Tissue Engineering of Cartilage; Can Cannabinoids Help?
Abstract
:1. Introduction
1.1. Tissue Engineering
1.2. Cartilage Disease
1.3. Cannabinoid System
2. Tissue Engineering Strategies to Solve Cartilage Disease
2.1. Bone Marrow Derived Mesenchymal Stem Cells and Chondrogenesis
3. Cannabinoid System Potential Use in Tissue-Engineered Cartilage
3.1. Cannabinoid System and Chondrogenesis
4. Is the Cannabinoid System a Potential Therapeutic Target in MSC-Based Tissue-Engineered Cartilage Regeneration Strategies?

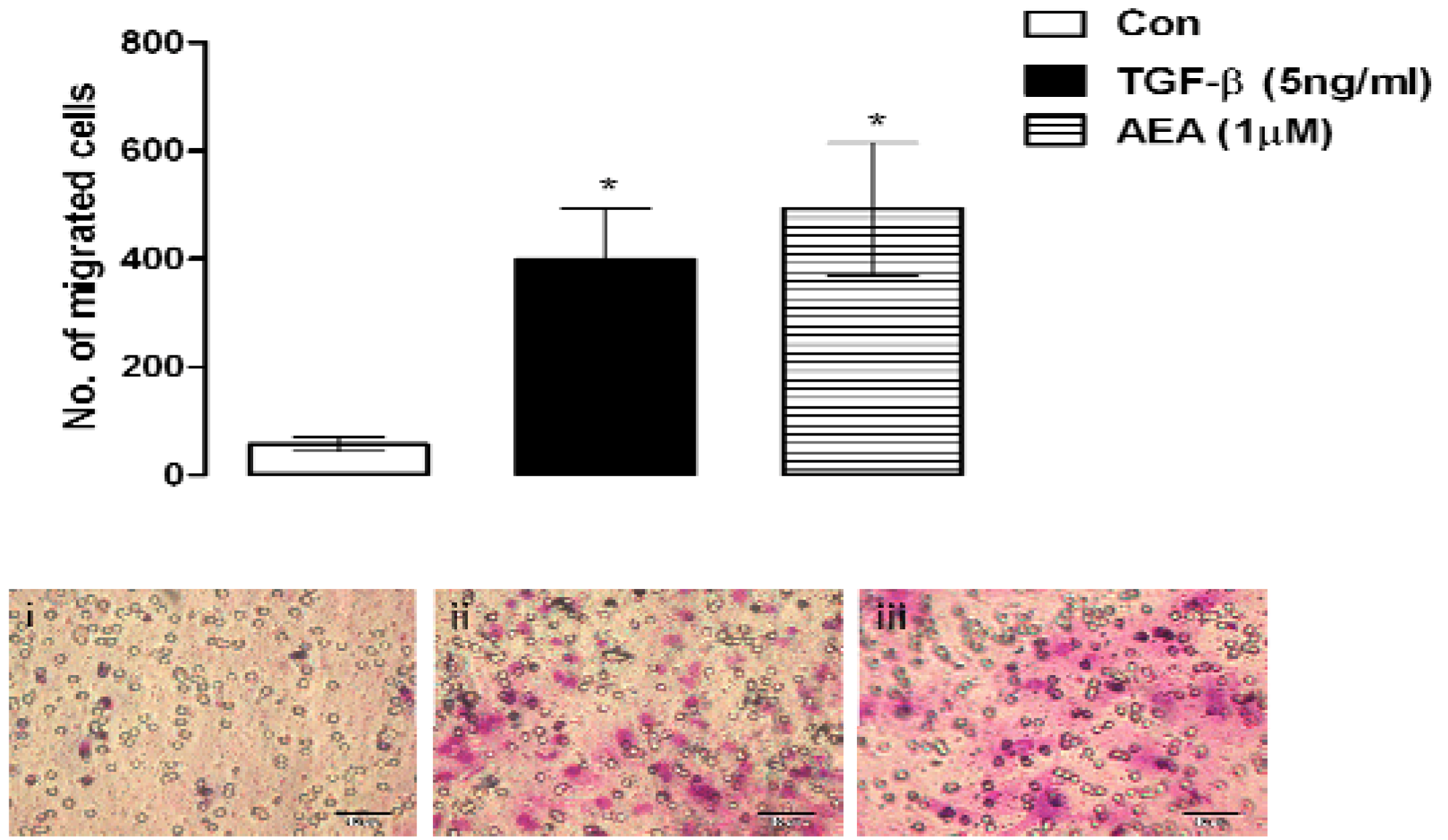

5. Conclusions and Future Directions
6. Methods
6.1. Culture of Mesenchymal Stem Cells
6.2. Drug Treatments
6.3. Cell Viability Assay
6.4. Cell Migration
6.5. Real-time PCR for Collagen II
6.6. Immunofluorescence for Collagen II
6.7. Safranin-O Histological Staining for Proteoglycans
Acknowledgements
References
- Idris, A.I.; van 't Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; Mechoulam, R.; Zimmer, A.; Bab, I. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar]
- Idris, A.I.; Ralston, S.H. Cannabinoids and Bone: Friend or Foe? Calcif. Tissue Int. 2010. [Google Scholar] [CrossRef]
- Ahrens, P.B.; Solursh, M.; Reiter, R.S. Stage-related capacity for limb chondrogenesis in cell culture. Dev. Biol. 1977, 60, 69–82. [Google Scholar]
- Guilak, F.; Awad, H.A.; Fermor, B.; Leddy, H.A.; Gimble, J.M. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology 2004, 41, 389–399. [Google Scholar]
- Mbvundula, E.C.; Bunning, R.A.; Rainsford, K.D. Effects of cannabinoids on nitric oxide production by chondrocytes and proteoglycan degradation in cartilage. Biochem. Pharmacol. 2005, 69, 635–640. [Google Scholar]
- Mbvundula, E.C.; Bunning, R.A.; Rainsford, K.D. Arthritis and cannabinoids: HU-210 and Win-55,212-2 prevent IL-1alpha-induced matrix degradation in bovine articular chondrocytes in vitro. J. Pharm. Pharmacol. 2006, 58, 351–358. [Google Scholar] [PubMed]
- Bidinger, B.; Torres, R.; Rossetti, R.G.; Brown, L.; Beltre, R.; Burstein, S.; Lian, J.B.; Stein, G.S.; Zurier, R.B. Ajulemic acid, a nonpsychoactive cannabinoid acid, induces apoptosis in human T lymphocytes. Clin. Immunol. 2003, 108, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; Stebulis, J.A.; Rossetti, R.G.; Burstein, S.H.; Zurier, R.B. Suppression of fibroblast metalloproteinases by ajulemic acid, a nonpsychoactive cannabinoid acid. J. Cell. Biochem. 2007, 100, 184–190. [Google Scholar]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar]
- McDougall, J.J.; Yu, V.; Thomson, J. In vivo effects of CB2 receptor-selective cannabinoids on the vasculature of normal and arthritic rat knee joints. Br. J. Pharmacol. 2008, 153, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.H. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000, 6, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Nesic, D.; Whiteside, R.; Brittberg, M.; Wendt, D.; Martin, I.; Mainil-Varlet, P. Cartilage tissue engineering for degenerative joint disease. Adv. Drug Deliv. Rev. 2006, 58, 300–322. [Google Scholar]
- Lu, L.; Zhu, X.; Valenzuela, R.G.; Currier, B.L.; Yaszemski, M.J. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin. Orthop. Relat. Res. 2001, S251–S270. [Google Scholar]
- Richter, W. Mesenchymal stem cells and cartilage in situ regeneration. J. Intern. Med. 2009, 266, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, M.J.; Mason, R.M. Bovine articular chondrocyte function in vitro depends upon oxygen tension. Osteoarthritis Cartilage 2000, 8, 386–392. [Google Scholar]
- Malda, J.; Martens, D.E.; Tramper, J.; van Blitterswijk, C.A.; Riesle, J. Cartilage tissue engineering: controversy in the effect of oxygen. Crit. Rev. Biotechnol. 2003, 23, 175–194. [Google Scholar]
- Marcus, R.E.; Srivastava, V.M. Effect of low oxygen tensions on glucose-metabolizing enzymes in cultured articular chondrocytes. Proc. Soc. Exp. Biol. Med. 1973, 143, 488–491. [Google Scholar]
- Brighton, C.T.; Heppenstall, R.B. Oxygen tension of the epiphyseal plate distal to an arteriovenous fistula. Clin. Orthop. Relat. Res. 1971, 80, 167–173. [Google Scholar]
- Brittberg, M.; Tallheden, T.; Sjogren-Jansson, B.; Lindahl, A.; Peterson, L. Autologous chondrocytes used for articular cartilage repair: an update. Clin. Orthop. Relat. Res. 2001, S337–S348. [Google Scholar]
- Haselgrove, J.C.; Shapiro, I.M.; Silverton, S.F. Computer modeling of the oxygen supply and demand of cells of the avian growth cartilage. Am. J. Physiol. 1993, 265, C497–C506. [Google Scholar]
- Shum, L.; Nuckolls, G. The life cycle of chondrocytes in the developing skeleton. Arthritis Res. 2002, 4, 94–106. [Google Scholar]
- DeLise, A.M.; Fischer, L.; Tuan, R.S. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 2000, 8, 309–334. [Google Scholar]
- Sandell, L.J.; Adler, P. Developmental patterns of cartilage. Front. Biosci. 1999, 4, D731–D742. [Google Scholar]
- Goldberg, V.M.; Caplan, A.I. Biological resurfacing: an alternative to total joint arthroplasty. Orthopedics 1994, 17, 819–821. [Google Scholar]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar]
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K.; Rodrigo, J.J. The microfracture technic in the management of complete cartilage defects in the knee joint. Orthopade 1999, 28, 26–32. [Google Scholar]
- Steadman, J.R.; Rodkey, W.G.; Briggs, K.K. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J. Knee. Surg. 2002, 15, 170–176. [Google Scholar] [PubMed]
- Kon, E.; Delcogliano, M.; Filardo, G.; Montaperto, C.; Marcacci, M. Second generation issues in cartilage repair. Sports Med. Arthrosc. 2008, 16, 221–229. [Google Scholar]
- McNickle, A.G.; Provencher, M.T.; Cole, B.J. Overview of existing cartilage repair technology. Sports Med. Arthrosc. 2008, 16, 196–201. [Google Scholar]
- Richter, W. Cell-based cartilage repair: illusion or solution for osteoarthritis. Curr. Opin. Rheumatol. 2007, 19, 451–456. [Google Scholar]
- Pertwee, R.G. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005, 7, E625–E654. [Google Scholar]
- Pertwee, R.G. Pharmacological actions of cannabinoids. Handb. Exp. Pharmacol. 2005, 1–51. [Google Scholar]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Mackie, K.; Martin, B.R.; Mechoulam, R.; Pertwee, R.G. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Hohmann, A.G. Cannabinoid mechanisms of pain suppression. Handb. Exp. Pharmacol. 2005, 168, 509–554. [Google Scholar]
- Goutopoulos, A.; Makriyannis, A. From cannabis to cannabinergics: new therapeutic opportunities. Pharmacol. Ther. 2002, 95, 103–117. [Google Scholar]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; Brusco, A.; Akinshola, B.E.; Liu, Q.R.; Hope, B.; Iwasaki, S.; Arinami, T.; Teasenfitz, L.; Uhl, G.R. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. NY Acad. Sci. 2006, 1074, 514–536. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Di Marzo, V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J. Neuroimmune Pharmacol. 2010, 5, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Scutt, A.; Williamson, E.M. Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors. Calcif. Tissue Int. 2007, 80, 50–59. [Google Scholar]
- Fowler, C.J.; Rojo, M.L.; Rodriguez-Gaztelumendi, A. Modulation of the endocannabinoid system: neuroprotection or neurotoxicity? Exp. Neurol. 2010, 224, 37–47. [Google Scholar] [PubMed]
- Steinmeyer, J.; Daufeldt, S. Pharmacological influence of antirheumatic drugs on proteoglycans from interleukin-1 treated articular cartilage. Biochem. Pharmacol. 1997, 53, 1627–1635. [Google Scholar]
- Cawston, T. Matrix metalloproteinases and TIMPs: properties and implications for the rheumatic diseases. Mol. Med. Today 1998, 4, 130–137. [Google Scholar]
- Steinmeyer, J.; Daufeldt, S.; Kalbhen, D.A. The proteoglycan metabolism, morphology and viability of articular cartilage treated with a synthetic matrix metalloproteinase inhibitor. Res. Exp. Med. (Berl.) 1997, 197, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Temenoff, J.S.; Mikos, A.G. Review: tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [PubMed]
- Friedenstein, A.J.; Piatetzky, S., II; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [Google Scholar]
- Owen, M. Marrow stromal stem cells. J. Cell Sci. 1988, 10, 63–76. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar]
- Barry, F.P.; Murphy, J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004, 36, 568–584. [Google Scholar]
- Lin, G.; Garcia, M.; Ning, H.; Banie, L.; Guo, Y.L.; Lue, T.F.; Lin, C.S. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008, 17, 1053–1063. [Google Scholar]
- De Bari, C.; Dell'Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar]
- Barry, F.P. Biology and clinical applications of mesenchymal stem cells. Birth Defects Res. C Embryo Today 2003, 69, 250–256. [Google Scholar]
- Barry, F.; Boynton, R.E.; Liu, B.; Murphy, J.M. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001, 268, 189–200. [Google Scholar]
- Barry, F.; Boynton, R.; Murphy, M.; Haynesworth, S.; Zaia, J. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2001, 289, 519–524. [Google Scholar]
- Bruder, S.P.; Jaiswal, N.; Ricalton, N.S.; Mosca, J.D.; Kraus, K.H.; Kadiyala, S. Mesenchymal stem cells in osteobiology and applied bone regeneration. Clin. Orthop. Relat. Res. 1998, S247–S256. [Google Scholar]
- Bruder, S.P.; Kraus, K.H.; Goldberg, V.M.; Kadiyala, S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J. Bone Joint Surg. Am. 1998, 80, 985–996. [Google Scholar]
- Digirolamo, C.M.; Stokes, D.; Colter, D.; Phinney, D.G.; Class, R.; Prockop, D.J. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999, 107, 275–281. [Google Scholar]
- Bunting, K.D.; Hawley, R.G. Integrative molecular and developmental biology of adult stem cells. Biol. Cell 2003, 95, 563–578. [Google Scholar]
- Salem, H.K.; Thiemermann, C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells 2010, 28, 585–596. [Google Scholar]
- Bianco, P.; Riminucci, M.; Gronthos, S.; Robey, P.G. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Kanichai, M.; Ferguson, D.; Prendergast, P.J.; Campbell, V.A. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J. Cell Physiol. 2008, 216, 708–715. [Google Scholar]
- McMahon, L.A.; Reid, A.J.; Campbell, V.A.; Prendergast, P.J. Regulatory effects of mechanical strain on the chondrogenic differentiation of MSCs in a collagen-GAG scaffold: experimental and computational analysis. Ann. Biomed. Eng. 2008, 36, 185–194. [Google Scholar]
- Sekiya, I.; Vuoristo, J.T.; Larson, B.L.; Prockop, D.J. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 4397–4402. [Google Scholar]
- Chen, C.W.; Tsai, Y.H.; Deng, W.P.; Shih, S.N.; Fang, C.L.; Burch, J.G.; Chen, W.H.; Lai, W.F. Type I and II collagen regulation of chondrogenic differentiation by mesenchymal progenitor cells. J. Orthop. Res. 2005, 23, 446–453. [Google Scholar]
- Pistoia, V.; Raffaghello, L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev. Clin. Immunol. 2010, 6, 211–218. [Google Scholar]
- Ross, R.A.; Brockie, H.C.; Stevenson, L.A.; Murphy, V.L.; Templeton, F.; Makriyannis, A.; Pertwee, R.G. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br. J. Pharmacol. 1999, 126, 665–672. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M. Expression of the endocannabinoid system in fibroblasts and myofascial tissues. J. Bodyw. Mov. Ther. 2008, 12, 169–182. [Google Scholar]
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; Canals, M.; Milligan, G.; Baker, D.; van't Hof, R.J.; Ralston, S.H. Cannabinoid receptor type 1 protects against age-related osteoporosis by regulating osteoblast and adipocyte differentiation in marrow stromal cells. Cell Metab. 2009, 10, 139–147. [Google Scholar]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar]
- Stebulis, J.A.; Johnson, D.R.; Rossetti, R.G.; Burstein, S.H.; Zurier, R.B. Ajulemic acid, a synthetic cannabinoid acid, induces an antiinflammatory profile of eicosanoids in human synovial cells. Life Sci. 2008, 83, 666–670. [Google Scholar] [PubMed]
- Burstein, S.H. Ajulemic acid (CT3): a potent analog of the acid metabolites of THC. Curr. Pharm. Des. 2000, 6, 1339–1345. [Google Scholar]
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar]
- Zurier, R.B.; Sun, Y.P.; George, K.L.; Stebulis, J.A.; Rossetti, R.G.; Skulas, A.; Judge, E.; Serhan, C.N. Ajulemic acid, a synthetic cannabinoid, increases formation of the endogenous proresolving and anti-inflammatory eicosanoid, lipoxin A4. FASEB J. 2009, 23, 1503–1509. [Google Scholar] [PubMed]
- Aguado, T.; Romero, E.; Monory, K.; Palazuelos, J.; Sendtner, M.; Marsicano, G.; Lutz, B.; Guzman, M.; Galve-Roperh, I. The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J. Biol. Chem. 2007, 282, 23892–23898. [Google Scholar]
- Berghuis, P.; Rajnicek, A.M.; Morozov, Y.M.; Ross, R.A.; Mulder, J.; Urban, G.M.; Monory, K.; Marsicano, G.; Matteoli, M.; Canty, A.; Irving, A.J.; Katona, I.; Yanagawa, Y.; Rakic, P.; Lutz, B.; Mackie, K.; Harkany, T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 2007, 316, 1212–1216. [Google Scholar]
- McPartland, J.M.; Skinner, E. The biodynamic model of osteopathy in the cranial field. Explore (NY) 2005, 1, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Tsai, S.; Kato, K.; Yamanouchi, D.; Wang, C.; Rafii, S.; Liu, B.; Kent, K.C. Transforming growth factor-beta promotes recruitment of bone marrow cells and bone marrow-derived mesenchymal stem cells through stimulation of MCP-1 production in vascular smooth muscle cells. J. Biol. Chem. 2009, 284, 17564–17574. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gowran, A.; McKayed, K.; Kanichai, M.; White, C.; Hammadi, N.; Campbell, V. Tissue Engineering of Cartilage; Can Cannabinoids Help? Pharmaceuticals 2010, 3, 2970-2985. https://doi.org/10.3390/ph3092970
Gowran A, McKayed K, Kanichai M, White C, Hammadi N, Campbell V. Tissue Engineering of Cartilage; Can Cannabinoids Help? Pharmaceuticals. 2010; 3(9):2970-2985. https://doi.org/10.3390/ph3092970
Chicago/Turabian StyleGowran, Aoife, Katey McKayed, Manoj Kanichai, Cillian White, Nissrin Hammadi, and Veronica Campbell. 2010. "Tissue Engineering of Cartilage; Can Cannabinoids Help?" Pharmaceuticals 3, no. 9: 2970-2985. https://doi.org/10.3390/ph3092970
APA StyleGowran, A., McKayed, K., Kanichai, M., White, C., Hammadi, N., & Campbell, V. (2010). Tissue Engineering of Cartilage; Can Cannabinoids Help? Pharmaceuticals, 3(9), 2970-2985. https://doi.org/10.3390/ph3092970



