3. Antiviral Activity of Cobalt(III) Complexes
The simple Co
3+ ion is unstable in water, but can be stabilized against reduction to Co
2+ by coordination to ligands or chelators. By far the most common ligand type used to stabilize the cobalt(III) ion in aqueous solution is the chelating N,O donor ligand. Surprisingly, cobalt(III) complexes derived from this ligand donor set have found application as antibacterial or antiviral agents. One of the most promising classes of Co(III) complexes containing N, O donor ligands is the CTC series of complexes
1 based on a chelating Schiff base (imd = imidazole; 2-mimd = 2-methylimidazole) (
Figure 1). In 1998, Epstein and coworkers reported that the cobalt complex CTC-96 (
1d) was effective in the treatment of epithelial herpetic keratitis, one of the major causes of blindness in industrial nations [
9]. Studies using the CTC class of drugs were performed using a rabbit eye model infected with Herpes Simplex Virus Type 1 (HSV-1) and all complexes inhibited HSV-1 replication in vitro with as little as 5 μg/mL required for strong antiviral activity.
Figure 1.
Structure of CTC-type cobalt(III) complexes (imd = imidazole, 2-mimd = 2-methylimidazole).
Figure 1.
Structure of CTC-type cobalt(III) complexes (imd = imidazole, 2-mimd = 2-methylimidazole).
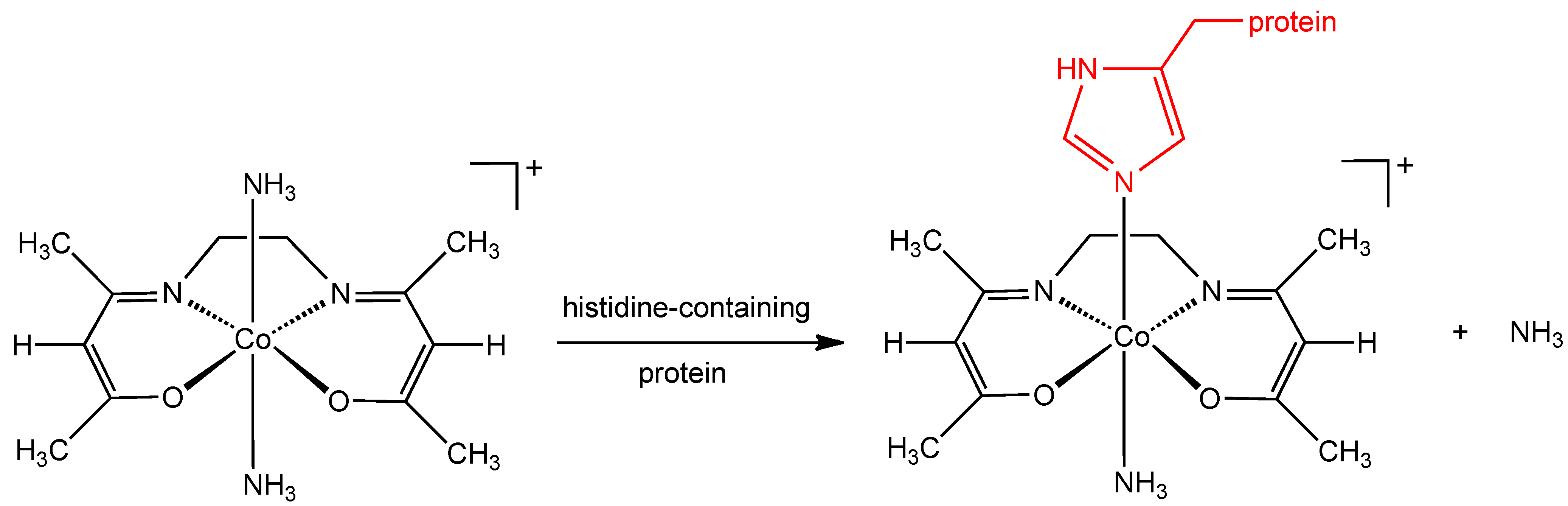
Although the mechanism of action of the CTC class of complexes has not been completely elucidated, it has been suggested that the molecular target is the herpes virus maturational protease, a serine protease containing large amounts of the amino acid, histidine. CTC complexes are known to bind strongly to the histidine molecule and the stability of Co(III) complexes with histidine in the axial position is particularly high (
Figure 2) [
10]. Supporting evidence for the involvement of this axial histidine interaction comes from the observation that a cobalt(III) chelate complex with an imidazole ligand already in the axial position (
1c, CTC-82), is inactive against HSV-1. There is evidence that CTC-96 inhibits membrane fusion events preventing virus entry, CTC-96 inhibited plaque formation by VSV (vesicular stomatitis virus) and VZV (varicella-zoster virus) [
11]. In 2006 Epstein reported the activity of CTC-96 (
1d) against adenovirus in a cell culture model and also against adenovirus keratoconjunctivitis in a rabbit model [
12]. The synthesis of CTC-96 was described by Böttcher
et al. [
13] and the drug formulation was developed and sold as Doxovir
TM; by the Redox Pharmaceutical Corporation [
14]. CTC compounds were also shown to inhibit Sp1, a DNA binding zinc finger protein and this has potential implications for the use of CTC compounds in the treatment of human immunodeficiency virus type 1 (HIV-1) [
15]. The CTC-23 complex (
1a) has superoxide scavenging properties [
16].
Figure 2.
Proposed interaction of histidine containing protein with CTC-complexes.
Figure 2.
Proposed interaction of histidine containing protein with CTC-complexes.
Hexamminecobalt(III) chloride, [Co(NH
3)
6]Cl
3 (
2, "Cohex") is an example of a classical Werner complex. Known for over a century, it is thermally and kinetically stable in aqueous solution and is easily synthesized. Cohex is a commercially available compound in which six ammonia ligands are arranged in an octahedral geometry about the Co(III) ion. In contrast to the CTC series of complexes, in which the axial ligands are readily exchanged for histidine ligand, the ammonia ligands on Cohex are inert towards ligand exchange [
17]. Despite the fact that Cohex lacks the ability to coordinate to, and hydrolyze phosphodiester bonds, it does possess the ability to hydrogen bond with the nitrogenous bases of nucleotides and the phosphate backbone of DNA [
17].
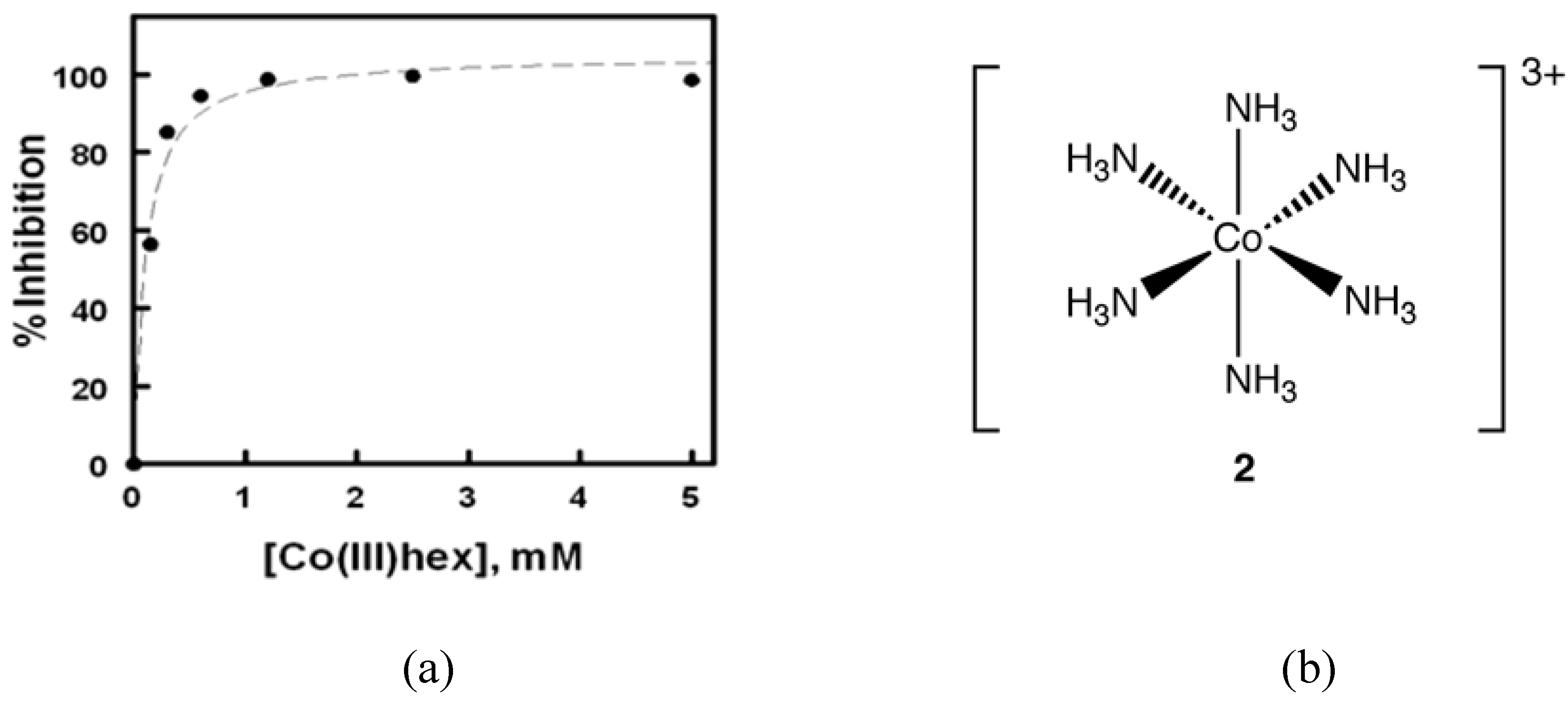
In 2008, Delehanty
et al. reported that Cohex significantly inhibited Sindbis virus replication in baby hamster kidney (BHK) cells in a dose and time-dependent manner (
Figure 3) [
18]. In plaque assays, the incubation of Cohex with Sindbis virus resulted in a dose-dependent decrease in virus replication when measured at both 24 and 48 hours post infection. Over the concentration range of 0 to 5 mM Cohex, the IC
50 for the inhibition of viral replication was determined to be 0.10 ± 0.4 mM at 48 hours. Additionally, when BHK cell monolayers were pretreated with Cohex for 6 hours prior to Sindbis infection, optimal cellular morphology and plasma membrane integrity were observed at 0.6 mM to 1.2 mM Cohex. Analysis by flow cytometry confirmed that Cohex mediated a concomitant dose-dependent increase in BHK cell viability and a decrease in the percentage of Sindbis virus-infected cells (IC
50 = 0.13 ± 0.4 mM). These findings demonstrated for the first time that Cohex possesses potent antiviral activity.
Figure 3.
(a) Degree of inhibition of Sindbis virus plaque formation plotted as a function of Cohex concentration for the 48 h data set. The IC50 for inhibition was determined to be 0.10 ± 0.04 mM Cohex using a one-site dose response logistic curve fit function. (b) Structure of Cohex cation 2 (chloride counterions omitted for clarity).
Figure 3.
(a) Degree of inhibition of Sindbis virus plaque formation plotted as a function of Cohex concentration for the 48 h data set. The IC50 for inhibition was determined to be 0.10 ± 0.04 mM Cohex using a one-site dose response logistic curve fit function. (b) Structure of Cohex cation 2 (chloride counterions omitted for clarity).
Although the detailed mechanism of Cohex activity remains elusive, certain observations as to the Cohex target in replicating viruses were made. Cohex exhibited significant antiviral activity and reduced synthesis of viral structural proteins as evidenced by EGFP reporter assays, and this information suggests that the cobalt complex inhibits viral replication via the inhibition of viral structural protein synthesis or some point further upstream in the viral replication cycle. Just as CTC-96 acts at the point of viral entry, it is possible that the high positive charge density of Cohex allows it to disrupt the interaction of Sindbis virus glycoproteins with highly negatively charged, polysulfonated heparan sulfate receptors. Another possible mechanism involves the ability of Cohex to compete with hydrated Mg(II) and interfere with virus assembly, based on the similar charge density to [Mg(OH
2)]
2+ [
19].
Carboranes are 12-vertex boron/carbon clusters, also known as dicarbollides. In 1965, Hawthorne reported a new class of cluster known as the metal bis(1,2-carbollides), formed when two dicarbollides sandwich a metal ion such as cobalt(III) [
20]. Cigler and Rezacova have described the use of cobalt bis(1,2-carbollides) as antiviral therapeutics, specifically against HIV protease [
21,
22]. Inhibition of proteases may be achieved either chemically, or via mutation, and this is an important step in the interruption of viral maturation. Viral maturation is dependant on the protease mediated viral polypeptide cleavage. In a random testing of compound libraries, Cigler identified the cobalt bis(1,2-carbollides) as HIV protease inhibitors. Antiviral activity was analyzed using PM-1 cells infected with an HIV-1 strain. Cobalt bis(1,2-carbollides)
3–
8 (
Figure 4) were synthesized and screened in tissue cultures.
Figure 4.
Structures of cobalt(III) carboranes used as HIV-1 inhibitors.
Figure 4.
Structures of cobalt(III) carboranes used as HIV-1 inhibitors.
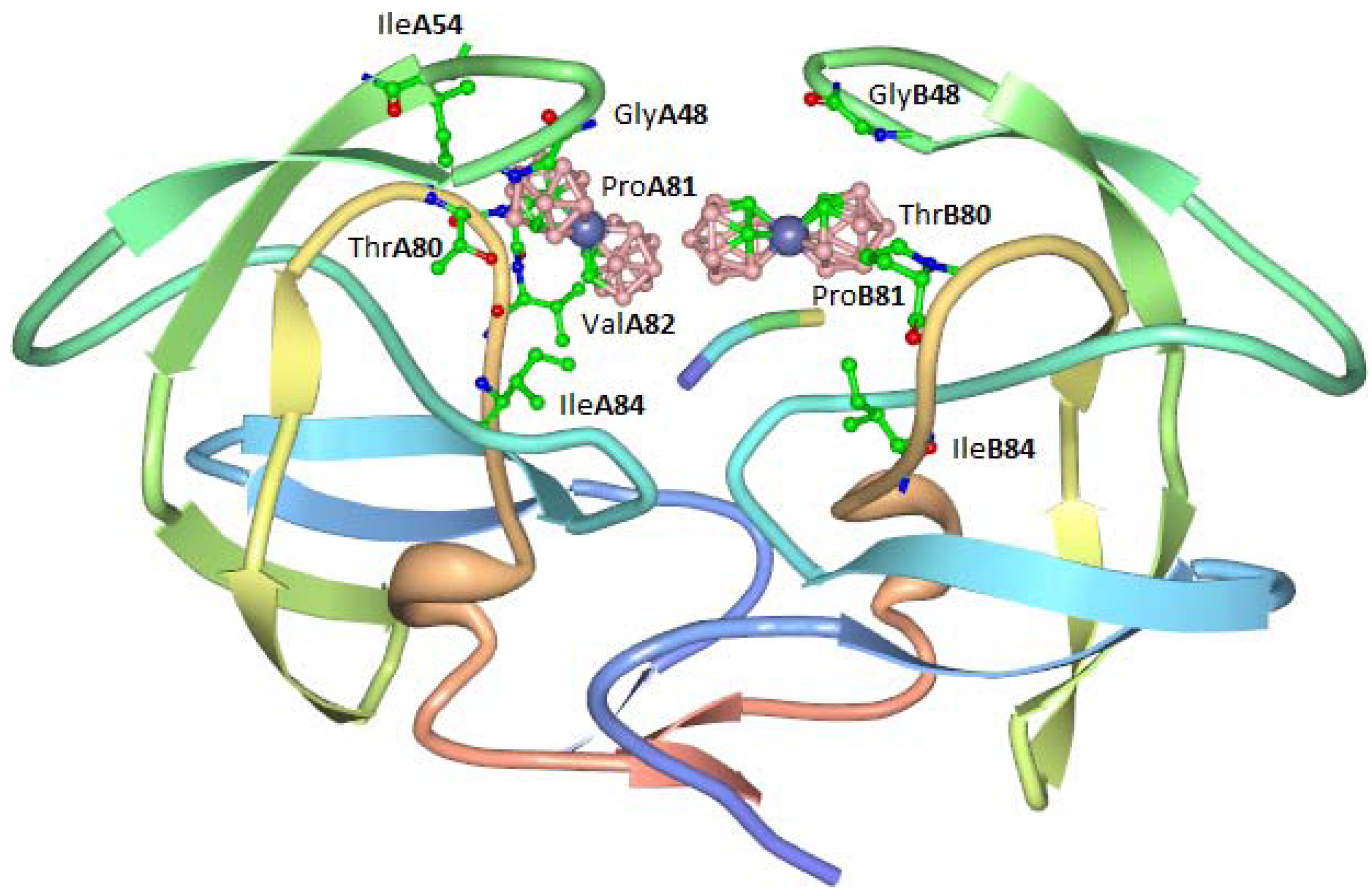
The highest activity was displayed by compound
8, which exhibited an EC
50 for inhibition of HIV-1 in tissue cultures of 250 nM (this is approximately 10× better than that observed for the related cobalt bis(1,2-carbollide)
7. The antiviral activity of cobalt bis(1,2-carbollides) was ascribed to the formation of a stable complex with HIV protease in which the two molecules of cobalt complex bind to a hydrophobic pocket in the flap-proximal region of the S3 and S3' subsites of the enzyme. An X-ray crystal structure of the complex was obtained (
Figure 5). Carboranes conjugated with porphyrins were also shown to inhibit HIV protease.
Figure 5.
Interaction of compound 3 with hydrophobic binding pocket of HIV protease.
Figure 5.
Interaction of compound 3 with hydrophobic binding pocket of HIV protease.
4. Antibacterial Activity of Cobalt(III) Complexes
A large number of reports on the antibacterial properties of cobalt complexes have appeared in the literature, with Co(II) complexes being the most studied, presumably due to their aqueous stability, availability, and ease of synthesis. However a number of examples of stable Co(III) complexes have also been reported. Although polydentate ligands with N, O and S donor atoms in the coordination sphere of cobalt are the most common ligands used to stabilize Co3+ ion in aqueous solution, a striking exception to this rule is the homoleptic hexammine cobalt(III) complex 2 described above which possesses high kinetic inertness in water. Reports on the antibacterial properties of cobalt(III) complexes frequently emphasize the increased effectiveness of cobalt ion coordination to a particular ligand when compared to the free ligand itself.
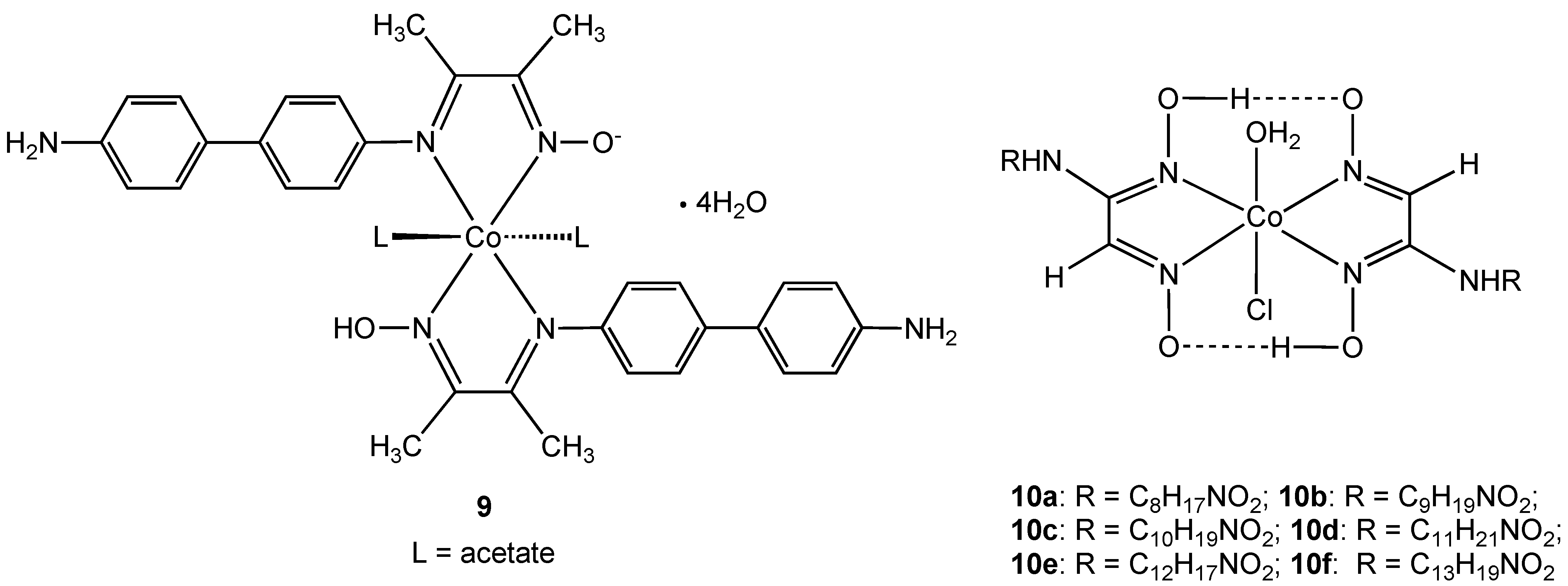
Complex
9, containing a new hybrid amine-imine-oxime ligand derived from the condensation reaction of diacetylmonoxime with benzidine, was shown to be effective against
Bacillius subtilis [
23]. However the same complex showed no activity towards
Staphylococcus aureus or the Gram-negative bacteria
Escherichia coli and
Enterobacter fecalis. Antibacterial activity was measured using simple zone inhibition techniques and activity was found to be less than the control antibiotics tetracycline and kanamycin (using μg of antibiotic per unit volume). Complex
9 is neutral due to the presence of one deprotonated oxime N-OH group and the oxidation state was assigned using magnetic moment measurements and absorption spectra. The related
vic-oxime complexes
10 were described by the same authors [
24]. Complexes
10a–f all showed antibacterial activity towards
Enterobacter aeruginosa,
E. coli, S. aureus, and
B. subtilis niger but were all less effective than the commonly used macrolide antibiotic azithromycin.
Figure 6.
Oxime cobalt(III) complexes 9 and 10.
Figure 6.
Oxime cobalt(III) complexes 9 and 10.
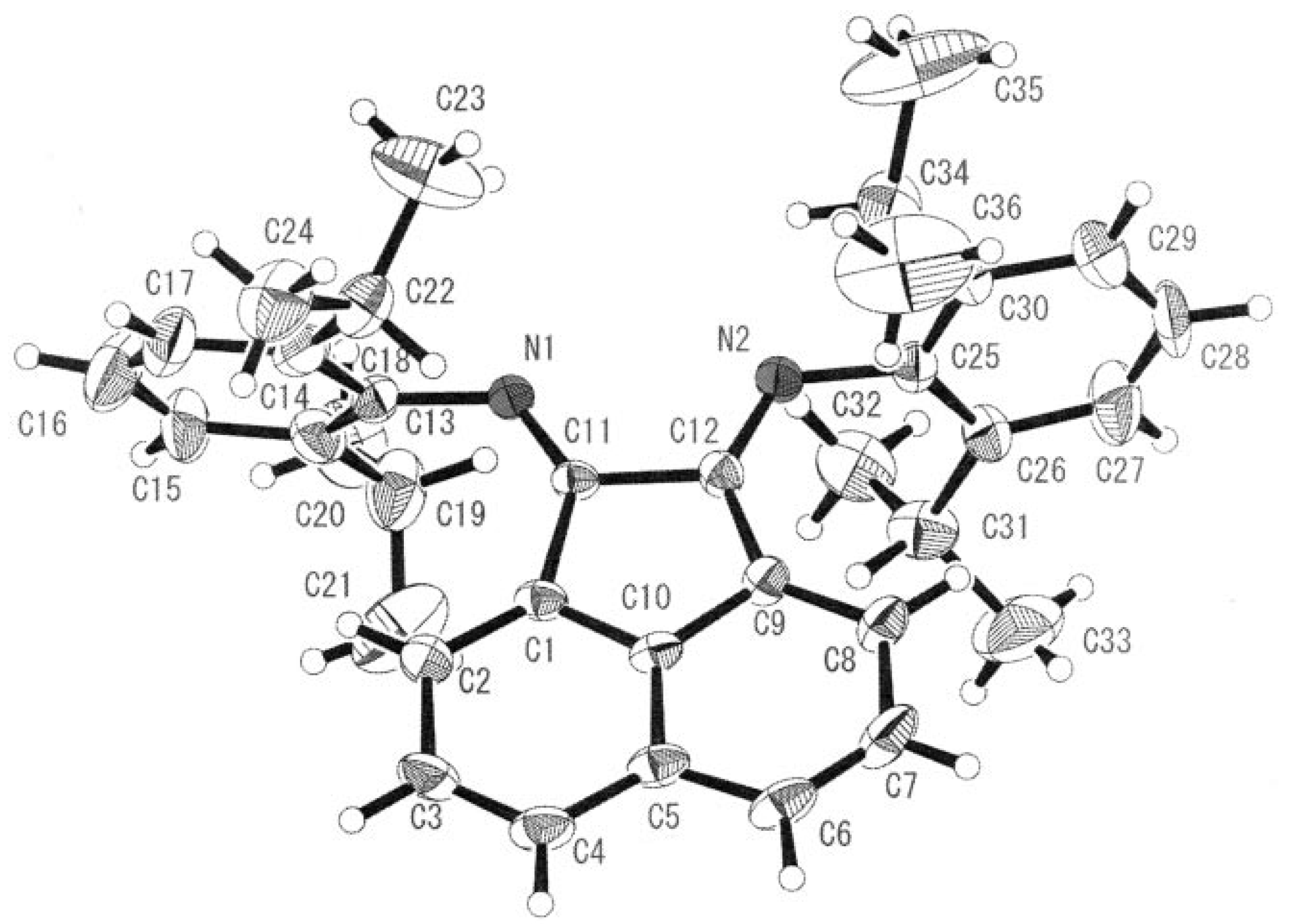
A cobalt(III) complex containing the rigid bidentate nitrogen ligand
bis[
N-(2,6-diisopropyl-phenyl)imino]acenaphthene (Pr-BIAN) demonstrated potent antibacterial activity towards
S. aureus and
E. coli (
Figure 7) [
25]. The authors noted the observed antimicrobial activity was highly dependant on the bulkiness of the
N-bisimine derivatives which increases the relative lipophilicity of the molecule. Chelation of a bulky ligand to a metal cation reduces the polarity of the ion due to ligand orbital overlap with the metal orbitals resulting in a delocalization of positive charge. An increase in lipophilicity of a metal complex enhances bacterial cell membrane penetration and blocking of metal binding sites on enzymes. Coordination of the
N-bisimine ligand to Co(II) instead of Co(III) had no appreciable effect on the potency of the complex supporting the hypothesis that hydrophobic shielding of the metal center by the chelating ligand is responsible for bioactivity.
Figure 7.
ORTEP representation of Pr-BIAN ligand (adapted from Ref. [
25]).
Figure 7.
ORTEP representation of Pr-BIAN ligand (adapted from Ref. [
25]).
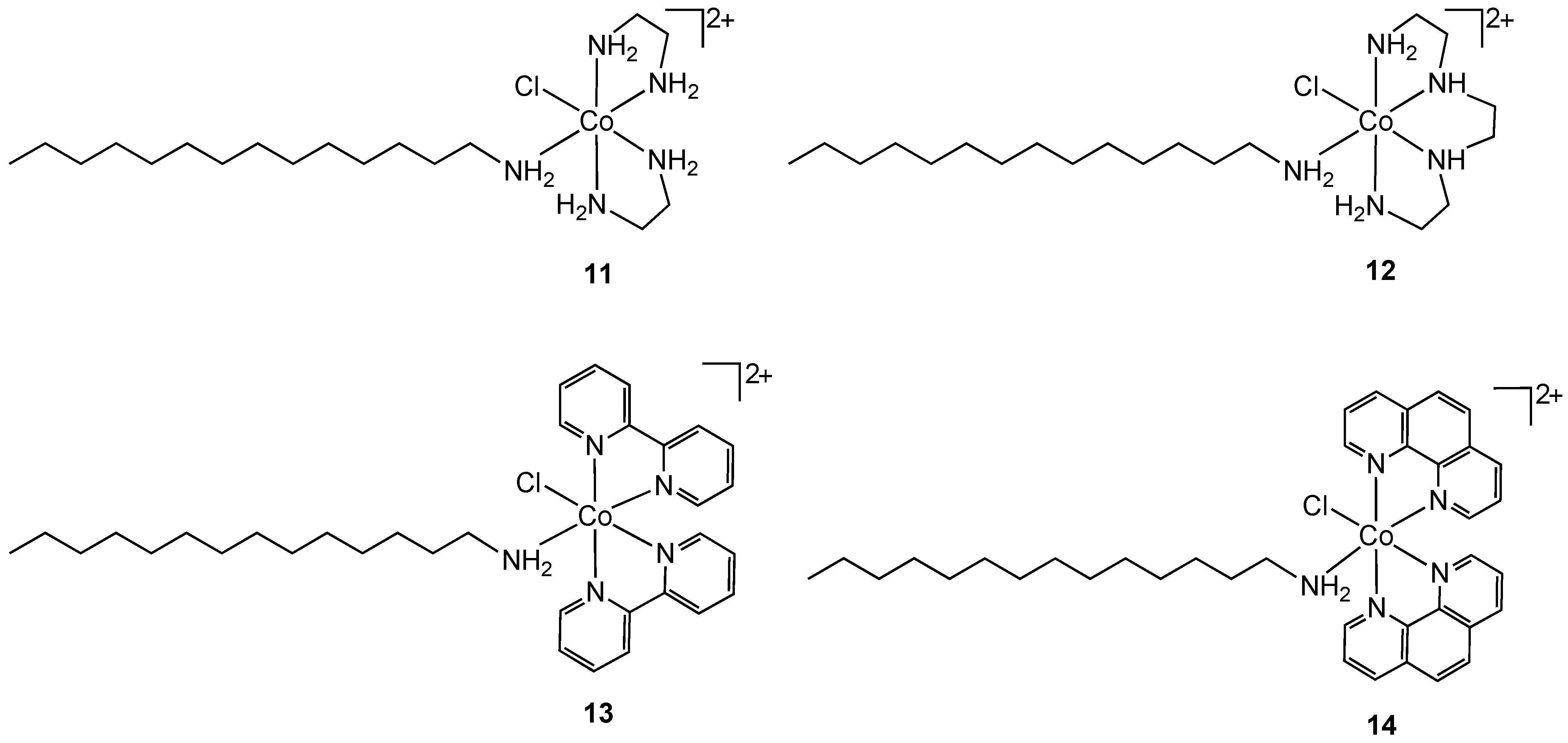
Another approach for improving the lipophilicity of metal complexes can be achieved by functionalization with surfactant-like ligands. Arunachalam reported the synthesis of metallosurfactant complexes of Co(III) containing ethylenediamine (
11), triethylenetetramine (
12), 2,2'-bipyridyl (
13) and 1,10-phenanthroline (
14) nitrogen chelates (
Figure 8) [
26]. The surfactant properties of the complexes were provided by a coordinated C-14 long chain amine.
Figure 8.
Cobalt(III) containing metallosurfactants.
Figure 8.
Cobalt(III) containing metallosurfactants.
Complexes
11–
14 were screened for antibacterial properties and activities compared with the antibiotic ciprofloxacin. All showed considerable activity against the Gram-positive bacteria
S. aureus and
B. subtilis and the Gram-negative bacteria
E. coli and
P. aeruginosa but did not achieve the same effect as ciprofloxacin. The authors did not compare the activities of
11–
14 to non-surfactant containing cobalt(III) analogs so it is difficult to reach conclusions about the mode of action. The hydrophobicity of the complexes can result in increased damage to bacterial cell walls. Complex binding to DNA was also suggested as a possible mode of action [
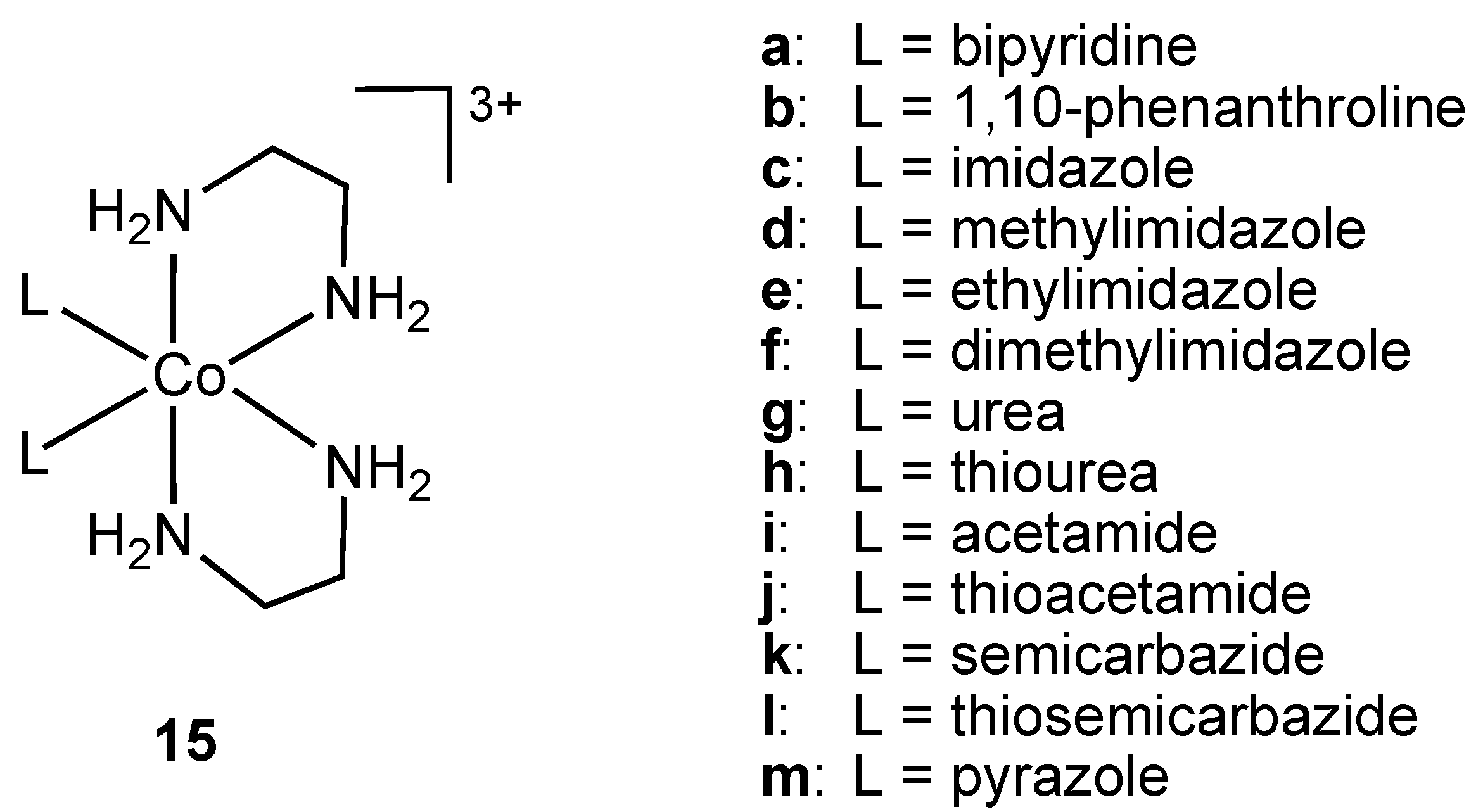
26]. Nagababu and co-workers screened a large number of bis(ethylenediamine)cobalt(III) cations
15 (
Figure 9) against Gram-positive and Gram-negative bacteria (
E. coli,
E. coli HB101,
Salmonella typhimurium,
Proteus vulgaris,
P.
aeruginosa,
S.
aureus,
S.
faecalis,
B.
subtilis) [
27].
Figure 9.
Ethylenediamine cobalt(III) cations 15 (bipyridine and 1,10-phenanthroline ligands are coordinated in bidentate chelating mode to the metal ion).
Figure 9.
Ethylenediamine cobalt(III) cations 15 (bipyridine and 1,10-phenanthroline ligands are coordinated in bidentate chelating mode to the metal ion).
The complexes were synthesized via chloride displacement from
trans-[CoCl
2(en)
2] (en = ethylenediamine) and subsequently characterized using visible absorption, IR and
1H-NMR spectroscopies. Antibacterial activities of
15 were determined using zone inhibition, minimum bactericidal concentration (MBC) and time period of lethal action. Cations
15a and
15b containing bidentate, chelating ligands were shown to possess the highest potency against
E. coli. The 1,10-phenanthroline containing cation
15b had higher activity than the antibiotics ampicillin, streptomycin, gentamicin, chloramphenicol and ciprofloxacin [
27].
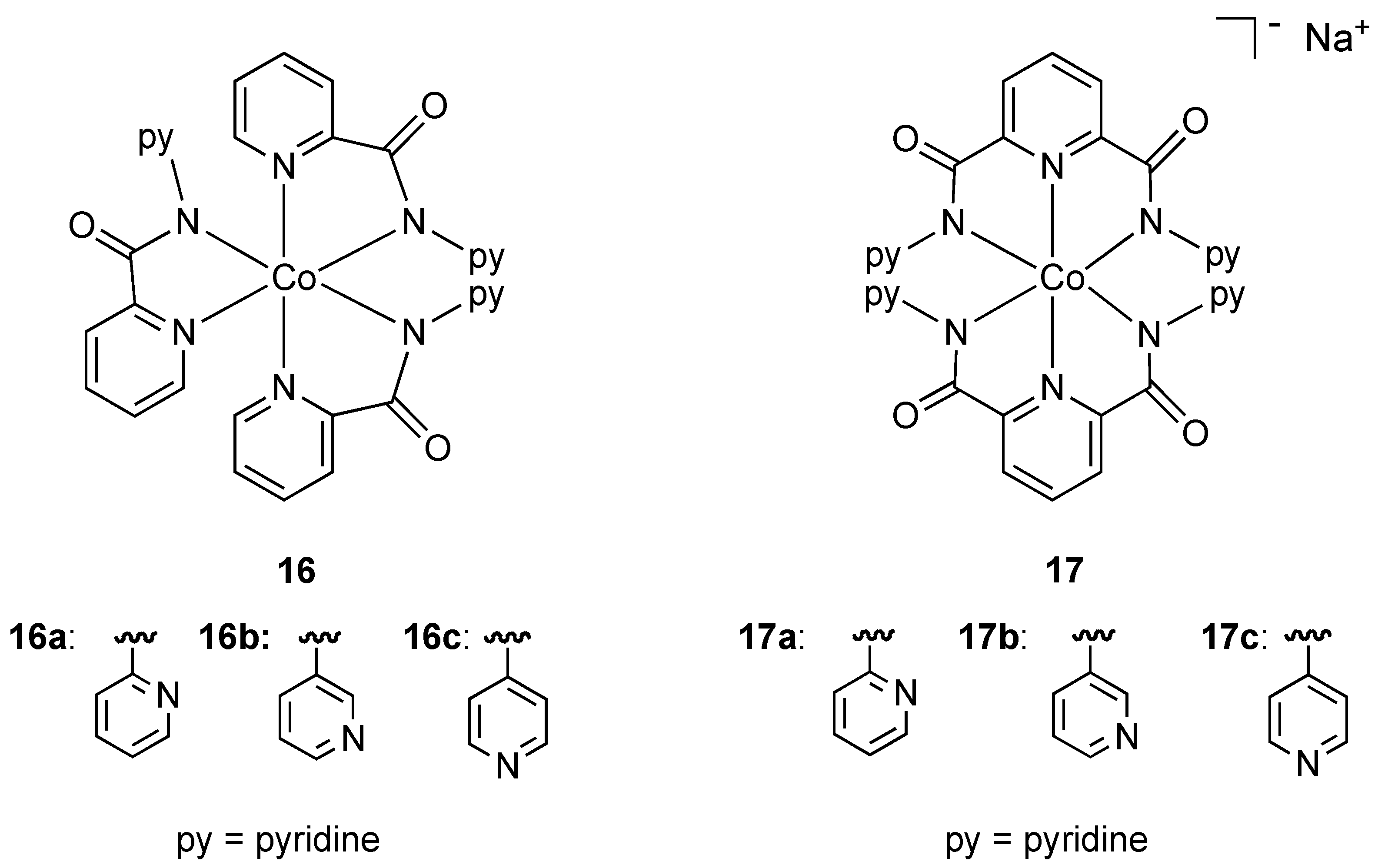
In an effort to increase the hydrophobic ligand shell around octahedral cobalt(III) and consequently improve membrane diffusion properties, Mishra and coworkers prepared the neutral and anionic mixed pyridine-amide complexes
16 and
17 (
Figure 10). All complexes contained the ligand binding through nitrogen donor atoms in a chelating fashion. The neutral anionic complexes
16 and
17a showed strong activity against resistant strains of
Pseudomonas,
E. coli, and standard strains of
Shigella and
Klebsiella while the anionic complexes
17b and
17c were also found to have activity against
Pseudomonas. The authors suggest that the presence and position of uncoordinated pyridine rings may be responsible for antibacterial properties. To the best of our knowledge these are the only examples of
anionic cobalt(III) complexes (
17a–c) being used as bactericides.
Figure 10.
Neutral and anionic pyridine-amide cobalt(III) complexes.
Figure 10.
Neutral and anionic pyridine-amide cobalt(III) complexes.
A number of cobalt(III) complexes containing N,O chelates have been shown to have antibacterial properties [
28,
29,
30,
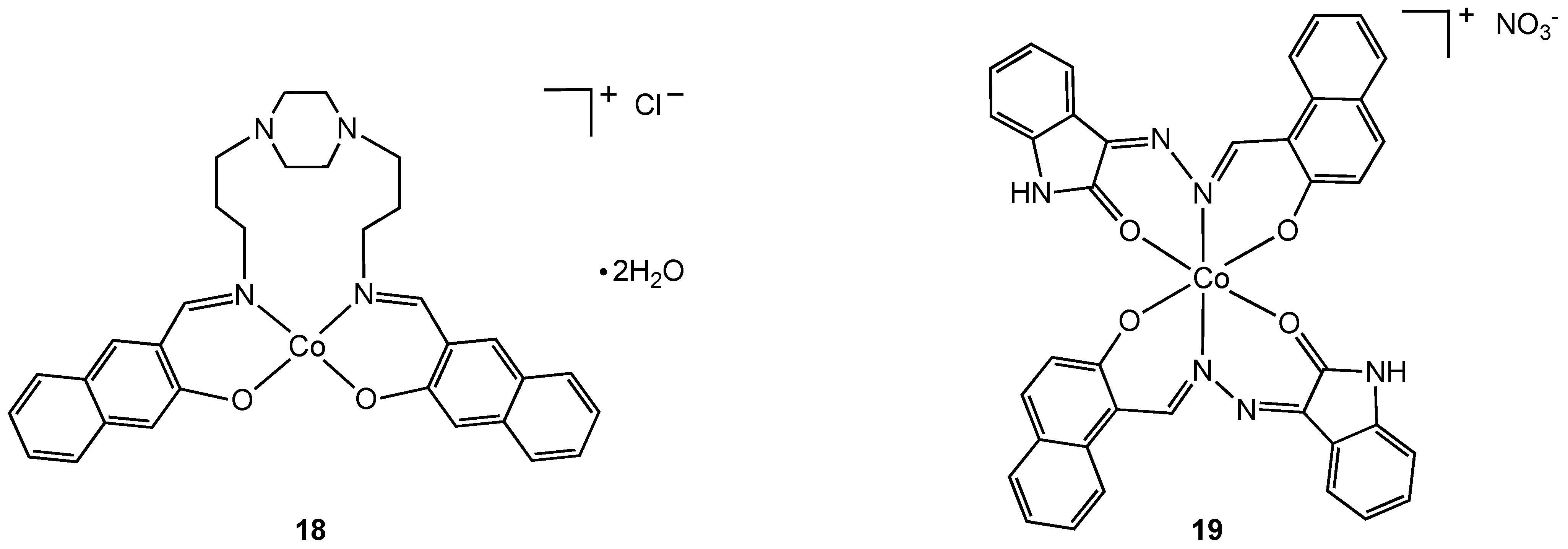
31]. An uncommon example of a tetragonal cobalt(III) complex (
18) was isolated from the reaction of Schiff base 1,4-bis[3-(2-hydroxy-1-naphthaldimine)propyl]piperazine (bappnaf) with cobalt(II) chloride in hot methanol (
Figure 11). Evidence for oxidation of cobalt(II) to cobalt(III) in this reaction was provided by elemental analysis, molar conductivity, thermal gravimetric analysis and mass spectroscopy [
31]. The related complex
19 was prepared directly from a cobalt(III) precursor, [Co(NH
3)(CO
3)]NO
3•0.5 H
2O, via ligand displacement [
29,
30] and screened for antibacterial properties. Complexes
18 and
19 exhibit broad-spectrum antibacterial activity with the metal complexes having higher activities than the free ligand. Enhanced lipophilicity due to metal coordination and chelation theory is used to explain the increase in bioactivity although clearly there may be other contributing factors.
Figure 11.
Tetragonal and octahedral cobalt (III) complexes containing N, O chelating Schiff base ligands.
Figure 11.
Tetragonal and octahedral cobalt (III) complexes containing N, O chelating Schiff base ligands.
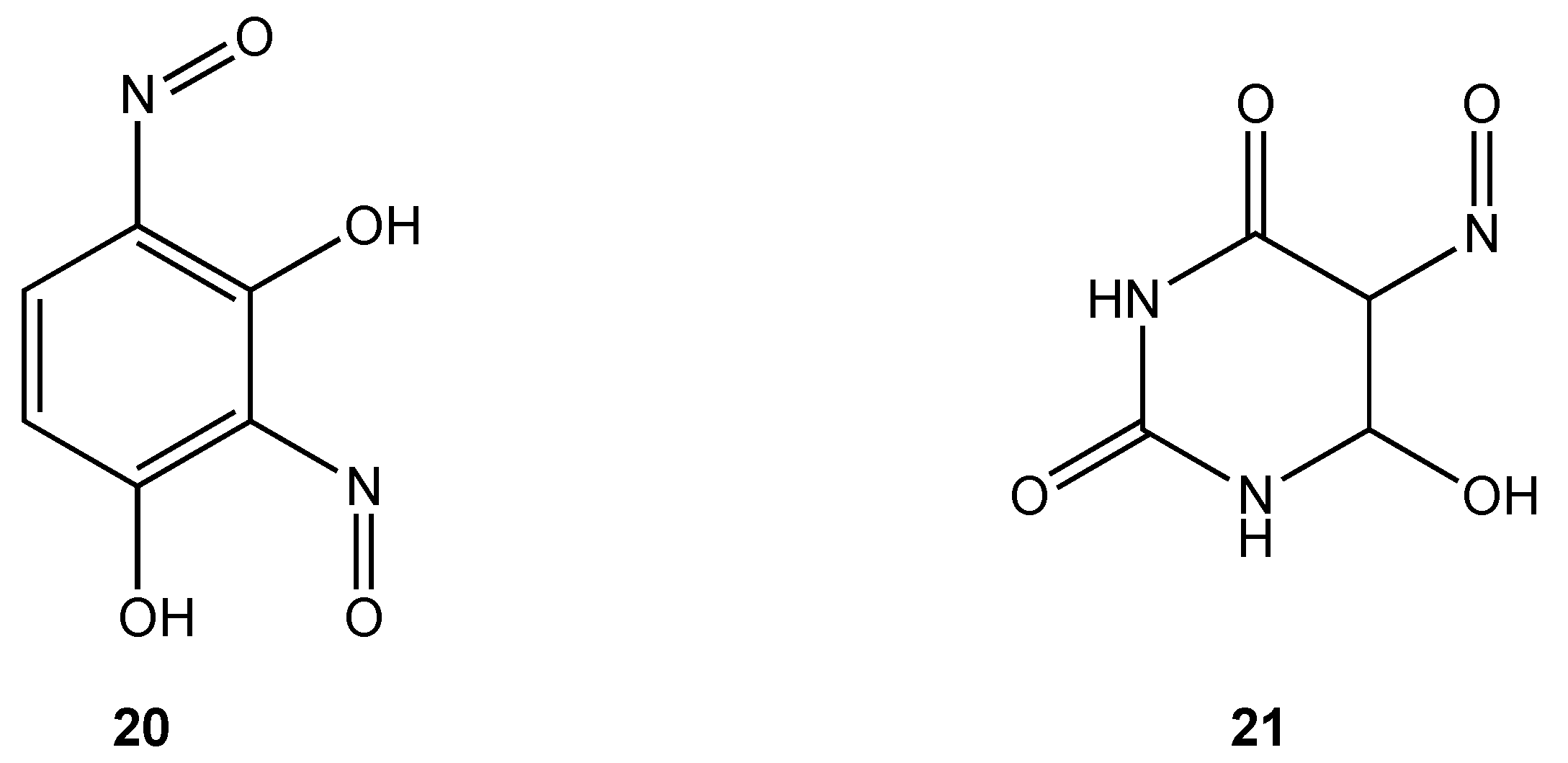
Only two cobalt(III) complexes containing O, O chelates have demonstrated antibacterial properties. Cobalt(III) complexes derived from dinitrosoresorcinol (
20) and violuric acid (
21) (
Figure 12) were synthesized by direct reaction of the ligands with cobalt(II) chloride in ethanol in varying molar ratios [
32]. Biocidal activity against
Serratia sp.,
Bacillus stearothermophilus,
B. subtilis and
Pseudomonas sp. was demonstrated to be higher for the metal complexes compared to either the free ligand or simple metal salts.
Figure 12.
Dinitrosoresorcinol and violuric acid ligands.
Figure 12.
Dinitrosoresorcinol and violuric acid ligands.
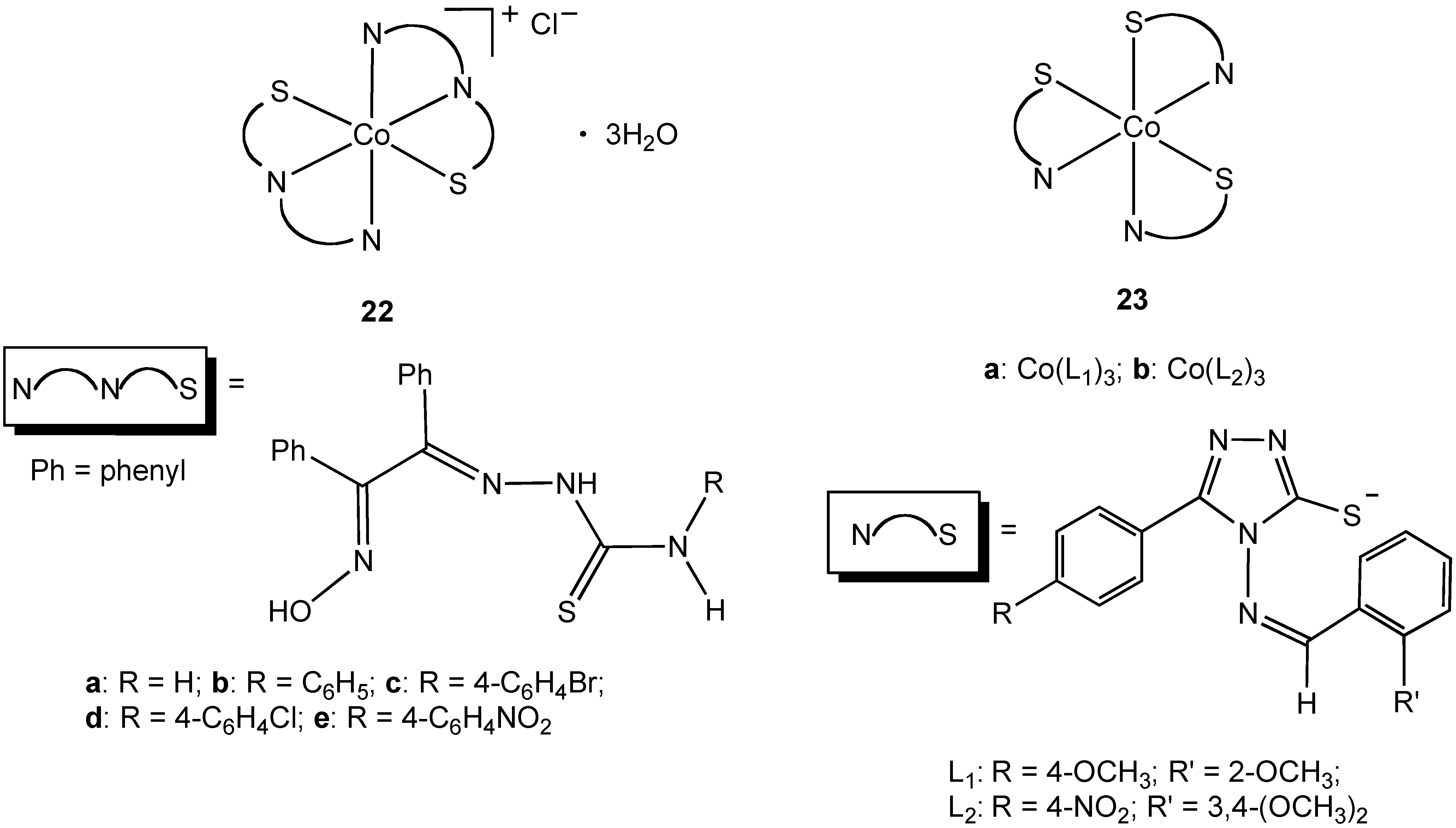
Complexes containing a sulfur atom as part of a ligand donor set represent the largest class of cobalt(III) compounds studied for their antibacterial properties [
33,
34,
35,
36,
37,
38,
39]. Sharma and coworkers synthesized octahedral Co(III) complexes
22a–e containing tridentate N, N, S thiosemicarbazone ligands (
Figure 13) [
35]. The thiosemicarbazones were of prior interest due to their extensive pharmacology including carcinostatic potency, antitumor, antibacterial, antiviral, antifungal, antimalarial, and antiamoebic properties. Complexes
22a–e proved to be toxic to
E. coli and
S. aureus with an increase in potency compared to the free thiosemicarbazone ligands [
35]. All of the complexes studied had less activity when compared to streptomycin. Complexes
23a–b showed moderate broad-spectrum antibacterial activity [
34]. The synthesis of
22 and
23 all required the use of hydrogen peroxide as an oxidant to convert Co(II) to Co(III), an alternative approach to the use of a pre-formed Co(III) precursor.
Figure 13.
Cobalt(III) complexes containing bidentate and tridentate nitrogen/sulfur donor ligands.
Figure 13.
Cobalt(III) complexes containing bidentate and tridentate nitrogen/sulfur donor ligands.
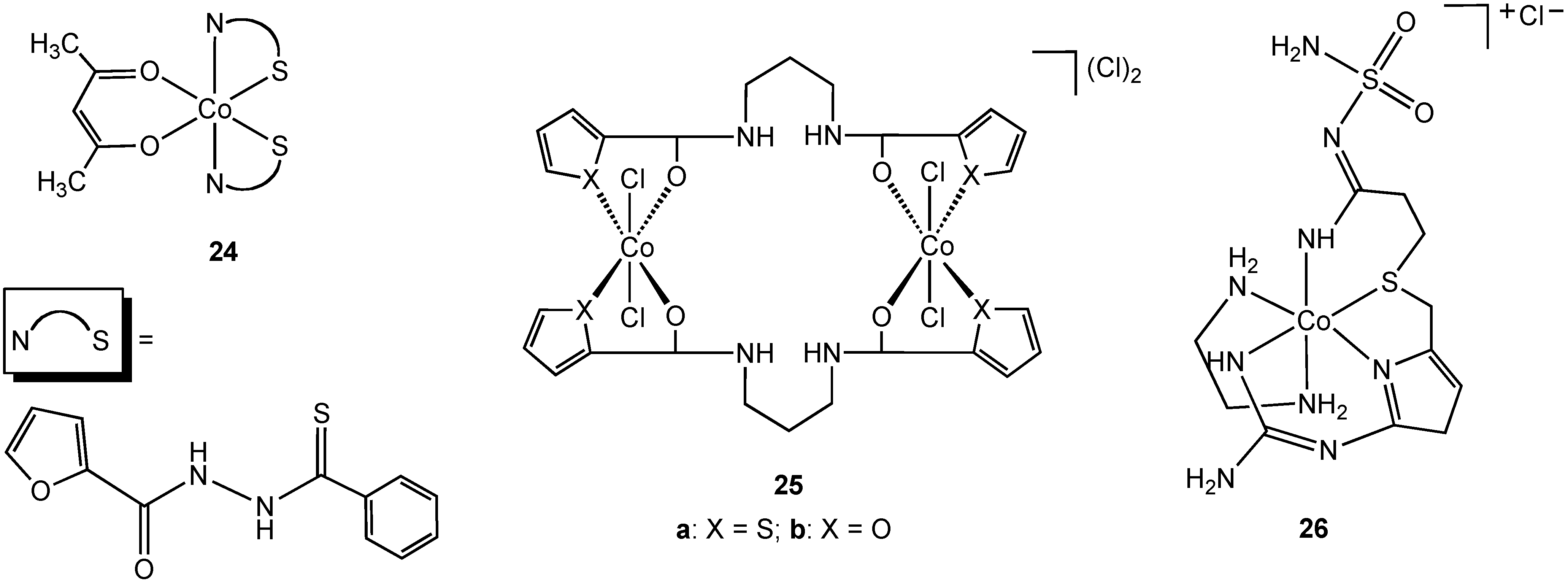
Singh reported the synthesis and characterization of the N,S chelate cobalt complex
24 derived from a 2-furanthiocarbohydrazide ligand (
Figure 14) [
39]. The complex is octahedral, two N, S ligands occupying four coordination sites in the complex, the fifth and sixth sites are completed with an acetylacetonate ligand. Due to the lack of solubility of
24 in water antibacterial screening using zone inhibition was performed using DMSO solution (blank DMSO/H
2O was used as a control). Complex
24 is active against
Staphylococcus albus and
Enterobactor. The free 2-furanthiocarbohydrazide ligand possesses no antibacterial activity. The bimetallic cobalt (III) complex
25a (
Figure 14) was shown to have antibacterial activity against
E. coli,
Yersinia enterocolitica,
Bacillus magaterium, and
B. subtilis. Surprisingly, the furan form of the complex (
25b), containing an oxygen donor atom in place of sulfur, demonstrated no activity towards the same group although no explanation was offered for this difference in activity.
Famotidine is a sulfur containing tetradentate N, N, N, S ligand and is a histamine H
2-receptor antagonist. In addition to being widely used in the treatment of peptic ulcers and reflux esophagitis, famotidine has also been used in the treatment of Zollinger-Ellison syndrome, Parkinson's disease and Alzheimer's disease. In 2006, Miodragović described the coordination of famotidine to cobalt(III) and reported the isolation of complex
26 (
Figure 14) [
36]. The octahedral geometry and tetradentate ligand coordination was confirmed using X-ray crystallography in which extensive intermolecular hydrogen bonding and non-covalent CH•••π and NH•••π interactions, as well as π-stacking interactions were observed. Complex
26 has antibacterial activity against
E. coli,
S. aureus and
Micrococcus lysodeikiticus, showing better growth inhibitory activity in comparison with famotidine alone.
Figure 14.
Cobalt(III) complexes containing bidentate and tetradentate nitrogen/sulfur donor ligands.
Figure 14.
Cobalt(III) complexes containing bidentate and tetradentate nitrogen/sulfur donor ligands.
Only two selenium containing cobalt(III) compounds have shown antibacterial activity [
40]. Complexes
27 and
28 in which the pyridine and quinoline derived ligands coordinate to cobalt via nitrogen and selenium donor atoms were synthesized and characterized using X-ray crystallography. Both complexes exhibited activity towards
E. coli,
B. subtilis, S. aureus and
Micrococcus lysodeikiticus.
Figure 15.
Selenium containing and organometallic cobalt(III) complexes.
Figure 15.
Selenium containing and organometallic cobalt(III) complexes.
Organometallic complexes containing a formal metal carbon bond are rare in biology, with vitamin B
12 representing a well-known example of this class of molecule. To the best of our knowledge, only one class of cobalt(III) containing organometallic complex has been studied with respect to chemotherapy. Navaneetha prepared the series of organometallic methyl cobalt(III) complexes
29a-
e in which the ligand
trans to the -CH
3 ligand was varied [
41]. All five complexes were active against
Klebsiella pneumoniea,
S. aureus,
E. coli and
B. subtilis.
5. Toxicity of Cobalt(III) Complexes
Cobalt is generally not considered to be a very toxic element. Most toxicity studies have been concerned with Co(II) metal ions, surgical implants, or cobalt metal dust [
42], with one notable example of cobalt-induced mortality from drinking large quantities of beer that contained cobalt chloride or cobalt sulfate as a foam stabilizer (“beer drinker's cardiomyopathy”) [
43]. There is much less known about toxicity of Co(III) complexes, with Cohex being the most studied. In general, it does not appear that Co(III) complexes are toxic at moderate levels of exposure, although some adverse effects on kidney function were reported.
The toxicity of metal-ion based therapeutics is a concern due to the intrinsic toxicity of some metal ions themselves. However, there are no open coordination sites on Cohex available for interacting with the environment. Further, the complex sequesters the inner-sphere ammonia ligands from most exchange-reactions in solution; therefore, interactions with solution molecules are by "outer-sphere" coordination via water bridges to the ammonia ligands and via the high charge-density of the Co(III) ion [
44,
45]. Due to the kinetic inertness of Co(III), we believe that the compound is stable such that its biological interaction will also reflect these modes of interactions. Thus, the toxicity of Cohex cannot be inferred from that of the Co
3+ ion itself.
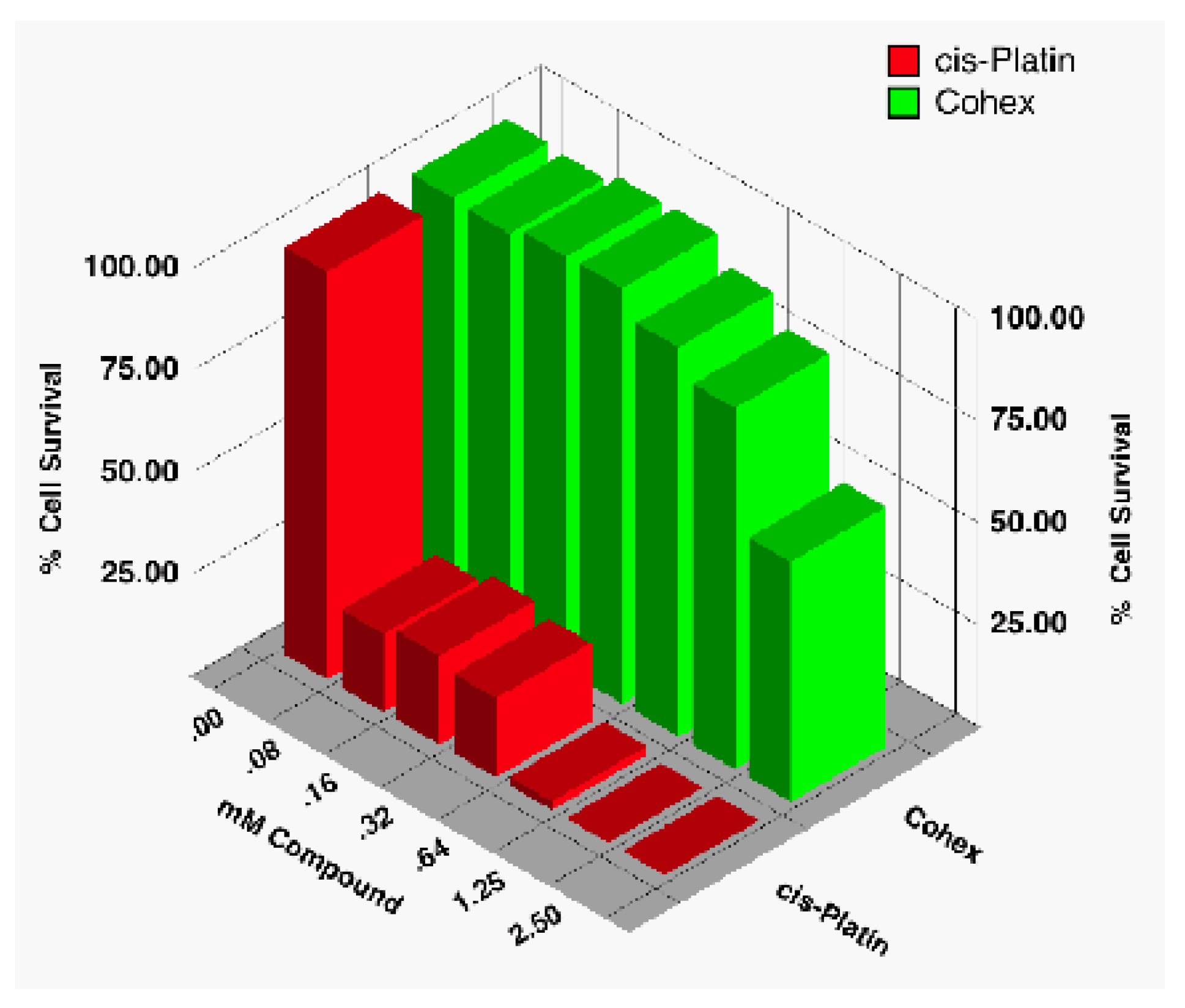
There are only a few sources dealing with the toxicity of Cohex. In our laboratory, we have found the compound to have low to moderate toxicity [
18], much less so than, say, cisplatin. In an early study, we assayed for the cytotoxicity of Cohex against BHK cells using CellTiter96® Proliferation Assay (Promega, Madison, WI) and compared it to that of cisplatin (
Figure 16).
Figure 16.
Comparison of cytotoxicity of Cohex against BHK cells with commercial drug cisplatin using CellTiter96.
Figure 16.
Comparison of cytotoxicity of Cohex against BHK cells with commercial drug cisplatin using CellTiter96.
As can be seen, Cohex cytotoxic effects are only evident around 1 mM or higher, whereas cisplatin was toxic even at the lowest concentration tested (80 μM). Since that study, we have routinely used up to 2.5 to 5 mM of compound in our flow cytometry studies without detecting a significant amount of cell death (
Table 1). In these studies, we measured the state of the cell (up to 10
4 cells) using propidium iodide for cell viability. Studies in other laboratories generally seem to confirm that toxicity starts around 1 mM but never seems to fully kill the cells—even at concentrations as high as 5 mM (unpublished data from this laboratory and private communications).
Table 1.
Typical flow cytometry results for uninfected BHK cells.
Table 1.
Typical flow cytometry results for uninfected BHK cells.
| mM Cohex | 0.00 | 0.60 | 1.25 | 2.50 | 5.00 |
| % Live | 75.4 | 73.6 | 74.8 | 78.2 | 73.5 |
Beyond the cytotoxicity studies, only two published reports exist of the toxicity of Cohex in small animal models (mice). An earlier paper reported on the effects of Cohex on oxidative stress-related parameters in male albino BALB/c mice [
46]. Mice were divided into four groups consisting of 6–8 animals each and one group served as the control. The other three groups of mice were administered Cohex in drinking water at concentrations of 25, 50, and 100 ppm for a period of 14 weeks. They found that tissue levels of the compound followed the trend kidney > liver > intestine > blood > spleen > lungs. Cobalt content (μg/g tissue) increased from 2.172 to 5.970 in kidney, 4.443 to 7.152 in liver, 1.571 to 2.828 in intestine, 0.305 to 0.504 in spleen, 1.289 to 1.455 in lung, and 0.286 to 1.185 (μg/mL) in blood with respect to a control. The concentrations of Cohex used for their experiments were apparently nontoxic since no treatment-related changes in daily food intake or growth rate were observed.
In a follow-up study on the toxicity of the compound at higher dosages, Naura and Sharma [
46] administered intraperitoneal injections once daily for 3 consecutive days at 5, 10, 20 mg/kg body weight and sacrificed the mice (male albino BALB/c) 24 h after last injection. They observed accumulation of Co in kidney > liver > spleen > lungs, with the increase of cobalt from 2.712 to 14.332 in kidney and 4.443 to 10.637 μg/g in liver tissues. In blood, the increase was from 1.269 to 1.729 μg/mL in blood when the dose was raised from zero to 20 mg/kg of body weight. Urea and creatine were observed to increase in the blood of the treated mice suggesting that Cohex can affect the normal functioning of the kidney following acute administration. However, none of the mice died before being sacrificed.
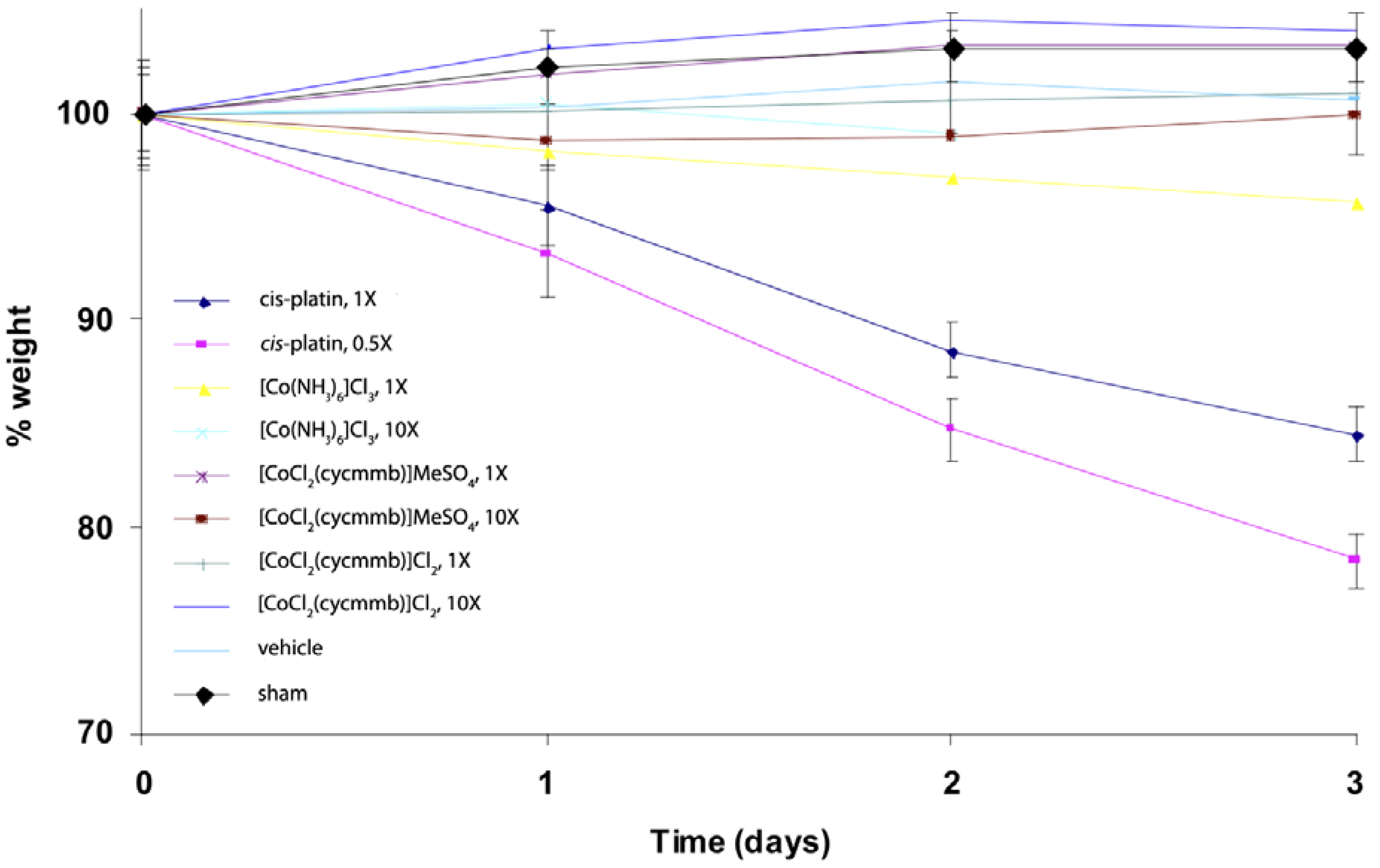
We have also done some studies of Cohex toxicity in mice. A preliminary mice toxicity study showed that similar results as the cytotoxicity work (
Figure 17) [
47]. For this study, mice were administered the agents at doses of 0.5, 1, 3 and/or 10X, where 1X is the molar equivalent of the LD
50 of cisplatin. Administration was by inter-peritoneal injection under light isoflurane anesthesia. The different compounds were dissolved in water at the desired concentration and all animals received 1 mL injection volume as a bolus. Vehicle shams received a 2 mL injection of water. Mouse weights were monitored daily, as were behavioral signs. A rough calculation indicates at 1× concentration for Cohex is about 1 mM Cohex in the blood volume.
Toxicity for the CTC series of cobalt complexes were briefly mentioned [
9] in a 1998 report stating that all the CTC complexes tested were non-toxic to Vero cells upon continuous exposure to ≤ 25 μg/mL, whereas 50 μg/mL of CTC 96, nine times daily, did not irritate uninfected rabbit eyes, but cytotoxicity was evident at ≥ 50 μg/mL. Cigler,
et al. [
21] reported on the potent, specific, and selective inhibition of HIV protease by parental and substituted metallacarboranes, specifically, cobalt bis(1,2-dicarbollides). Antiviral activity was analyzed by using PM-1 cells infected with HIV-1 strain NL4-3 modified from a published procedure ref. PM-1 cells were infected by co-culture and washed 4 h after infection, and compounds
3–
8 or the solvent DMSO, respectively, was added after the wash. No significant toxicity of tested compounds in tissue cultures was observed in the concentration range up to 50 μM. Mishra,
et al. [
48] studied several pyridine-amide based cobalt(III) complexes for their antimicrobial activity. Cytotoxic activity of the metal complexes was also determined via growth inhibition by MTT assay. The final concentrations ranged from 1,500–0.73 μg/mL. They found that cobalt(III) complexes were less cytotoxic than that of gentamycin. The cytotoxic activity results suggested that these cobalt complexes were less cytotoxic at these moderate concentrations.
Figure 17.
Weight loss of C57BL/6J mice exposed to platinum(II) and cobalt(III) complexes. 1X is equivalent to approx. 1 mM [Co(NH3)6]Cl3 in blood volume of the mice. Cycmmb = methyl 4-[(1,4,7,10-tetraazacyclododec-1-yl)methylbenzoate.
Figure 17.
Weight loss of C57BL/6J mice exposed to platinum(II) and cobalt(III) complexes. 1X is equivalent to approx. 1 mM [Co(NH3)6]Cl3 in blood volume of the mice. Cycmmb = methyl 4-[(1,4,7,10-tetraazacyclododec-1-yl)methylbenzoate.