Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain
Abstract
:1. Gastrointestinal Pain
2. Sensory Afferents Innervating the Gastrointestinal Tract
3. What Is a Gut-Innervating Nociceptor?
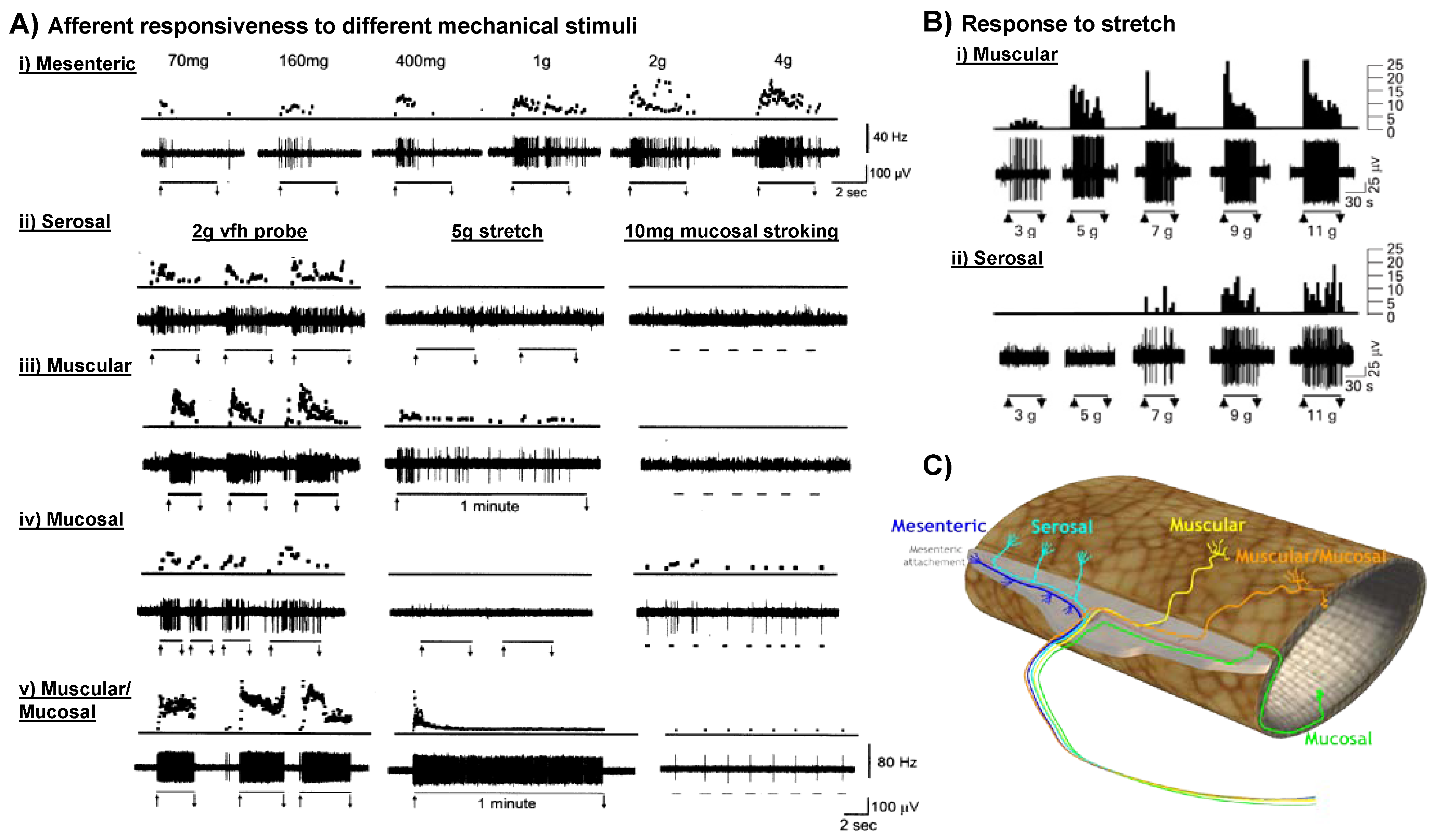
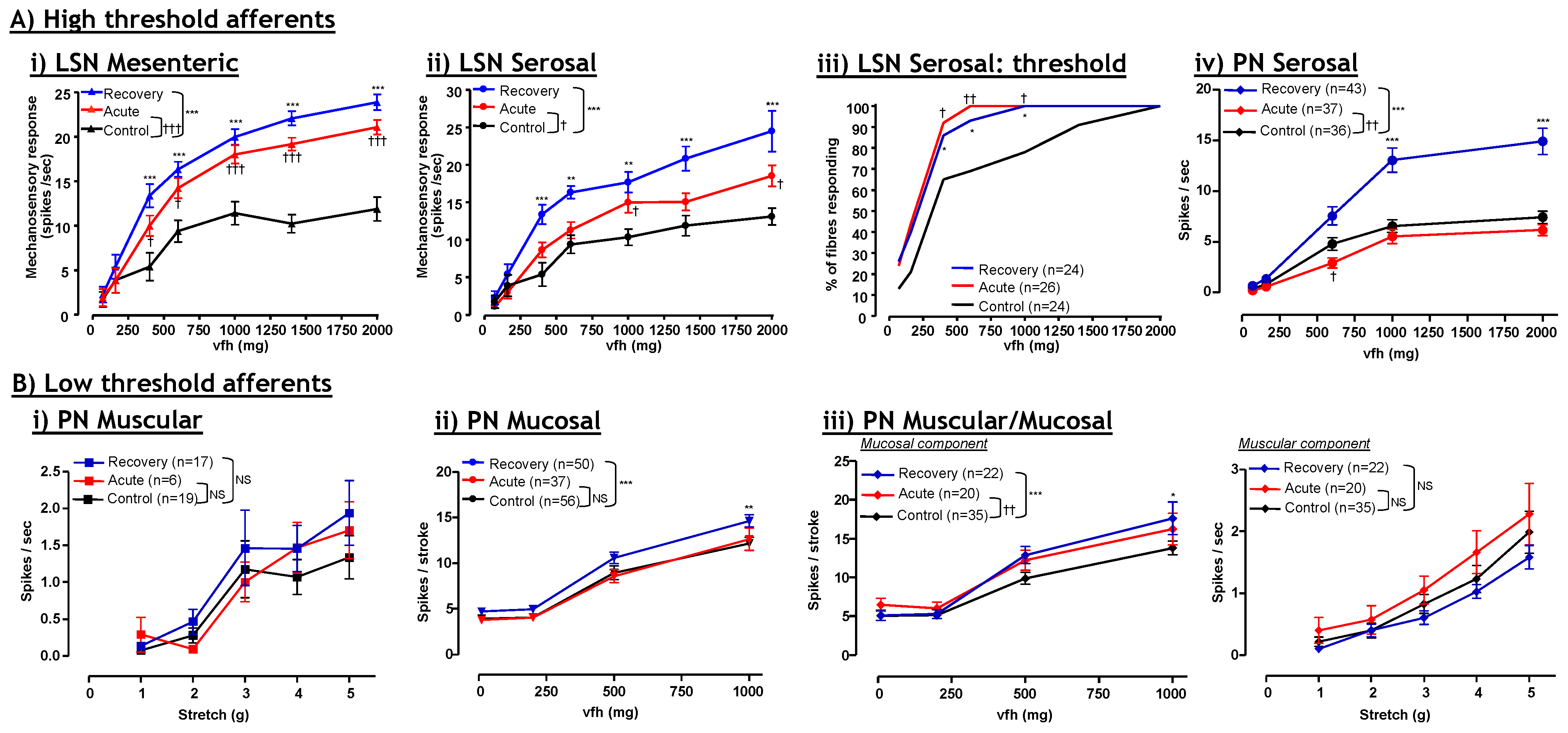
4. Visceral Hypersensitivity
5. TRPV1
| Functional role for channel in healthy: | Isolated gut neurons | Afferent gut fibres | Whole animal | Is channel function in gut neurons altered by inflammatory mediators? |
|---|---|---|---|---|
| TRPV1 | √ | √ | √ | √; 5-HT ↑, PAR2 ↑ |
| TRPV4 | √ | √ | √ | √; 5-HT ↑, PAR2 ↑, Histamine ↑, PAR4↓ |
| TRPA1 | √ | √ | √ | √; Bradykinin ↑, 4-HNE ↑, PAR2↔ or ↑ |
| ASIC1 | ND | √ | √ | ND |
| ASIC2 | ND | √ | √ | ND |
| ASIC3 | ND | √ | √ | ND |
| NaV1.5 | X | ND | ND | ND |
| NaV1.7 | √ | ND | ND | ND |
| NaV1.8 | √ | ND | √ | √; TNFα ↑ |
| NaV1.9 | √ | ND | X | ND |
| Kv | √ | ND | ND | √; TNFα ↓ |
5.1. TRPV1: Contribution to Inflammation
5.2. TRPV1: Contribution to Visceral Hyperalgesia
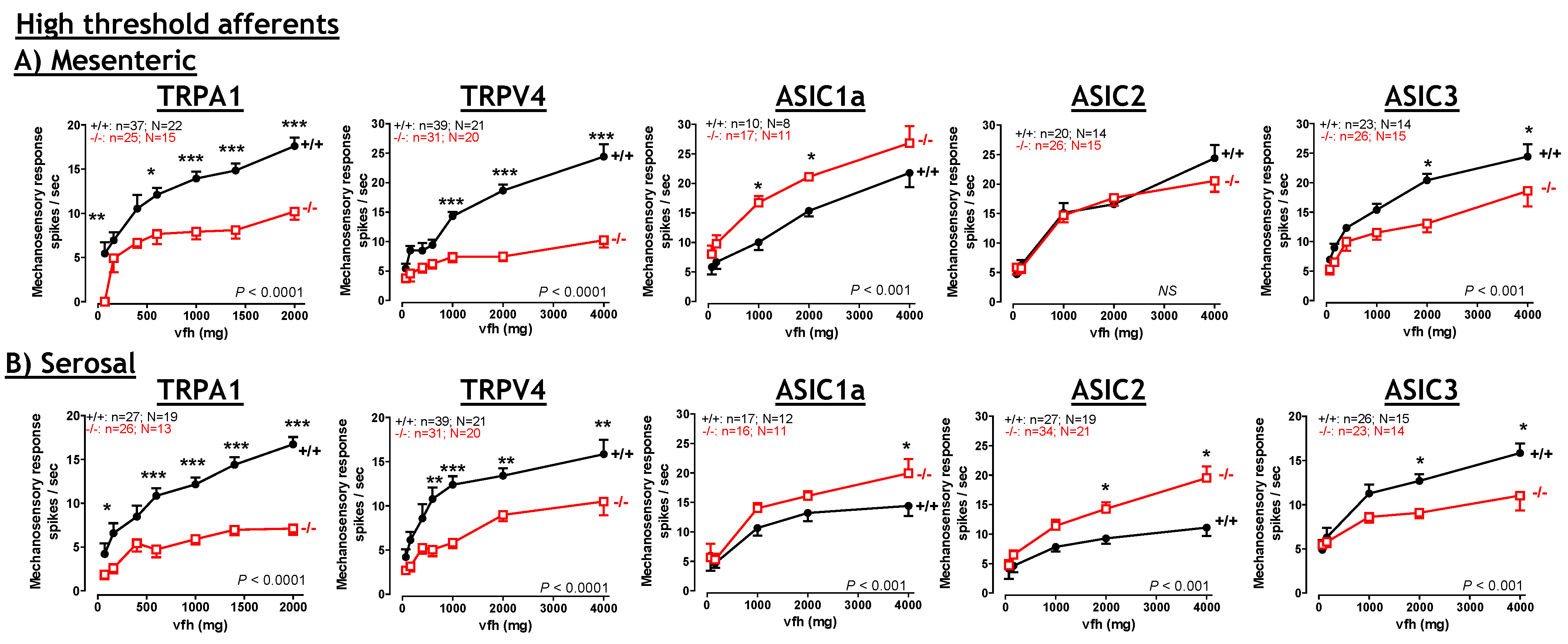
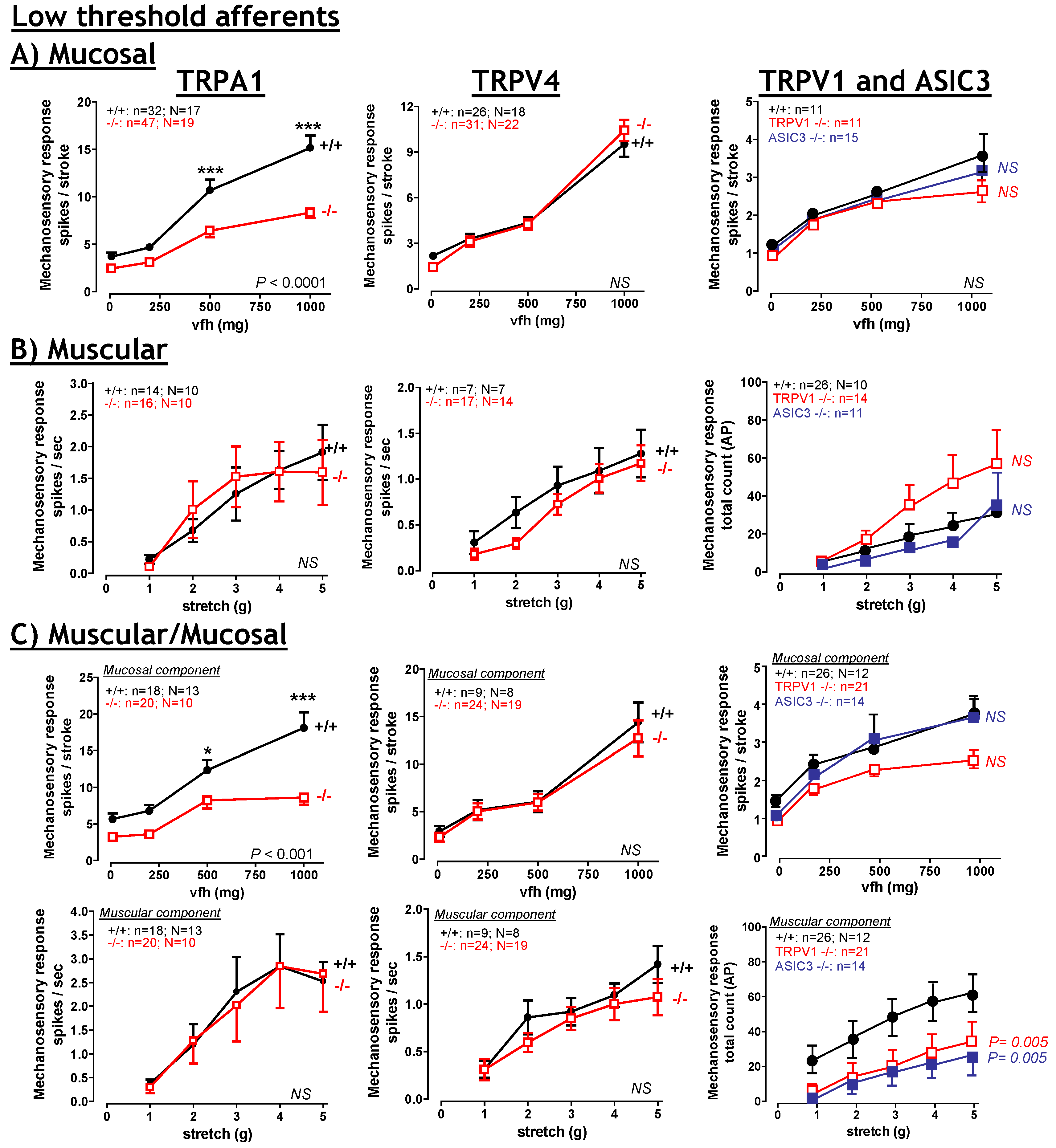
5.3. TRPV1: Therapeutic potential?
| Altered channel function or expression in gut based inflammatory models? | Isolated gut neurons | Afferent gut fibres | Whole animal | Is channel expression or function altered in gut based human diseases? |
|---|---|---|---|---|
| TRPV1 | √ | √ | √ | √ |
| TRPV4 | √ | √ | √ | √ |
| TRPA1 | ND | √ | √ | ND |
| ASIC1 | ND | ND | ND | ND |
| ASIC2 | ND | ND | ND | ND |
| ASIC3 | ND | √ | √ | √ |
| NaV1.5 | ND | ND | ND | √ |
| NaV1.7 | X | ND | ND | √ |
| NaV1.8 | √ | ND | √ | ND |
| NaV1.9 | X | ND | √ | ND |
| Kv | √ | ND | ND | ND |
6. TRPA1
6.1. TRPA1: Contribution to Inflammation
6.2. TRPA1: Contribution to visceral hyperalgesia.
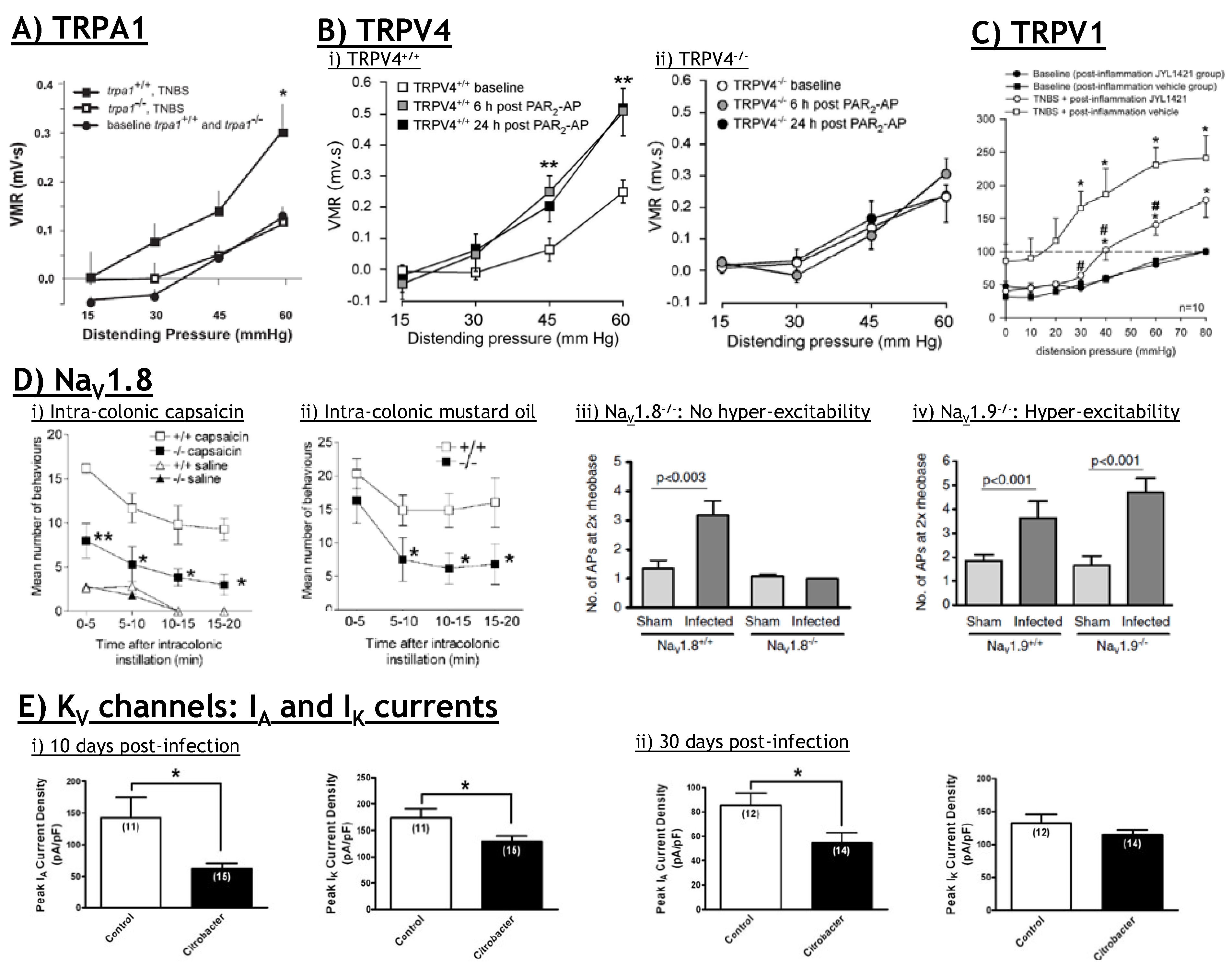
6.3. TRPA1: Therapeutic potential?
7. TRPV4
7.1. TRPV4: Contribution to visceral hyperalgesia.
7.2. TRPV4: Therapeutic potential?
8. Acid Sensing Ion Channels
8.1. ASICs: Therapeutic Potential?
9. Voltage Gated Sodium and Potassium Channels (NaV and KV)
9.1. NaV and KV: Contribution to Visceral Hyperalgesia.
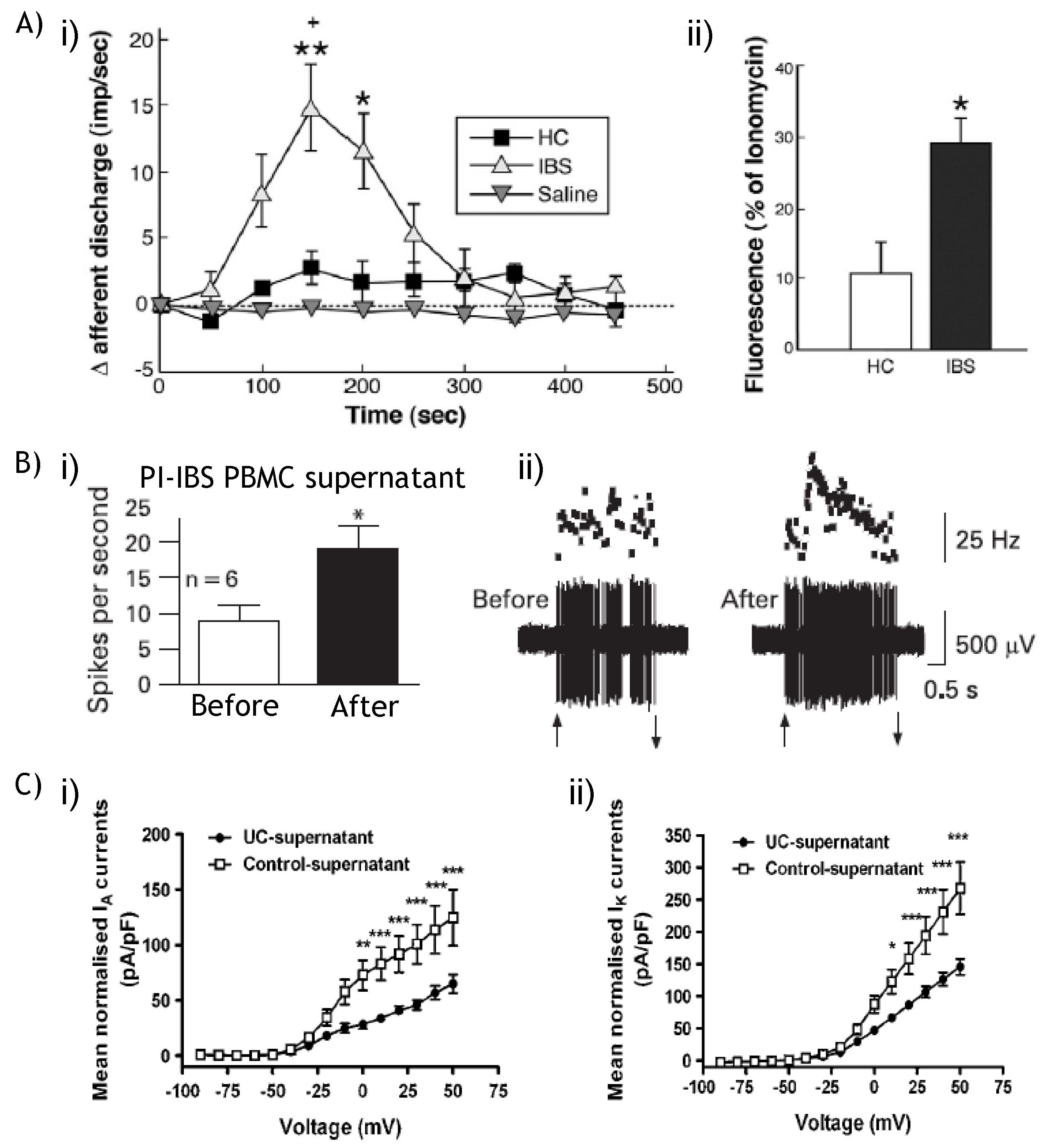
9.2. NaV and KV: Therapeutic potential?
10. Concluding Remarks
Acknowledgements
References
- Richter, J.E. Noncardiac (Unexplained) Chest Pain. Curr. Treat. Options Gastroenterol. 2000, 3, 329–334. [Google Scholar]
- Richter, J.E. Chest pain and gastroesophageal reflux disease. J. Clin. Gastroenterol. 2000, 30, S39–S41. [Google Scholar]
- Tack, J.; Talley, N.J. Gastroduodenal disorders. Am. J. Gastroenterol. 2010, 105, 757–763. [Google Scholar]
- Tack, J.; Talley, N.J.; Camilleri, M.; Holtmann, G.; Hu, P.; Malagelada, J.R.; Stanghellini, V. Functional gastroduodenal disorders. Gastroenterology 2006, 130, 1466–1479. [Google Scholar]
- Geeraerts, B.; Tack, J. Functional dyspepsia: past, present, and future. J. Gastroenterol. 2008, 43, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Camilleri, M.; Farrugia, G.; McCallum, R.W.; Bharucha, A.E.; Mayer, E.A.; Tack, J.F.; Spiller, R.; Horowitz, M.; Vinik, A.I.; et al. Gastroparesis and functional dyspepsia: excerpts from the AGA/ANMS meeting. Neurogastroenterol. Motil. 2010, 22, 113–133. [Google Scholar] [PubMed]
- Camilleri, M. Management of the Irritable Bowel Syndrome. Gastroenterology 2001, 120, 652–668. [Google Scholar]
- Spiller, R. Clinical update: irritable bowel syndrome. Lancet 2007, 369, 1586–1588. [Google Scholar]
- Spiller, R. Review article: probiotics and prebiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 385–396. [Google Scholar]
- Spiller, R.; Aziz, Q.; Creed, F.; Emmanuel, A.; Houghton, L.; Hungin, P.; Jones, R.; Kumar, D.; Rubin, G.; Trudgill, N.; et al. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 2007, 56, 1770–1798. [Google Scholar] [PubMed]
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar]
- Hou, J.K.; El-Serag, H.; Thirumurthi, S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: a systematic review. Am. J. Gastroenterol. 2009, 104, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory bowel disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar]
- Beyak, M.J.; Vanner, S. Inflammation-induced hyperexcitability of nociceptive gastrointestinal DRG neurones: the role of voltage-gated ion channels. Neurogastroenterol. Motil. 2005, 17, 175–186. [Google Scholar]
- Blackshaw, L.A.; Brookes, S.J.H.; Grundy, D.; Schemann, M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol. Motil. 2007, 19, 1–19. [Google Scholar]
- Brierley, S.M. Molecular basis of mechanosensitivity. Auton. Neurosci. 2010, 153, 58–68. [Google Scholar]
- Kirkup, A.J.; Brunsden, A.M.; Grundy, D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G787–G794. [Google Scholar] [PubMed]
- Blackshaw, L.A.; Gebhart, G.F. The pharmacology of gastrointestinal nociceptive pathways. Curr. Opin. Pharmacol. 2002, 2, 642–649. [Google Scholar]
- Hughes, P.A.; Brierley, S.M.; Blackshaw, L.A. Post-inflammatory modification of colonic afferent mechanosensitivity. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1034–1040. [Google Scholar]
- Sperber, A.D.; Drossman, D.A. Functional abdominal pain syndrome: constant or frequently recurring abdominal pain. Am. J. Gastroenterol. 2010, 105, 770–774. [Google Scholar]
- Azpiroz, F.; Bouin, M.; Camilleri, M.; Mayer, E.A.; Poitras, P.; Serra, J.; Spiller, R.C. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol. Motil. 2007, 19, 62–88. [Google Scholar]
- Barbara, G.; De Giorgio, R.; Stanghellini, V.; Cremon, C.; Corinaldesi, R. A role for inflammation in irritable bowel syndrome? Gut 2002, 51, i41–i44. [Google Scholar] [PubMed]
- Barbara, G.; Stanghellini, V.; De Giorgio, R.; Cremon, C.; Cottrell, G.; Santini, D.; Pasquinelli, G.; Morselli-Labate, A.; Grady, E.; Bunnett, N.; et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004, 126, 693–702. [Google Scholar] [PubMed]
- Barbara, G.; Wang, B.; Stanghellini, V.; de Giorgio, R.; Cremon, C.; Di Nardo, G.; Trevisani, M.; Campi, B.; Geppetti, P.; Tonini, M.; et al. Mast Cell-Dependent Excitation of Visceral-Nociceptive Sensory Neurons in Irritable Bowel Syndrome. Gastroenterology 2007, 132, 26–37. [Google Scholar] [PubMed]
- Liebregts, T.; Adam, B.; Bredack, C.; Roth, A.; Heinzel, S.; Lester, S.; Downie-Doyle, S.; Smith, E.; Drew, P.; Talley, N.J.; et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007, 132, 913–920. [Google Scholar] [PubMed]
- Spiller, R.; Garsed, K. Infection, inflammation, and the irritable bowel syndrome. Dig. Liver Dis. 2009, 41, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.R.; Blackshaw, L.A.; Brookes, S.J.; Grundy, D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol. Motil. 2004, 16 (Suppl. 1), 28–33. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F.; Laird, J.M. Visceral pain. Lancet 1999, 353, 2145–2148. [Google Scholar]
- Brierley, S.M.; Jones, R.C., 3rd; Gebhart, G.F.; Blackshaw, L.A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 2004, 127, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Lynn, P.A.; Blackshaw, L.A. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J. Physiol. 1999, 518 (Pt. 1), 271–282. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Blackshaw, L.A. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J. Physiol. 1998, 512 (Pt. 3), 907–916. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Martin, C.M.; Blackshaw, L.A. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J. Neurophysiol. 2002, 87, 2095–2103. [Google Scholar]
- Brierley, S.M.; Hughes, P.A.; Page, A.J.; Kwan, K.Y.; Martin, C.M.; O'Donnell, T.A.; Cooper, N.J.; Harrington, A.M.; Adam, B.; Liebregts, T.; et al. The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology 2009, 137, 2084–2095, e3.. [Google Scholar] [PubMed]
- Brierley, S.M.; Page, A.J.; Hughes, P.A.; Adam, B.; Liebregts, T.; Cooper, N.J.; Holtmann, G.; Liedtke, W.; Blackshaw, L.A. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology 2008, 134, 2059–2069. [Google Scholar]
- Hughes, P.A.; Brierley, S.M.; Martin, C.M.; Brookes, S.J.; Linden, D.R.; Blackshaw, L.A. Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut 2009, 58, 1333–1341. [Google Scholar]
- Berthoud, H.R.; Kressel, M.; Raybould, H.E.; Neuhuber, W.L. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat. Embryol. (Berl.) 1995, 191, 203–212. [Google Scholar] [PubMed]
- Berthoud, H.R.; Powley, T.L. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol. 1992, 319, 261–276. [Google Scholar]
- Lynn, P.A.; Olsson, C.; Zagorodnyuk, V.; Costa, M.; Brookes, S.J. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology 2003, 125, 786–794. [Google Scholar]
- Zagorodnyuk, V.P.; Brookes, S.J. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J. Neurosci. 2000, 20, 6249–6255. [Google Scholar]
- Zagorodnyuk, V.P.; Chen, B.N.; Costa, M.; Brookes, S.J. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J. Physiol. 2003, 553, 575–587. [Google Scholar]
- Booth, C.E.; Kirkup, A.J.; Hicks, G.A.; Humphrey, P.P.; Grundy, D. Somatostatin sst (2) receptor-mediated inhibition of mesenteric afferent nerves of the jejunum in the anesthetized rat. Gastroenterology 2001, 121, 358–369. [Google Scholar]
- Sengupta, J.N.; Saha, J.K.; Goyal, R.K. Stimulus-response function studies of esophageal mechanosensitive nociceptors in sympathetic afferents of opossum. J. Neurophysiol. 1990, 64, 796–812. [Google Scholar]
- Blumberg, H.; Haupt, P.; Janig, W.; Kohler, W. Encoding of visceral noxious stimuli in the discharge patterns of visceral afferent fibres from the colon. Pflug. Arch. Eur. J. Physiol. 1983, 398, 33–40. [Google Scholar]
- Morrison, J.F.B. Splanchnic slowly adapting mechanoreceptors with punctate receptove fields in the mesentery and the gastrointestinal tract of the cat. J. Physiol. 1973, 233, 349–361. [Google Scholar]
- Song, X.; Chen, B.N.; Zagorodnyuk, V.P.; Lynn, P.A.; Blackshaw, L.A.; Grundy, D.; Brunsden, A.M.; Costa, M.; Brookes, S.J. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 2009, 137, 274–284, 284 e1.. [Google Scholar] [PubMed]
- Brierley, S.M.; Jones, R.C.W., III; Xu, L.; Gebhart, G.F.; Blackshaw, L.A. Activation of splanchnic and pelvic colonic afferents by bradykinin in mice. Neurogastroenterol. Motil. 2005, 17, 854–862. [Google Scholar]
- Brierley, S.M.; Jones, R.C.W., III; Xu, L.; Robinson, D.R.; Hicks, G.A.; Gebhart, G.F.; Blackshaw, L.A. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J. Physiol. 2005, 567, 267–281. [Google Scholar]
- Lewin, G.R.; Moshourab, R. Mechanosensation and Pain. J. Neurobiol. 2004, 61, 30–44. [Google Scholar]
- Bielefeldt, K.; Davis, B.M. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G130–G138. [Google Scholar]
- Jones, R.C., 3rd; Xu, L.; Gebhart, G.F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J. Neurosci. 2005, 25, 10981–10989. [Google Scholar] [PubMed]
- Malin, S.A.; Christianson, J.A.; Bielefeldt, K.; Davis, B.M. TPRV1 Expression Defines Functionally Distinct Pelvic Colon Afferents. J. Neurosci. 2009, 29, 743–752. [Google Scholar]
- Yu, S.; Undem, B.J.; Kollarik, M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J. Physiol. 2005, 563, 831–842. [Google Scholar]
- Gebhart, G.F. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G834–G838. [Google Scholar] [PubMed]
- Fioramonti, J.; Gebhart, G.F. In vivo and transgenic animal models used to study visceral hypersensitivity. Neurogastroenterol. Motil. 2007, 19, 20–28. [Google Scholar] [CrossRef]
- De Schepper, H.U.; De Winter, B.Y.; Van Nassauw, L.; Timmermans, J.P.; Herman, A.G.; Pelckmans, P.A.; De Man, J.G. TRPV1 receptors on unmyelinated C-fibres mediate colitis-induced sensitization of pelvic afferent nerve fibres in rats. J. Physiol. 2008, 586, 5247–5258. [Google Scholar]
- Lynn, P.A.; Chen, B.N.; Zagorodnyuk, V.P.; Costa, M.; Brookes, S.J. TNBS-induced inflammation modulates the function of one class of low-threshold rectal mechanoreceptors in the guinea pig. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G862–G871. [Google Scholar]
- Sengupta, J.N.; Snider, A.; Su, X.; Gebhart, G.F. Effects of kappa opioids in the inflamed rat colon. Pain 1999, 79, 175–185. [Google Scholar]
- Caterina, M.J. Vanilloid receptors take a TRP beyond the sensory afferent. Pain 2003, 105, 5–9. [Google Scholar]
- Caterina, M.J.; Julius, D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar]
- Akiba, Y.; Mizumori, M.; Kuo, M.; Ham, M.; Guth, P.H.; Engel, E.; Kaunitz, J.D. CO2 chemosensing in rat oesophagus. Gut 2008, 57, 1654–1664. [Google Scholar]
- Blackshaw, L.A.; Page, A.J.; Partosoedarso, E.R. Acute effects of capsaicin on gastrointestinal vagal afferents. Neuroscience 2000, 96, 407–416. [Google Scholar]
- Brierley, S.M.; Carter, R.; Jones, W., 3rd; Xu, L.; Robinson, D.R.; Hicks, G.A.; Gebhart, G.F.; Blackshaw, L.A. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. J. Physiol. 2005, 567, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Spencer, N.J.; Kerrin, A.; Singer, C.A.; Hennig, G.W.; Gerthoffer, W.T.; McDonnell, O. Identification of capsaicin-sensitive rectal mechanoreceptors activated by rectal distension in mice. Neuroscience 2008, 153, 518–534. [Google Scholar]
- Christianson, J.A.; McIlwrath, S.L.; Koerber, H.R.; Davis, B.M. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience 2006, 140, 247–257. [Google Scholar]
- Robinson, D.R.; McNaughton, P.A.; Evans, M.L.; Hicks, G.A. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol. Motil. 2004, 16, 113–124. [Google Scholar]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar]
- Rong, W.; Hillsley, K.; Davis, J.B.; Hicks, G.; Winchester, W.J.; Grundy, D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J. Physiol. 2004, 560, 867–881. [Google Scholar]
- Kindt, S.; Vos, R.; Blondeau, K.; Tack, J. Influence of intra-oesophageal capsaicin instillation on heartburn induction and oesophageal sensitivity in man. Neurogastroenterol. Motil. 2009, 21, 1032–1082. [Google Scholar]
- Laird, J.; Martinez-Caro, L.; Garcia-Nicas, E.; Cervero, F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001, 92, 335–342. [Google Scholar]
- Laird, J.M.; Souslova, V.; Wood, J.N.; Cervero, F. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. J. Neurosci. 2002, 22, 8352–8356. [Google Scholar] [PubMed]
- Levine, J.D.; Alessandri-Haber, N. TRP channels: targets for the relief of pain. Biochim. Biophys. Acta. 2007, 1772, 989–1003. [Google Scholar] [PubMed]
- Sugiura, T.; Bielefeldt, K.; Gebhart, G.F. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J. Neurosci. 2004, 24, 9521–9530. [Google Scholar]
- Amadesi, S.; Cottrell, G.S.; Divino, L.; Chapman, K.; Grady, E.F.; Bautista, F.; Karanjia, R.; Barajas-Lopez, C.; Vanner, S.; Vergnolle, N.; et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J. Physiol. 2006, 575, 555–571. [Google Scholar] [PubMed]
- Amadesi, S.; Nie, J.; Vergnolle, N.; Cottrell, G.S.; Grady, E.F.; Trevisani, M.; Manni, C.; Geppetti, P.; McRoberts, J.A.; Ennes, H.; et al. Protease-Activated Receptor 2 Sensitizes the Capsaicin Receptor Transient Receptor Potential Vanilloid Receptor 1 to Induce Hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [PubMed]
- Fujino, K.; de la Fuente, S.G.; Takami, Y.; Takahashi, T.; Mantyh, C.R. Attenuation of acid induced oesophagitis in VR-1 deficient mice. Gut 2006, 55, 34–40. [Google Scholar]
- Fujino, K.; Takami, Y.; de la Fuente, S.G.; Ludwig, K.A.; Mantyh, C.R. Inhibition of the vanilloid receptor subtype-1 attenuates TNBS-colitis. J. Gastrointest. Surg. 2004, 8, 842–847. [Google Scholar]
- Miranda, A.; Nordstrom, E.; Mannem, A.; Smith, C.; Banerjee, B.; Sengupta, J.N. The role of transient receptor potential vanilloid 1 in mechanical and chemical visceral hyperalgesia following experimental colitis. Neuroscience 2007, 148, 1021–1032. [Google Scholar]
- Kihara, N.; de la Fuente, S.G.; Fujino, K.; Takahashi, T.; Pappas, T.N.; Mantyh, C.R. Vanilloid receptor-1 containing primary sensory neurones mediate dextran sulphate sodium induced colitis in rats. Gut 2003, 52, 713–719. [Google Scholar]
- Kimball, E.S.; Wallace, N.H.; Schneider, C.R.; D'Andrea, M.R.; Hornby, P.J. Vanilloid receptor 1 antagonists attenuate disease severity in dextran sulphate sodium-induced colitis in mice. Neurogastroenterol. Motil. 2004, 16, 811–818. [Google Scholar]
- Martelli, L.; Ragazzi, E.; di Mario, F.; Martelli, M.; Castagliuolo, I.; Dal Maschio, M.; Palu, G.; Maschietto, M.; Scorzeto, M.; Vassanelli, S.; et al. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol. Motil. 2007, 19, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Sibaev, A.; Marsicano, G.; Blaudzun, H.; Storr, M.; Lutz, B. Vanilloid receptor (TRPV1)-deficient mice show increased susceptibility to dinitrobenzene sulfonic acid induced colitis. J. Mol. Med. 2006, 84, 142–146. [Google Scholar]
- Hong, S.; Fan, J.; Kemmerer, E.S.; Evans, S.; Li, Y.; Wiley, J.W. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut 2009, 58, 202–210. [Google Scholar]
- Jones, R.C., 3rd; Otsuka, E.; Wagstrom, E.; Jensen, C.S.; Price, M.P.; Gebhart, G.F. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 2007, 133, 184–194. [Google Scholar] [PubMed]
- Winston, J.; Shenoy, M.; Medley, D.; Naniwadekar, A.; Pasricha, P.J. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology 2007, 132, 615–627. [Google Scholar]
- De Schepper, H.U.; De Man, J.G.; Ruyssers, N.E.; Deiteren, A.; Van Nassauw, L.; Timmermans, J.P.; Martinet, W.; Herman, A.G.; Pelckmans, P.A.; De Winter, B.Y. TRPV1 receptor signaling mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Am. J. Physiol. GI. Liver Physiol. 2008, 294, G245–G253. [Google Scholar]
- Yang, J.; Li, Y.; Zuo, X.; Zhen, Y.; Yu, Y.; Gao, L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neurosci. Lett. 2008, 440, 237–241. [Google Scholar]
- Adam, B.; Liebregts, T.; Gschossmann, J.M.; Krippner, C.; Scholl, F.; Ruwe, M.; Holtmann, G. Severity of mucosal inflammation as a predictor for alterations of visceral sensory function in a rat model. Pain 2006, 123, 179–186. [Google Scholar]
- Menozzi, A.; Pozzoli, C.; Poli, E.; Lazzaretti, M.; Grandi, D.; Coruzzi, G. Long-term study of TNBS-induced colitis in rats: focus on mast cells. Inflamm. Res. 2006, 55, 416–422. [Google Scholar]
- Matthews, P.J.; Aziz, Q.; Facer, P.; Davis, J.B.; Thompson, D.G.; Anand, P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur. J. Gastroenterol. Hepatol. 2004, 16, 897–902. [Google Scholar]
- Bhat, Y.M.; Bielefeldt, K. Capsaicin receptor (TRPV1) and non-erosive reflux disease. Eur. J. Gastroenterol. Hepatol. 2006, 18, 263–270. [Google Scholar]
- Guarino, M.P.; Cheng, L.; Ma, J.; Harnett, K.; Biancani, P.; Altomare, A.; Panzera, F.; Behar, J.; Cicala, M. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol. Motil. 2010, 22, 746–751. [Google Scholar]
- Hammer, J.; Fuhrer, M.; Pipal, L.; Matiasek, J. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterol. Motil. 2008, 20, 125–133. [Google Scholar]
- Fuhrer, M.; Hammer, J. Effect of repeated, long term capsaicin ingestion on intestinal chemo- and mechanosensation in healthy volunteers. Neurogastroenterol. Motil. 2009, 21, 521–527, e7.. [Google Scholar] [CrossRef] [PubMed]
- Tahara, T.; Shibata, T.; Nakamura, M.; Yamashita, H.; Yoshioka, D.; Hirata, I.; Arisawa, T. Homozygous TRPV1 315C influences the susceptibility to functional dyspepsia. J. Clin. Gastroenterol. 2010, 44, e1–e7. [Google Scholar]
- Yiangou, Y.; Facer, P.; Dyer, N.H.; Chan, C.L.; Knowles, C.; Williams, N.S.; Anand, P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet 2001, 357, 1338–1339. [Google Scholar]
- Akbar, A.; Yiangou, Y.; Facer, P.; Walters, J.R.; Anand, P.; Ghosh, S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008, 57, 923–929. [Google Scholar]
- Chan, C.L.; Facer, P.; Davis, J.B.; Smith, G.D.; Egerton, J.; Bountra, C.; Williams, N.S.; Anand, P. Sensory fibres expressing capsaicin receptor TRPV1 in patients with rectal hypersensitivity and faecal urgency. Lancet 2003, 361, 385–391. [Google Scholar]
- Holzer, P. TRPV1: a new target for treatment of visceral pain in IBS? Gut 2008, 57, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar]
- Garami, A.; Shimansky, Y.P.; Pakai, E.; Oliveira, D.L.; Gavva, N.R.; Romanovsky, A.A. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J. Neurosci. 2010, 30, 1435–1440. [Google Scholar]
- Dent, J. Pathogenesis of gastro-oesophageal reflux disease and novel options for its therapy. Neurogastroenterol. Motil. 2008, 20 (Suppl. 1), 91–102. [Google Scholar] [CrossRef] [PubMed]
- Dent, J. Landmarks in the understanding and treatment of reflux disease. J. Gastroenterol. Hepatol. 2009, 24 (Suppl. 3), S5–S14. [Google Scholar] [CrossRef] [PubMed]
- Dent, J.; El-Serag, H.B.; Wallander, M.A.; Johansson, S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 2005, 54, 710–717. [Google Scholar]
- Corey, D.P.; Garcia-Anoveros, J.; Holt, J.R.; Kwan, K.Y.; Lin, S.-Y.; Vollrath, M.A.; Amalfitano, A.; Cheung, E.L.-M.; Derfler, B.H.; Duggan, A.; et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 2004, 432, 723–730. [Google Scholar] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar]
- Cattaruzza, F.; Spreadbury, I.; Miranda-Morales, M.; Grady, E.F.; Vanner, S.J.; Bunnett, N.W. Transient Receptor Potential Ankyrin-1 has a Major Role in Mediating Visceral Pain in Mice. Am. J. Physiol. GI. Liver Physiol. 2009, 298, G81–G91. [Google Scholar]
- Laird, J.M.; Olivar, T.; Roza, C.; De_Felipe, C.; Hunt, S.P.; Cervero, F. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience 2000, 98, 345–352. [Google Scholar]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andre, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar]
- Kimball, E.S.; Prouty, S.P.; Pavlick, K.P.; Wallace, N.H.; Schneider, C.R.; Hornby, P.J. Stimulation of neuronal receptors, neuropeptides and cytokines during experimental oil of mustard colitis. Neurogastroenterol. Motil. 2007, 19, 390–400. [Google Scholar]
- Yu, S.; Ouyang, A. TRPA1 in bradykinin-induced mechanical hypersensitivity of vagal C fibers in guinea pig esophagus. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G255–G265. [Google Scholar]
- Page, A.J.; Brierley, S.M.; Martin, C.M.; Price, M.P.; Symonds, E.; Butler, R.; Wemmie, J.A.; Blackshaw, L.A. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 2005, 54, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Doihara, H.; Nozawa, K.; Kojima, R.; Kawabata-Shoda, E.; Yokoyama, T.; Ito, H. QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol. Cell. Biochem. 2009, 331, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Kawabata-Shoda, E.; Doihara, H.; Kojima, R.; Okada, H.; Mochizuki, S.; Sano, Y.; Inamura, K.; Matsushime, H.; Koizumi, T.; et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl. Acad. Sci. USA 2009, 106, 3408–3413. [Google Scholar]
- Purhonen, A.K.; Louhivuori, L.M.; Kiehne, K.; Kerman, K.E.; Herzig, K.H. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008, 582, 229–232. [Google Scholar]
- Kwan, K.Y.; Allchorne, A.J.; Vollrath, M.A.; Christensen, A.P.; Zhang, D.-S.; Woolf, C.J.; Corey, D.P. TRPA1 Contributes to Cold, Mechanical, and Chemical Nociception but Is Not Essential for Hair-Cell Transduction. Neuron 2006, 50, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Prober, D.A.; Zimmerman, S.; Myers, B.R.; McDermott, B.M., Jr.; Kim, S.-H.; Caron, S.; Rihel, J.; Solnica-Krezel, L.; Julius, D.; Hudspeth, A.J.; et al. Zebrafish TRPA1 Channels Are Required for Chemosensation But Not for Thermosensation or Mechanosensory Hair Cell Function. J. Neurosci. 2008, 28, 10102–10110. [Google Scholar] [PubMed]
- Kerstein, P.C.; del Camino, D.; Moran, M.M.; Stucky, C.L. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol. Pain 2009, 5, 19. [Google Scholar]
- Kwan, K.Y.; Glazer, J.M.; Corey, D.P.; Rice, F.L.; Stucky, C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009, 29, 4808–4819. [Google Scholar]
- Kremeyer, B.; Lopera, F.; Cox, J.; Momin, A.; Rugiero, F.; Marsh, S.; Woods, C.; Jones, N.; Paterson, K.; Fricker, F.; et al. A Gain-of-Function Mutation in TRPA1 Causes Familial Episodic Pain Syndrome. Neuron 2010, 66, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Sipe, W.E.B.; Brierley, S.M.; Martin, C.M.; Phillis, B.D.; Cruz, F.B.; Grady, E.F.; Liedtke, W.; Cohen, D.M.; Vanner, S.; Blackshaw, L.A.; et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1288–G1298. [Google Scholar] [PubMed]
- Cenac, N.; Altier, C.; Chapman, K.; Liedtke, W.; Zamponi, G.; Vergnolle, N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology 2008, 135, 937–946, 946 e1-e2.. [Google Scholar] [CrossRef] [PubMed]
- Cenac, N.; Altier, C.; Motta, J.P.; d'Aldebert, E.; Galeano, S.; Zamponi, G.W.; Vergnolle, N. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut 2010, 59, 481–488. [Google Scholar]
- Karanjia, R.; Spreadbury, I.; Bautista-Cruz, F.; Tsang, M.E.; Vanner, S. Activation of protease-activated receptor-4 inhibits the intrinsic excitability of colonic dorsal root ganglia neurons. Neurogastroenterol. Motil. 2009, 21, 1218–1221. [Google Scholar]
- Auge, C.; Balz-Hara, D.; Steinhoff, M.; Vergnolle, N.; Cenac, N. Protease-activated receptor-4 (PAR 4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol. Motil. 2009, 21, 1189–1107. [Google Scholar]
- Bueno, L. Protease activated receptor 2: a new target for IBS treatment. Eur. Rev. Med. Pharmacol. Sci. 2008, 12 (Suppl. 1), 95–102. [Google Scholar] [PubMed]
- Cenac, N.; Andrews, C.N.; Holzhausen, M.; Chapman, K.; Cottrell, G.; Andrade-Gordon, P.; Steinhoff, M.; Barbara, G.; Beck, P.; Bunnett, N.W.; et al. Role for protease activity in visceral pain in irritable bowel syndrome. J. Clin. Invest. 2007, 117, 636–647. [Google Scholar] [PubMed]
- Faure, C.; Patey, N.; Gauthier, C.; Brooks, E.M.; Mawe, G.M. Serotonin Signaling Is Altered in Irritable Bowel Syndrome With Diarrhea But Not in Functional Dyspepsia in Pediatric Age Patients. Gastroenterology 2010, 139, 249–258. [Google Scholar]
- Hillsley, K.; Lin, J.H.; Stanisz, A.; Grundy, D.; Aerssens, J.; Peeters, P.J.; Moechars, D.; Coulie, B.; Stead, R.H. Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice. J. Physiol. 2006, 576, 257–267. [Google Scholar] [PubMed]
- Ibeakanma, C.; Miranda-Morales, M.; Richards, M.; Bautista-Cruz, F.; Martin, N.; Hurlbut, D.; Vanner, S. Citrobacter rodentium colitis evokes post-infectious hyperexcitability of mouse nociceptive colonic dorsal root ganglion neurons. J. Physiol. 2009, 587, 3505–3521. [Google Scholar]
- Liedtke, W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J. Physiol. 2005, 567, 53–58. [Google Scholar]
- Liedtke, W. TRPV4 as osmosensor: a transgenic approach. Pflugers Archiv. European. Journal of Physiology 2005, 451, 176–180. [Google Scholar]
- Liedtke, W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann. N.Y. Acad. Sci. 2008, 1144, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Yeh, J.J.; Parada, C.A.; Reichling, D.B.; Levine, J.D. Transient Receptor Potential Vanilloid 4 Is Essential in Chemotherapy-Induced Neuropathic Pain in the Rat. J. Neurosci. 2004, 24, 4444–4452. [Google Scholar]
- Alessandri-Haber, N.; Joseph, E.; Dina, O.A.; Liedtke, W.; Levine, J.D. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 2005, 118, 70–79. [Google Scholar]
- Alessandri-Haber, N.; Yeh, J.J.; Boyd, A.E.; Parada, C.A.; Chen, X.; Reichling, D.B.; Levine, J.D. Hypotonicity Induces TRPV4-Mediated Nociception in Rat. Neuron 2003, 39, 497–511. [Google Scholar]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578, 715–733. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired Pressure Sensation in Mice Lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar]
- Hughes, P.A.; Brierley, S.M.; Young, R.L.; Blackshaw, L.A. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J. Comp. Neurol. 2007, 500, 863–875. [Google Scholar] [PubMed]
- Page, A.J.; Brierley, S.M.; Martin, C.M.; Hughes, P.A.; Blackshaw, L.A. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain 2007, 133, 150–160. [Google Scholar]
- Page, A.J.; Brierley, S.M.; Martin, C.M.; Martinez-Salgado, C.; Wemmie, J.A.; Brennan, T.J.; Symonds, E.; Omari, T.; Lewin, G.R.; Welsh, M.J.; et al. The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology 2004, 127, 1739–1747. [Google Scholar] [PubMed]
- Wultsch, T.; Painsipp, E.; Shahbazian, A.; Mitrovic, M.; Edelsbrunner, M.; Lazdunski, M.; Waldmann, R.; Holzer, P. Deletion of the acid-sensing ion channel ASIC3 prevents gastritis-induced acid hyperresponsiveness of the stomach-brainstem axis. Pain 2008, 134, 245–253. [Google Scholar]
- Benson, C.J.; Xie, J.; Wemmie, J.A.; Price, M.P.; Henss, J.M.; Welsh, M.J.; Snyder, P.M. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 2338–2343. [Google Scholar]
- Welsh, M.J.; Price, M.P.; Xie, J. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J. Biol. Chem. 2002, 277, 2369–2372. [Google Scholar]
- Yiangou, Y.; Facer, P.; Smith, J.A.; Sangameswaran, L.; Eglen, R.; Birch, R.; Knowles, C.; Williams, N.; Anand, P. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. Eur. J. Gastroenterol. Hepatol. 2001, 13, 891–896. [Google Scholar]
- Diochot, S.; Salinas, M.; Baron, A.; Escoubas, P.; Lazdunski, M. Peptides inhibitors of acid-sensing ion channels. Toxicon 2007, 49, 271–284. [Google Scholar]
- Jensen, J.E.; Durek, T.; Alewood, P.F.; Adams, D.J.; King, G.F.; Rash, L.D. Chemical synthesis and folding of APETx2, a potent and selective inhibitor of acid sensing ion channel 3. Toxicon 2009, 54, 56–61. [Google Scholar]
- Lu, Y.; Ma, X.; Sabharwal, R.; Snitsarev, V.; Morgan, D.; Rahmouni, K.; Drummond, H.A.; Whiteis, C.A.; Costa, V.; Price, M.; et al. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron 2009, 64, 885–897. [Google Scholar] [PubMed]
- Ziemann, A.E.; Allen, J.E.; Dahdaleh, N.S.; Drebot, II; Coryell, M.W.; Wunsch, A.M.; Lynch, C.M.; Faraci, F.M.; Howard, M.A., 3rd; Welsh, M.J.; et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell 2009, 139, 1012–1021. [Google Scholar] [PubMed]
- Cummins, T.R.; Sheets, P.L.; Waxman, S.G. The roles of sodium channels in nociception: Implications for mechanisms of pain. Pain 2007, 131, 243–257. [Google Scholar]
- Dib-Hajj, S.D.; Black, J.A.; Waxman, S.G. Voltage-gated sodium channels: therapeutic targets for pain. Pain Med. 2009, 10, 1260–1269. [Google Scholar]
- Momin, A.; Wood, J.N. Sensory neuron voltage-gated sodium channels as analgesic drug targets. Curr. Opin. Neurobiol. 2008, 18, 383–388. [Google Scholar]
- Waxman, S.G. Sodium channel blockers and axonal protection in neuroinflammatory disease. Brain 2005, 128, 5–6. [Google Scholar]
- King, D.E.; Macleod, R.J.; Vanner, S.J. Trinitrobenzenesulphonic acid colitis alters Na 1.8 channel expression in mouse dorsal root ganglia neurons. Neurogastroenterol. Motil. 2009, 21, 880–e64. [Google Scholar] [CrossRef] [PubMed]
- Beyak, M.J.; Ramji, N.; Krol, K.M.; Kawaja, M.D.; Vanner, S.J. Two TTX-resistant Na+ currents in mouse colonic dorsal root ganglia neurons and their role in colitis-induced hyperexcitability. Am. J. Physiol. GI. Liver Physiol. 2004, 287, G845–G855. [Google Scholar]
- Wulff, H.; Castle, N.A.; Pardo, L.A. Voltage-gated potassium channels as therapeutic targets. Nat. Rev. Drug Discov. 2009, 8, 982–1001. [Google Scholar]
- Dang, K.; Bielefeldt, K.; Gebhart, G.F. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G573–G579. [Google Scholar]
- Gebhart, G.F.; Bielefeldt, K.; Ozaki, N. Gastric hyperalgesia and changes in voltage gated sodium channel function in the rat. Gut 2002, 51 (Suppl. 1), i15–i18. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt, K.; Ozaki, N.; Gebhart, G.F. Mild gastritis alters voltage-sensitive sodium currents in gastric sensory neurons in rats. Gastroenterology 2002, 122, 752–761. [Google Scholar]
- Bielefeldt, K.; Ozaki, N.; Gebhart, G.F. Experimental ulcers alter voltage-sensitive sodium currents in rat gastric sensory neurons. Gastroenterology 2002, 122, 394–405. [Google Scholar]
- Moore, B.A.; Stewart, T.M.; Hill, C.; Vanner, S.J. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G1045–G1051. [Google Scholar]
- Stewart, T.; Beyak, M.J.; Vanner, S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J. Physiol. 2003, 552, 797–807. [Google Scholar]
- Keating, C.; Beyak, M.; Foley, S.; Singh, G.; Marsden, C.; Spiller, R.; Grundy, D. Afferent hypersensitivity in a mouse model of post-inflammatory gut dysfunction: role of altered serotonin metabolism. J. Physiol. 2008, 586, 4517–4530. [Google Scholar]
- Martinez, V.; Melgar, S. Lack of colonic-inflammation-induced acute visceral hypersensitivity to colorectal distension in Na(v)1.9 knockout mice. Eur. J. Pain 2008, 12, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.A.; Brierley, S.M.; Martin, C.M.; Liebregts, T.; Persson, J.; Adam, B.; Holtmann, G.; Blackshaw, L.A. TRPV1-expressing sensory fibres and IBS: links with immune function. Gut 2009, 58, 465–466. [Google Scholar]
- Ibeakanma, C.; Vanner, S. TNFalpha is a key mediator of the pronociceptive effects of mucosal supernatant from human ulcerative colitis on colonic DRG neurons. Gut 2010, 59, 612–621. [Google Scholar]
- Saito, Y.A.; Strege, P.R.; Tester, D.J.; Locke, G.R., 3rd; Talley, N.J.; Bernard, C.E.; Rae, J.L.; Makielski, J.C.; Ackerman, M.J.; Farrugia, G. Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G211–G218. [Google Scholar] [PubMed]
- Fertleman, C.R.; Baker, M.D.; Parker, K.A.; Moffatt, S.; Elmslie, F.V.; Abrahamsen, B.; Ostman, J.; Klugbauer, N.; Wood, J.N.; Gardiner, R.M.; et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron 2006, 52, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Fertleman, C.R.; Ferrie, C.D. What's in a name--familial rectal pain syndrome becomes paroxysmal extreme pain disorder. J. Neurol. Neurosurg. Psychiatry 2006, 77, 1294–1295. [Google Scholar]
- Fertleman, C.R.; Ferrie, C.D.; Aicardi, J.; Bednarek, N.A.; Eeg-Olofsson, O.; Elmslie, F.V.; Griesemer, D.A.; Goutieres, F.; Kirkpatrick, M.; Malmros, I.N.; et al. Paroxysmal extreme pain disorder (previously familial rectal pain syndrome). Neurology 2007, 69, 586–595. [Google Scholar] [PubMed]
- Yiangou, Y.; Facer, P.; Chessell, I.P.; Bountra, C.; Chan, C.; Fertleman, C.; Smith, V.; Anand, P. Voltage-gated ion channel Nav1.7 innervation in patients with idiopathic rectal hypersensitivity and paroxysmal extreme pain disorder (familial rectal pain). Neurosci. Lett. 2007, 427, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Verne, G.N.; Sen, A.; Price, D.D. Intrarectal lidocaine is an effective treatment for abdominal pain associated with diarrhea-predominant irritable bowel syndrome. J. Pain 2005, 6, 493–496. [Google Scholar]
- Akopian, A.N.; Souslova, V.; England, S.; Okuse, K.; Ogata, N.; Ure, J.; Smith, A.; Kerr, B.J.; McMahon, S.B.; Boyce, S.; et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 1999, 2, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.R.; Dib-Hajj, S.D.; Black, J.A.; Akopian, A.N.; Wood, J.N.; Waxman, S.G. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J. Neurosci. 1999, 19, RC43. [Google Scholar]
- Nassar, M.A.; Levato, A.; Stirling, L.C.; Wood, J.N. Neuropathic pain develops normally in mice lacking both Na(v)1.7 and Na(v)1.8. Mol. Pain 2005, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Amaya, F.; Wang, H.; Costigan, M.; Allchorne, A.J.; Hatcher, J.P.; Egerton, J.; Stean, T.; Morisset, V.; Grose, D.; Gunthorpe, M.J.; et al. The voltage-gated sodium channel Na(v)1.9 is an effector of peripheral inflammatory pain hypersensitivity. J. Neurosci. 2006, 26, 12852–12860. [Google Scholar] [PubMed]
- Jarvis, M.F.; Honore, P.; Shieh, C.C.; Chapman, M.; Joshi, S.; Zhang, X.F.; Kort, M.; Carroll, W.; Marron, B.; Atkinson, R.; et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc. Natl. Acad. Sci. USA 2007, 104, 8520–8525. [Google Scholar]
- Nassar, M.A.; Stirling, L.C.; Forlani, G.; Baker, M.D.; Matthews, E.A.; Dickenson, A.H.; Wood, J.N. Nociceptor-specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc. Natl. Acad. Sci. USA 2004, 101, 12706–12711. [Google Scholar]
- Schmalhofer, W.A.; Calhoun, J.; Burrows, R.; Bailey, T.; Kohler, M.G.; Weinglass, A.B.; Kaczorowski, G.J.; Garcia, M.L.; Koltzenburg, M.; Priest, B.T. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol. Pharmacol. 2008, 74, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.P.; MacFarlane, J.; MacDonald, M.L.; Thompson, J.; Dube, M.P.; Mattice, M.; Fraser, R.; Young, C.; Hossain, S.; Pape, T.; et al. Loss-of-function mutations in the Nav1.7 gene underlie congenital indifference to pain in multiple human populations. Clin. Genet. 2007, 71, 311–319. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Brierley, S.M.; Hughes, P.A.; Harrington, A.M.; Rychkov, G.Y.; Blackshaw, L.A. Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain. Pharmaceuticals 2010, 3, 2768-2798. https://doi.org/10.3390/ph3092768
Brierley SM, Hughes PA, Harrington AM, Rychkov GY, Blackshaw LA. Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain. Pharmaceuticals. 2010; 3(9):2768-2798. https://doi.org/10.3390/ph3092768
Chicago/Turabian StyleBrierley, Stuart M., Patrick A. Hughes, Andrea M. Harrington, Grigori Y. Rychkov, and L. Ashley Blackshaw. 2010. "Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain" Pharmaceuticals 3, no. 9: 2768-2798. https://doi.org/10.3390/ph3092768
APA StyleBrierley, S. M., Hughes, P. A., Harrington, A. M., Rychkov, G. Y., & Blackshaw, L. A. (2010). Identifying the Ion Channels Responsible for Signaling Gastro-Intestinal Based Pain. Pharmaceuticals, 3(9), 2768-2798. https://doi.org/10.3390/ph3092768




