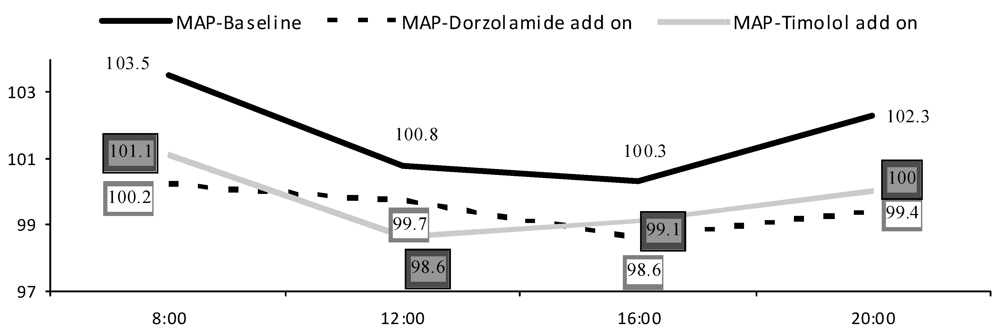
6. Discussion
7. Conclusions
Conflicts of Interest
Acknowledgements
References
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Bengtsson, B.; Hussein, M. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression (Results from the Early Manifest Glaucoma Trial). Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar]
- Leske, C.M.; Heijl, A.; Hyman, L.; Bengtsson, B.; Dong, L.; Yang, Z. the EMGT Group. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology 2007, 114, 1965–1972. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Harris, A. Clinical Revalence of Ocular Blood Flow (OBF) Measurements Icluding Effects of General Medications of Specific Glaucoma Treatment. In Ocular Blood Flow in Glaucoma, 6th ed; Kugler Publications: Amsterdam, The Netherlands, 2009; pp. 60–126. [Google Scholar]
- Klein, B.E.; Klein, R.; Knudtson, M.D. Intraocular pressure and systemic blood pressure: Longitudinal prospective the Beaver Dam Eye Study. Br. J. Ophthalmol. 2005, 89, 284–287. [Google Scholar] [CrossRef]
- Harris, A.; Jonescu-Cuypers, C.; Martin, B.; Kagemann, L.; Zalish, M.; Garzozi, H.J. Simultaneous management of blood flow and IOP in glaucoma. Acta Ophthalmol. Scand. 2001, 79, 336–341. [Google Scholar] [CrossRef]
- Plange, N.; Kaup, M.; Daneljan, L.; Predel, H.G.; Remky, A.; Arend, O. 24-h Blood pressure monitoring in normal tension glaucoma: Night-time blood pressure variability. J. Hum. Hypertens. 2006, 20, 137–142. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, X.; Kripke, D.F.; Weinreb, R.N. Twenty-four hour intraocular pressure pattern associated with early glaucomatous changes. Invest. Ophthalmol. Vis. Sci. 2003, 44, 1586–1590. [Google Scholar] [CrossRef]
- Asrani, S.; Zeimer, R.; Wilesnsky, J.; Gieser, D.; Vitale, S.; Lindenmuth, K. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J. Glaucoma 2000, 9, 134–142. [Google Scholar] [CrossRef]
- Quaranta, L.; Pizzolante, T.; Riva, I.; Haidich, A.B.; Konstas, A.G.; Stewart, W.C. Twenty-four-hour intraocular pressure and blood pressure levels with bimatoprost versus latanoprost in patients with normal-tension glaucoma. Br. J. Ophthalmol. 2008, 92, 1227–1231. [Google Scholar]
- Elena, P.P.; Denis, P.; Kosina-Boix, M.; Saraux, H.; Lapalus, P. Beta adrenergic binding sites in the human eye: An autoradiographic study. J. Ocul. Pharmacol. 1990, 6, 143–149. [Google Scholar] [CrossRef]
- Cohen, J.S.; Khatana, A.K.; Greff, L.J. Evolving paradigms in the medical treatment of glaucoma. Int. Ophthalmol. 2004, 25, 253–265. [Google Scholar] [CrossRef]
- Moore, D.; Harris, A.; Wudunn, D.; Kheradiya, N.; Siesky, B. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma? Clin. Ophthalmol. 2008, 2, 849–861. [Google Scholar]
- Januleviciene, I.; Ehrlich, R.; Siesky, B.; Nedzelskiene, I.; Harris, A. Visual function, optic nerve structure, and ocular blood flow parameters after 1 year of glaucoma treatment with fixed combinations. Eur. J. Ophthalmol. 2009, 5, 790–797. [Google Scholar]
- Agis Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000, 130, 429–440. [CrossRef]
- Costa, V.P.; Harris, A.; Stefánsson, E.; Flammer, J.; Krieglstein, G.K.; Orzalesi, N.; Heijl, A.; Renard, J.P.; Serra, L.M. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog. Retin. Eye Res. 2003, 22, 769–805. [Google Scholar] [CrossRef]
- Duke-Elder, S. The phasic variation in the ocular tension in primary glaucoma. Am. J. Ophthalmol. 1959, 35, 1–21. [Google Scholar]
- Sacca, S.C.; Rolando, M.; Marletta, A.; Macrì, A.; Cerqueti, P.; Ciurlo, G. Fluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjects. Ophthalmologica 1998, 212, 115–119. [Google Scholar]
- Kaiser, H.J.; Flammer, J.; Graft, T.; Stumfig, D. Systemic blood pressure in primary glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1993, 231, 677–680. [Google Scholar] [CrossRef]
- Siesky, B.; Harris, A.; Kagemann, L.; Stefansson, E.; McCranor, L.; Miller, B.; Bwatwa, J.; Regev, G.; Ehrlich, R. Ocular blood flow and oxygen delivery to the retina in primary open-angle glaucoma patients: The addition of dorzolamide to timolol monotherapy. Acta Ophthalmol. 2010, 88, 142–149. [Google Scholar] [CrossRef]
- Harris, A.; Arend, O.; Arend, S.; Martin, B. Effects of topical dorzolamide on retinal and retrobulbar hemodynamics. Acta Ophthalmol. Scand. 1996, 74, 569–572. [Google Scholar]
- Martinez, A.; Gonzalez, F.; Capeans, C.; Perez, R.; Sanchez-Salorio, M. Dorzolamide effect on ocular blood flow. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1270–1275. [Google Scholar]
- Moss, A.M.; Harris, A.; Siesky, B.; Rusia, D.; Williamson, K.M.; Shoshani, Y. Update and critical appraisal of combined timolol and carbonic anhydrase inhibitors and the effect on ocular blood flow in glaucoma patients. Clin. Ophthalmol. 2010, 4, 233–241. [Google Scholar] [Green Version]
- Siesky, B.; Harris, A.; Brizendine, E.; Marques, C.; Loh, J.; Mackey, J.; Overton, J.; Netland, P. Literature review and meta-analysis of topical carbonic anhydrase inhibitors and ocular blood flow. Surv. Ophthalmol. 2009, 54, 33–46. [Google Scholar] [CrossRef]
- Harris, A.; Jonescu-Cuypers, C.P.; Kagemann, L.; Nowacki, E.A.; Garzozi, H.; Cole, C.; Martin, B. Effect of dorzolamide timolol combination versus timolol 0.5% on ocular blood flow in patients with primary open-angle glaucoma. Am. J. Ophthalmol. 2001, 132, 490–495. [Google Scholar] [CrossRef]
- Bernd, A.S.; Pillunat, L.E.; Böhm, A.G.; Schmidt, K.G.; Richard, G. Ocular hemodynamics and visual field in glaucoma treated with dorzolamide. Ophthalmologe 2001, 98, 451–455. [Google Scholar] [CrossRef]
- Pillunat, L.E.; Bohm, A.G.; Koller, A.U.; Schmidt, K.G.; Klemm, M.; Richard, G. Effect of topical dorzolamide on optic nerve head blood flow. Graefes Arch. Clin. Exp. Ophthalmol. 1999, 237, 495–500. [Google Scholar] [CrossRef]
- Bergstrand, I.C.; Heijl, A.; Harris, A. Dorzolamide and ocular blood flow in previously untreated glaucoma patients: A controlled double-masked study. Acta Ophthalmol. Scand. 2002, 80, 176–182. [Google Scholar] [CrossRef]
- Harris, A.; Evans, D.; Martin, B.; Zalish, M.; Kagemann, L.; McCranor, L.; Garzozi, H. Nocturnal blood pressure reduction: Effect on retrobulbar hemodynamics in glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 372–378. [Google Scholar]
- Galambos, P.; Vafiadis, J.; Vilchez, S.E.; Wagenfeld, L.; Matthiessen, E.T.; Richard, G.; Klemm, M.; Zeitz, O. Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology 2006, 113, 1832–1836. [Google Scholar] [CrossRef]
- Grunwald, J.E.; Mathur, S.; DuPont, J. Effects of dorzolamide hydrochloride 2% on the retinal circulation. Acta Ophthalmol. Scand. 1997, 75, 236–238. [Google Scholar]
- Faingold, D.; Hudson, C.; Flanagan, J.; Guan, K.; Rawji, M.; Buys, Y.M.; Trope, G.E. Assessment of retinal hemodynamics with the Canon laser blood flowmeter after a single dose of 2% dorzolamide hydrochloride eyedrops. Can. J. Ophthalmol. 2004, 39, 506–510. [Google Scholar]
- Harris, A.; Spaeth, G.; Wilson, R.; Moster, M.; Sergott, R.; Martin, B. Nocturnal ophthalmic arterial hemodynamics in primary open-angle glaucoma. J. Glaucoma 1997, 6, 170–174. [Google Scholar]
- Nicolela, M.T.; Buckley, A.R.; Walman, B.E.; Drance, S.M. A comparative study of the effects of timolol and latanoprost on blood flow velocity of the retrobulbar vessels. Am. J. Ophthalmol. 1996, 122, 784–789. [Google Scholar]
- Evans, D.W.; Harris, A.; Cantor, L.B. Primary open-angle glaucoma patients characterized by ocular vasospasm demonstrate a different ocular vascular response to timolol versus betaxolol. J. Ocul. Pharmacol. Ther. 1999, 15, 479–487. [Google Scholar] [CrossRef]
- Galassi, F.; Sodi, A.; Renieri, G.; Ucci, F.; Pieri, B.; Harris, A.; Siesky, B. Effects of timolol and dorzolamide on retrobulbar hemodynamics in patients with newly diagnosed primary open-angle glaucoma. Ophthalmologica 2002, 216, 123–128. [Google Scholar] [CrossRef]
- Harris, A.; Spaeth, G.L.; Sergott, R.C.; Katz, L.J.; Cantor, L.B.; Martin, B.J. Retrobulbar arterial hemodynamic effects of betaxolol and timolol in normal-tension glaucoma. Am. J. Ophthalmol. 1995, 120, 168–175. [Google Scholar]
- Stankiewicz, A.; Misiuk-Hojło, M.; Grabska-Liberek, I.; Romanowska-Dixon, B.; Wierzbowska, J.; Mulak, M.; Szuścik, I.; Sierdziński, J.; Ehrlich, R.; Harris, A. Intraocular pressure and ocular hemodynamics in patients with primary open-angle glaucoma treated with the combination of morning dosing of bimatoprost and dorzolamide hydrochloride. Acta Ophthalmol. 2011, 89, 57–63. [Google Scholar] [CrossRef]
- Siesky, B.; Harris, A.; Kagemann, L.; Stefansson, E.; McCranor, L.; Miller, B.; Bwatwa, J.; Regev, G.; Ehrlich, R. Ocular blood flow and oxygen delivery to the retina in primary open-angle glaucoma patients: The addition of dorzolamide to timolol monotherapy. Acta Ophthalmol. 2010, 88, 142–149. [Google Scholar] [CrossRef]
- Steigerwalt, R.D.; Belcaro, G.V.; Laurora, G.; Cesarone, M.R.; de Sanctis, M.T.; Incandela, L. Ocular and orbital blood flow in patients with essential hypertension treated with trandolapril. Retina 1998, 18, 539–545. [Google Scholar]
- Spicher, T.; Orgul, S.; Gugleta, K.; Teuchner, B.; Flammer, J. The effect of losartan potassium on choroidal haemodynamics in healthy subjects. J. Glaucoma 2002, 11, 177–182. [Google Scholar] [CrossRef]
- Resch, H.; Weigert, G.; Karl, K.; Pemp, B.; Garhofer, G.; Schmetterer, L. Effect of systemic moxaverine on ocular blood flow in humans. Acta Ophthalmol. 2009, 87, 731–735. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Januleviciene, I.; Siaudvytyte, L.; Diliene, V.; Barsauskaite, R.; Paulaviciute-Baikstiene, D.; Siesky, B.; Harris, A. Comparison of Intraocular Pressure, Blood Pressure, Ocular Perfusion Pressure and Blood Flow Fluctuations During Dorzolamide Versus Timolol Add-On Therapy in Prostaglandin Analogue Treated Glaucoma Subjects. Pharmaceuticals 2012, 5, 325-338. https://doi.org/10.3390/ph5030325
Januleviciene I, Siaudvytyte L, Diliene V, Barsauskaite R, Paulaviciute-Baikstiene D, Siesky B, Harris A. Comparison of Intraocular Pressure, Blood Pressure, Ocular Perfusion Pressure and Blood Flow Fluctuations During Dorzolamide Versus Timolol Add-On Therapy in Prostaglandin Analogue Treated Glaucoma Subjects. Pharmaceuticals. 2012; 5(3):325-338. https://doi.org/10.3390/ph5030325
Chicago/Turabian StyleJanuleviciene, Ingrida, Lina Siaudvytyte, Vaida Diliene, Ruta Barsauskaite, Daiva Paulaviciute-Baikstiene, Brent Siesky, and Alon Harris. 2012. "Comparison of Intraocular Pressure, Blood Pressure, Ocular Perfusion Pressure and Blood Flow Fluctuations During Dorzolamide Versus Timolol Add-On Therapy in Prostaglandin Analogue Treated Glaucoma Subjects" Pharmaceuticals 5, no. 3: 325-338. https://doi.org/10.3390/ph5030325
APA StyleJanuleviciene, I., Siaudvytyte, L., Diliene, V., Barsauskaite, R., Paulaviciute-Baikstiene, D., Siesky, B., & Harris, A. (2012). Comparison of Intraocular Pressure, Blood Pressure, Ocular Perfusion Pressure and Blood Flow Fluctuations During Dorzolamide Versus Timolol Add-On Therapy in Prostaglandin Analogue Treated Glaucoma Subjects. Pharmaceuticals, 5(3), 325-338. https://doi.org/10.3390/ph5030325





