Pyrazole Carbohydrazide Derivatives of Pharmaceutical Interest
Abstract
:1. Introduction
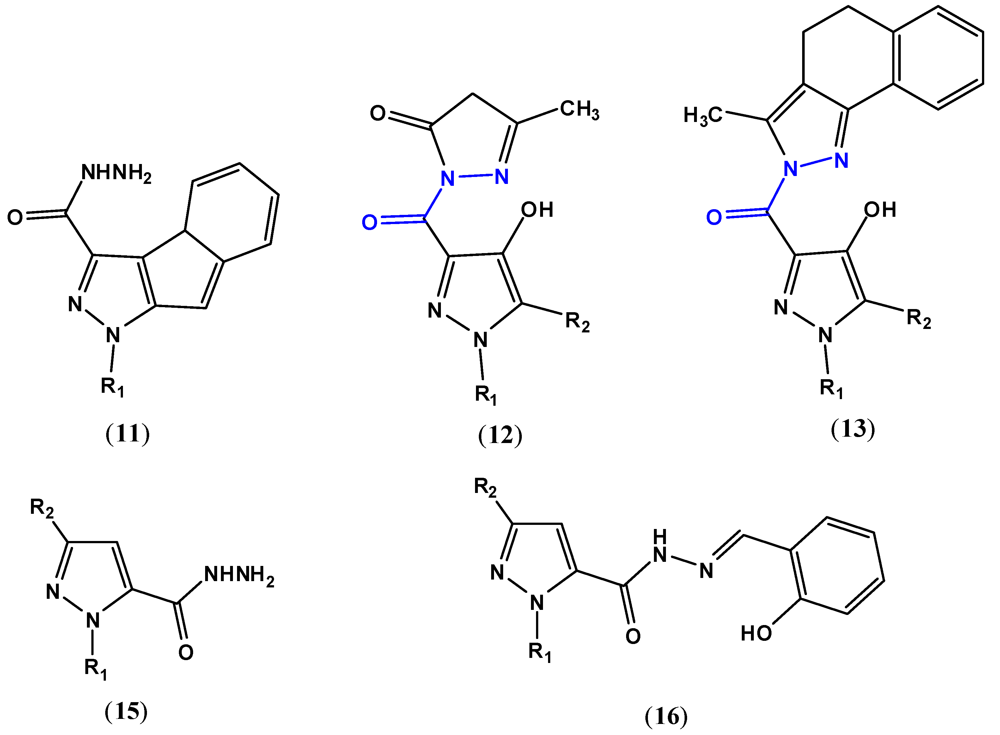
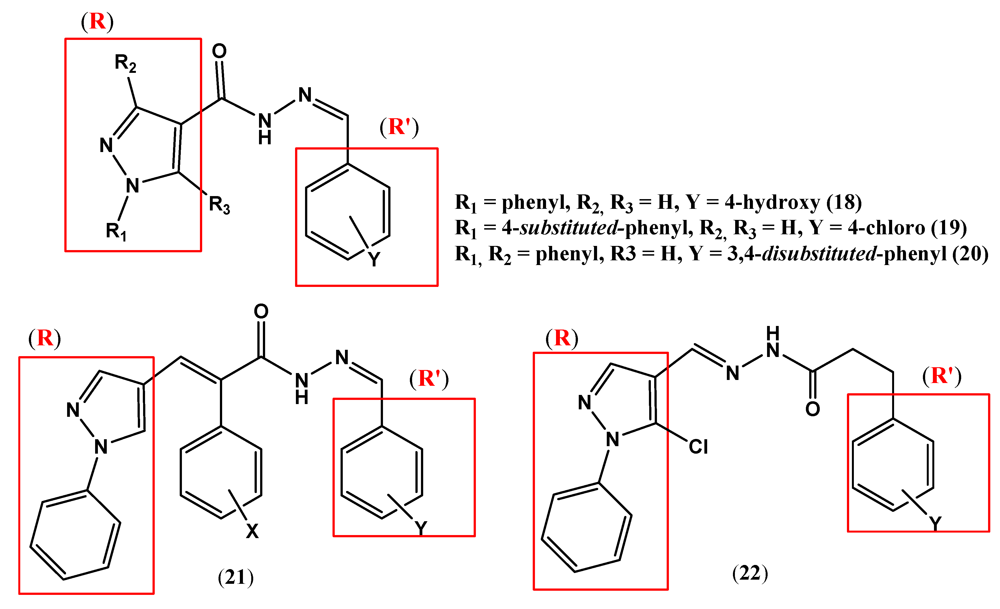
2. Pyrazole Compounds with Carbohydrazide Moiety Internalized
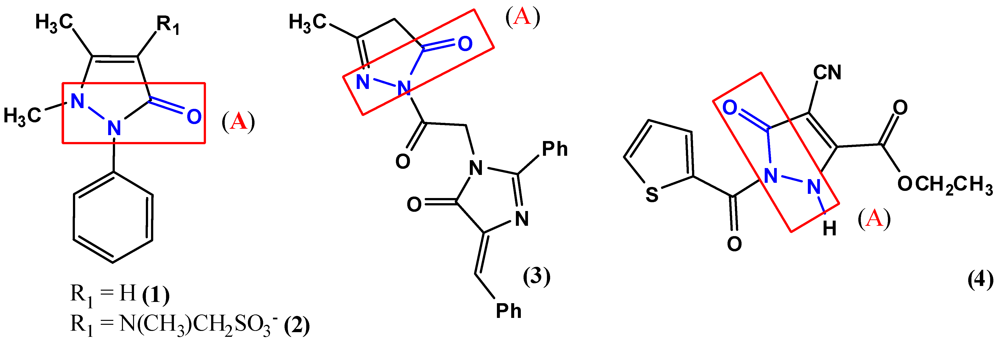
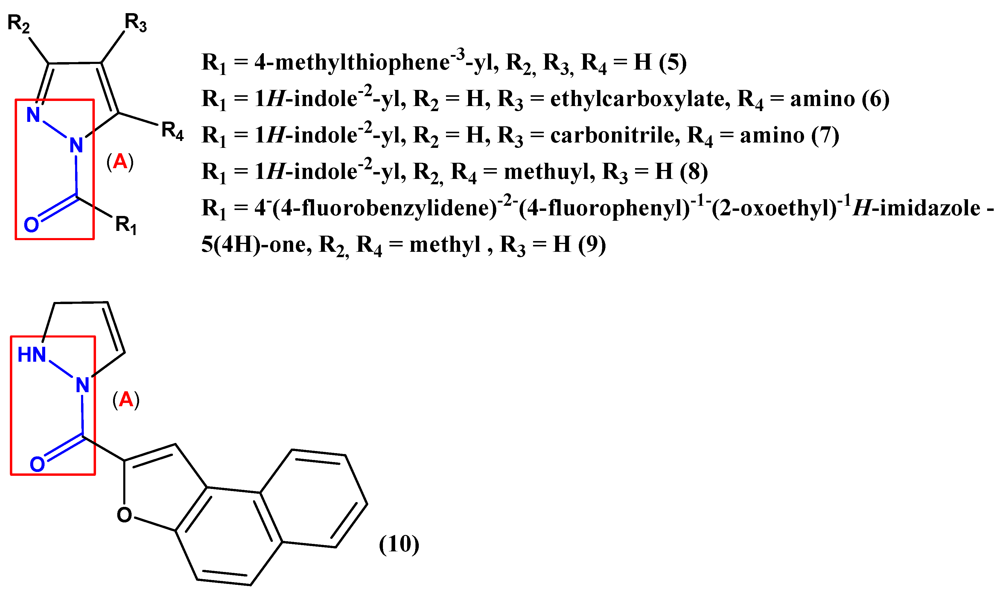
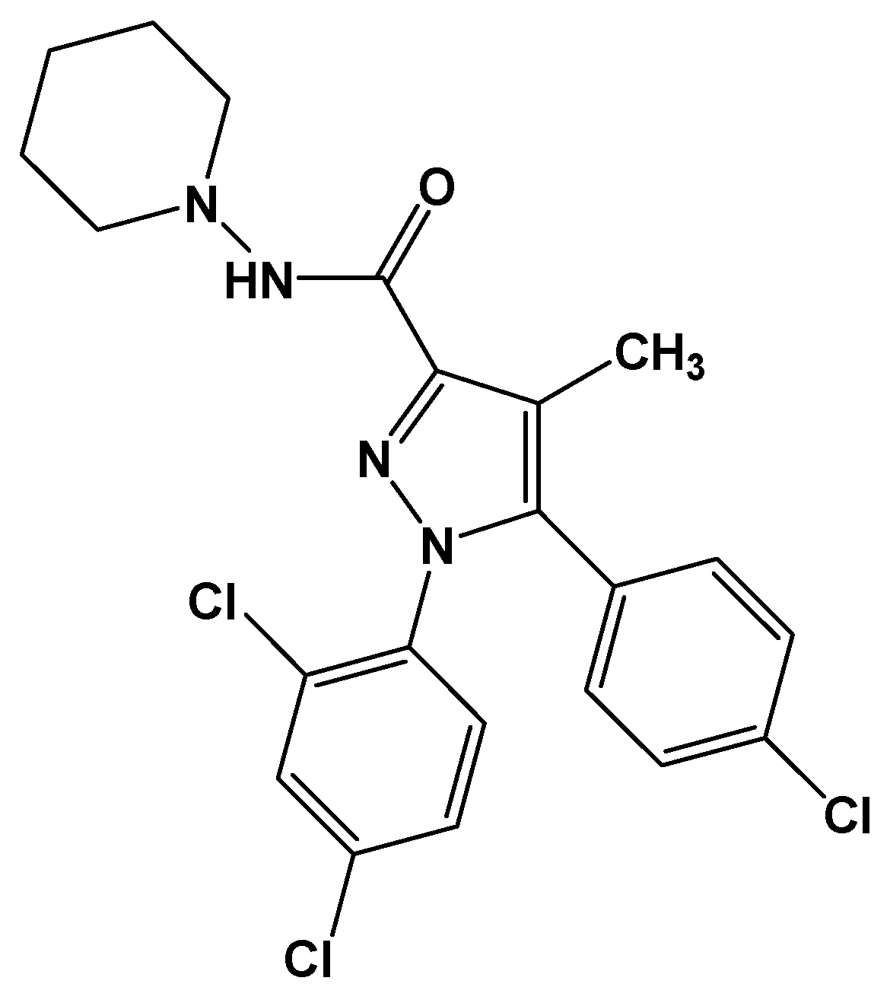
3. Biological Activities of Carbohydrazides Derived from Pyrazole
4. Conclusions
Acknowledgements
References
- Mansour, A.K.; Eid, M.M.; Khalil, N.S.A.M. Synthesis and reactions of some new heterocyclic carbohydrazides and related compounds as potential anticancer agents. Molecules 2003, 8, 744–755. [Google Scholar] [CrossRef]
- Metwally, K.A.; Abdel-Aziz, L.M.; Lashine, E.-S.M.; Husseiny, M.I.; Badawy, R.H. Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: Synthesis and preliminary evaluation as antimicrobial agents. Bioorg. Med. Chem. 2006, 14, 8675–8682. [Google Scholar]
- Ghiglieri-Bertez, C.; Coquelet, C.; Alazet, A.; Bonne, C. Dual inhibitors of the cyclooxygenase and lipoxygenase pathways: Synthesis and activity of hydrazone derivatives. [Inhibiteurs mixtes des voies de la cyclooxygénase et des lipoxygénases: Synthèse et activité de dérivés hydrazoniques]. Eur. J. Med. Chem. 1987, 22, 147–152. [Google Scholar] [CrossRef]
- Combs, D.W.; Rampulla, M.S.; Demers, J.P.; Falotico, R.; Moore, J.B. Heteroatom analogues of bemoradan: Chemistry and cardiotonic activity of l,4-benzot hiazinylpyridazinones. J. Med. Chem. 1992, 35, 172–176. [Google Scholar] [CrossRef]
- Montanari, M.L.C.; Montanari, C.A. Comparative QSAR of antimicrobial hydrazides. [Validação lateral em relações quantitativas entre estrutura e atividade farmacológica, QSAR.]. Quím. Nova 2002, 25, 231–240. [Google Scholar] [CrossRef]
- Farghaly, A.R.A.H. Synthesis, reactions and antimicrobial activity of some new indolyl-1,3,4-oxadiazole, triazole and pyrazole derivatives. J. Chin. Chem. Soc. 2004, 51, 147–156. [Google Scholar]
- Sapijanskaite, B.; Mickevièius, V. Synthesis of 1-(9-butylcarbazol-3-yl)-5-oxopyrrolidine-3-carboxylic acid derivatives. Chem. Heterocycl. Compd. 2008, 44, 807–814. [Google Scholar]
- Xia, Y.; Fan, X.D.; Zhao, B.X.; Zhao, J.; Shin, D.S.; Miao, J.Y. Synthesis and structure activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives as potential agents against A549 lung cancer cells. Eur. J. Med. Chem. 2008, 43, 2347–2353. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.; Abuo-Rahma, E.D.A.; Hassan, A.A. Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur. J. Med. Chem. 2009, 44, 3480–3487. [Google Scholar] [CrossRef]
- Khalifa, M.M.; Abdelbaky, N.A. Synthesis of new imidazolyl acetic acid derivatives with antiinflammatory and analgesic activities. Arch. Pharm. Res. 2008, 31, 419–423. [Google Scholar] [CrossRef]
- Hafez, H.N.; El-Gazzara, A.B.A. Design and synthesis of 3-pyrazolyl-thiophene, thieno[2,3-d]pyrimidines as new bioactive and pharmacological activities. Bioorg. Med. Chem. Lett. 2008, 18, 5222–5227. [Google Scholar]
- Kumaraswamy, M.N.; Chandrashekhar, C.; Shivakumar, H.; Mathias, D.A.P.; Mahadevan, K.M.; Vaidya, V.P. Synthesis and activity evaluation of 2-(1-naphtho[2,1-b]furan-2-yl-carbonyl)-3,5-disubstituted-2,3-dihydro-1H-pyrazoles. Indian J. Pharm. Sci. 2008, 70, 715–720. [Google Scholar] [CrossRef]
- Hegazi, B.; Mohamed, H.A.; Dawood, K.M.; Badria, F.A.R. Cytotoxicity and utility of 1-indanone in the synthesis of some new heterocycles. Chem. Pharm. Bull. 2010, 58, 479–483. [Google Scholar]
- Rostom, S.A.F. Polysubstituted pyrazoles, part 6. Synthesis of some 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazol-3-carbonyl derivatives linked to nitrogenous heterocyclic ring systems as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 2767–2776. [Google Scholar] [CrossRef]
- Francisco, M.E.Y.; Seltzman, H.H.; Gilliam, A.F.; Mitchell, R.A.; Rider, S.L.; Pertwee, R.G.; Stevenson, L.A.; Thomas, B.F. Synthesis and structure-activity relationships of amide and hydrazide analogues of the cannabinoid CB1 receptor antagonist N-(Piperidinyl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716). J. Med. Chem. 2002, 45, 2708–2719. [Google Scholar] [CrossRef]
- Padgett, L.W. Recent developments in cannabinoid ligands. Life Sci. 2005, 77, 1767–1798. [Google Scholar] [CrossRef]
- Fong, T.M.; Heymsfield, S.B. Cannabinoid-1 receptor inverse agonists: Current understanding of mechanism of action and unanswered questions CB1R mechanism of action. Int. J. Obes. 2009, 33, 947–955. [Google Scholar] [CrossRef]
- Soyka, M.; Koller, G.; Schmidt, P.; Lesch, O.M.; Leweke, M.; Fehr, C.; Gann, H.; Mann, K.F. ACTOL Study Investigators. Cannabinoid receptor 1 blocker rimonabant (SR 141716) for treatment of alcohol dependence: Results from a placebo-controlled, double-blind trial. J. Clin. Psychopharmacol. 2008, 28, 317–324. [Google Scholar] [CrossRef]
- Xia, Y.; Dong, Z.W.; Zhao, B.X.; Ge, X.; Meng, N.; Shinc, D.S.; Miaob, J.Y. Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg. Med. Chem. 2007, 15, 6893–6899. [Google Scholar] [CrossRef]
- Fan, C.D.; Zhao, B.X.; Wei, F.; Zhang, G.H.; Dong, W.L.; Miao, J.Y. Synthesis and discovery of autophagy inducers for A549 and H460 lung cancer cells, novel 1-(20-hydroxy-30-aroxypropyl)-3-aryl-1H-pyrazole-5-carbohydrazide derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 3860–3864. [Google Scholar]
- Fan, C.; Zhao, J.; Zhao, B.; Zhang, S.; Miao, J. Novel complex of copper and a salicylaldehyde pyrazole hydrazone derivative induces apoptosis through up-regulating integrin in vascular endothelial cells. Chem Res. Toxicol. 2009, 22, 1517–1525. [Google Scholar]
- Fan, C.D.; Su, H.; Zhao, J.; Zhao, B.X.; Zhang, S.L.; Miao, J.Y. A novel copper complex of salicylaldehyde pyrazole hydrazone induces apoptosis through up-regulating integrin b4 in H322 lung carcinoma cells. Eur. J. Med. Chem. 2010, 45, 1438–1446. [Google Scholar]
- Li, Z.M.; Chen, H.S.; Zhao, W.G.; Zhang, K.; Huang, X.S. Synthesis and biological activity of pyrazole derivatives. Chem. J. Chin. Univ. 1997, 18, 1794–1799. [Google Scholar]
- Chen, H.S.; Li, Z.M. Synthesis of some heteroaryl pyrazole derivatives and their biological activities. Chin. J. Chem. 2000, 18, 596–602. [Google Scholar]
- Lian, S.; Su, H.; Zhao, B.X.; Liu, W.Y.; Zheng, L.W.; Miao, J.Y. Synthesis and discovery of pyrazole-5-carbohydrazide N-glycosides as inducer of autophagy in A549 lung cancer cells. Bioorg. Med. Chem. 2009, 17, 7085–7092. [Google Scholar] [CrossRef]
- Zheng, L.W.; Wub, L.L.; Zhao, B.X.; Dong, W.L.; Miao, J.Y. Synthesis of novel substituted pyrazole-5-carbohydrazide hydrazone derivatives and discovery of a potent apoptosis inducer in A549 lung cancer cells. Bioorg. Med. Chem. 2009, 17, 1957–1962. [Google Scholar]
- Matheus, M.E.; Oliveira, L.F.; Freitas, A.C.; Carvalho, A.M.; Barreiro, E.J. Antinociceptive property of new 4-acyl-arylhydrazone pyrazole compounds. Braz. J. Med. Biol. Res. 1991, 24, 1219–1222. [Google Scholar]
- Matheus, M.E.; Oliveira, L.F.; Freitas, A.C.C. Antinociceptive activity of new pyrazole derivatives. Rev. Port. Farm. 1998, XLVIII, 153–158. [Google Scholar]
- Sant'anna, C.M.R.; Alencastro, R.B.; Rodrigues, C.R.; Barreiro, G.; Barreiro, E.J.; Motta Neto, J.D.; Freitas, A.C.C. A semiempirical study of pyrazole acylhydrazones as potential antimalarial agents. Int. J. Quantum Chem. 1996, 60, 1835–1843. [Google Scholar] [CrossRef]
- Bernardino, A.M.R.; Gomes, A.O.; Machado, G.M.C.; Canto-cavalheiro, M.M.; Leon, L.; Amaral, V.; Charret, K.S.; Freitas, A.C.C. Synthesis and leishmanicidal activities of 1-(4-X-phenyl)-N'-[(4-Y-phenyl)methylene]-1H-pyrazole-4-carbohydrazides. Eur. J. Med. Chem. 2006, 41, 80–87. [Google Scholar] [CrossRef]
- Charret, K.S.; Rodrigues, R.F.; Gomes, A.O.; Bernardino, A.M.R.; Canto-Cavaleiro, M.; Leon, L.L.; Amaral, V.F. Effect of the oral treatment with pyrazole carbohydrazides derivatives in the murine infection by leishmania amazonensis. Am. J. Trop. Med. Hyg. 2009, 80, 568–573. [Google Scholar]
- Chornous, V.A.; Bratenko, M.K.; Vovk, M.V.; Sidorchuk, I.I. Synthesis and antimicrobial activity of pyrazole-4-carboxylic acid hydrazides and n-(4-pyrazoyl) hydrazones of aromatic and heteroaromatic aldehydes. Pharm. Chem. J. 2001, 35, 203–205. [Google Scholar] [CrossRef]
- Vera-DiVaio, M.A.F.; Freitas, A.C.C.; Castro, H.C.; Albuquerque, S.; Cabral, L.M.; Rodrigues, C.R.; Albuquerque, M.G.; Martins, R.C.A.; Henriques, M.G.M.O; Dias, L.R.S. Synthesis, antichagasic in vitro evaluation, cytotoxicity assays, molecular modeling and SAR/QSAR studies of a 2-phenyl-3-(1-phenyl-1H-pyrazol-4-yl)-acrylic acid benzylidene-carbohydrazide series. Bioorg. Med. Chem. 2009, 17, 295–302. [Google Scholar]
- Kaushik, D.; Khan, S.A.; Chawla, G.; Kumar, S. N'-[(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene] 2/4-substituted hydrazides: Synthesis and anticonvulsant activity. Eur. J. Med. Chem. 2010, 45, 3943–3949. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dias, L.R.S.; Salvador, R.R.S. Pyrazole Carbohydrazide Derivatives of Pharmaceutical Interest. Pharmaceuticals 2012, 5, 317-324. https://doi.org/10.3390/ph5030317
Dias LRS, Salvador RRS. Pyrazole Carbohydrazide Derivatives of Pharmaceutical Interest. Pharmaceuticals. 2012; 5(3):317-324. https://doi.org/10.3390/ph5030317
Chicago/Turabian StyleDias, Luiza Rosaria Sousa, and Raquel Rocha Silva Salvador. 2012. "Pyrazole Carbohydrazide Derivatives of Pharmaceutical Interest" Pharmaceuticals 5, no. 3: 317-324. https://doi.org/10.3390/ph5030317
APA StyleDias, L. R. S., & Salvador, R. R. S. (2012). Pyrazole Carbohydrazide Derivatives of Pharmaceutical Interest. Pharmaceuticals, 5(3), 317-324. https://doi.org/10.3390/ph5030317



