Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities
Abstract
:1. Introduction
2. Synthetic Peptides in Targeted Photodynamic Therapy
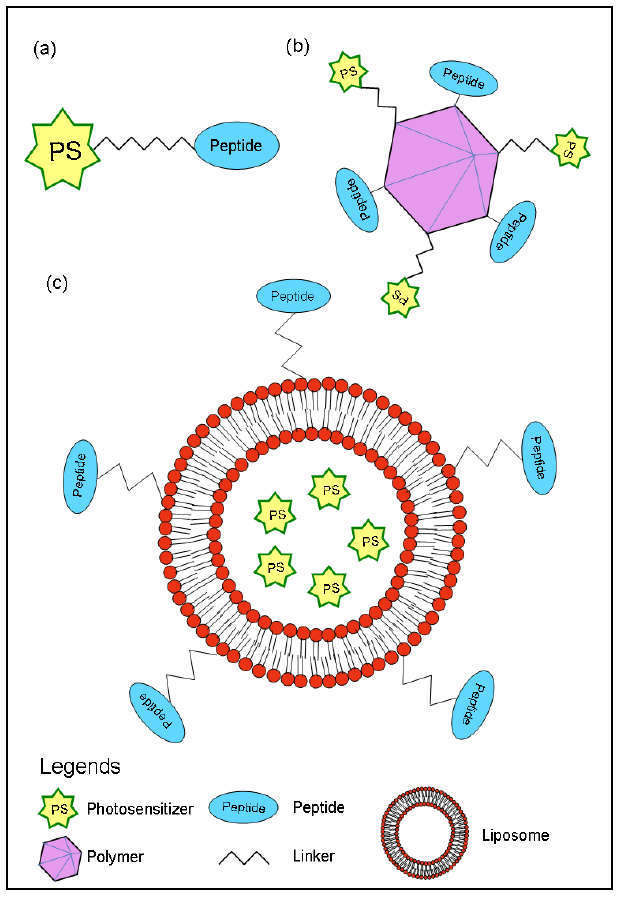
2.1. Cellular-targeted PDT with synthetic peptides
2.2. Vascular-targeted PDT with synthetic peptides
3. Nanoparticle-Based Drug Delivery and Targeting in PDT
3.1. Cellular-targeted nanotherapy
4. Vascular and Anti-Angiogenesis Targeted Photodynamic Therapy
4.1. Vascular-targeted PDT
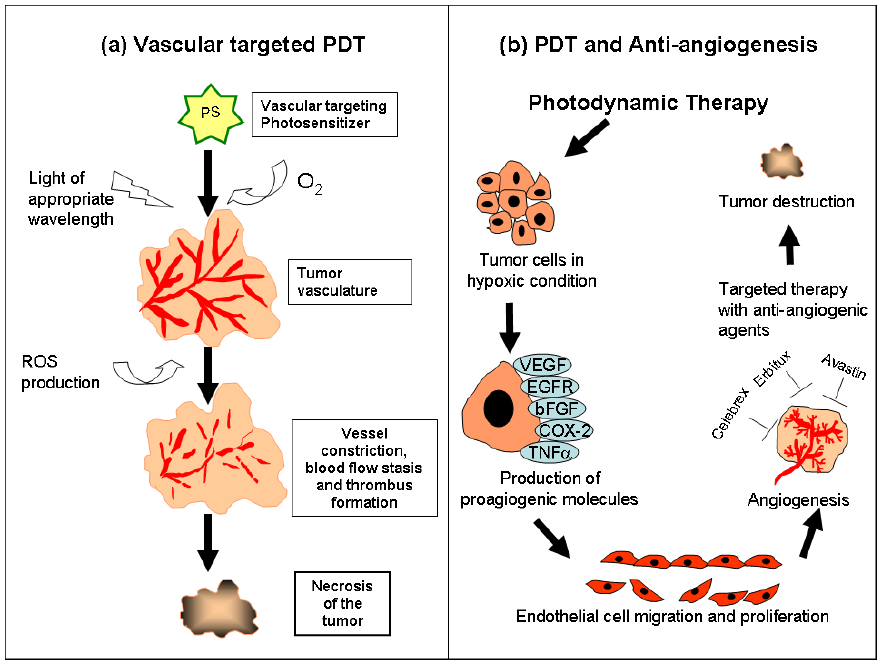
4.2. Cellular-targeted PDT and anti-angiogenesis therapy
5. Photodynamic Therapy-Mediated Immune Response
5.1. Pre-clinical studies
5.2. PDT-generated anti-tumor vaccines
5.3. Clinical studies
6. Conclusions
References
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar]
- Fabris, C.; Valduga, G.; Miotto, G.; Borsetto, L.; Jori, G.; Garbisa, S.; Reddi, E. Photosensitization with zinc (II) phthalocyanine as a switch in the decision between apoptosis and necrosis. Cancer Res. 2001, 61, 7495–7500. [Google Scholar]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar]
- Gollnick, S.O.; Brackett, C.M. Enhancement of anti-tumor immunity by photodynamic therapy. Immunol. Res. 2010, 46, 216–226. [Google Scholar]
- Kalyn, R. Overview of targeted therapies in oncology. J. Oncol. Pharm. Pract. 2007, 13, 199–205. [Google Scholar]
- Josefsen, L.B.; Boyle, R.W. Photodynamic therapy: novel third-generation photosensitizers one step closer? Br. J. Pharmacol 2008, 154, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Sharman, W.M.; van Lier, J.E.; Allen, C.M. Targeted photodynamic therapy via receptor mediated delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 53–76. [Google Scholar]
- Bechet, D.; Couleaud, P.; Frochot, C.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. Nanoparticles as vehicles for delivery of photodynamic therapy agents. Trends Biotechnol. 2008, 26, 612–621. [Google Scholar]
- Abels, C. Targeting of the vascular system of solid tumours by photodynamic therapy (PDT). Photochem. Photobiol. Sci. 2004, 3, 765–771. [Google Scholar]
- Kamrava, M.; Bernstein, M.B.; Camphausen, K.; Hodge, J.W. Combining radiation, immunotherapy, and antiangiogenesis agents in the management of cancer: the Three Musketeers or just another quixotic combination? Mo. Biosyst. 2009, 5, 1262–1270. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. Targeting EGFR with photodynamic therapy in combination with Erbitux enhances in vivo bladder tumor response. Mol. Cancer 2009, 8, 94. [Google Scholar] [PubMed]
- Rahimipour, S.; Ben-Aroya, N.; Ziv, K.; Chen, A.; Fridkin, M.; Koch, Y. Receptor-mediated targeting of a photosensitizer by its conjugation to gonadotropin-releasing hormone analogues. J. Med. Chem. 2003, 46, 3965–3974. [Google Scholar]
- De Luca, S.; Tesauro, D.; Di Lello, P.; Fattorusso, R.; Saviano, M.; Pedone, C.; Morelli, G. Synthesis and solution characterization of a porphyrin-CCK8 conjugate. J. Pept. Sci. 2001, 7, 386–394. [Google Scholar]
- Renno, R.Z.; Terada, Y.; Haddadin, M.J.; Michaud, N.A.; Gragoudas, E.S.; Miller, J.W. Selective photodynamic therapy by targeted verteporfin delivery to experimental choroidal neovascularization mediated by a homing peptide to vascular endothelial growth factor receptor-2. Arch. Ophthalmol. 2004, 122, 1002–1011. [Google Scholar]
- Ichikawa, K.; Hikita, T.; Maeda, N.; Yonezawa, S.; Takeuchi, Y.; Asai, T.; Namba, Y.; Oku, N. Antiangiogenic photodynamic therapy (PDT) by using long-circulating liposomes modified with peptide specific to angiogenic vessels. Biochim. Biophys. Acta 2005, 1669, 69–74. [Google Scholar]
- Oku, N.; Ishii, T. Antiangiogenic photodynamic therapy with targeted liposomes. Methods Enzymol. 2009, 465, 313–330. [Google Scholar]
- Hamblin, M.R.; Miller, J.L.; Hasan, T. Effect of charge on the interaction of site-specific photoimmunoconjugates with human ovarian cancer cells. Cancer Res. 1996, 56, 5205–5210. [Google Scholar]
- Krinick, N.L.; Sun, Y.; Joyner, D.; Spikes, J.D.; Straight, R.C.; Kopecek, J. A polymeric drug delivery system for the simultaneous delivery of drugs activatable by enzymes and/or light. J. Biomater. Sci. Polym. Ed. 1994, 5, 303–324. [Google Scholar]
- Chaleix, V.; Sol, V.; Huang, Y.; Guilloton, M.; Granet, R.; Blais, J.-C.; Krausz, P. RGD Porphyrin Conjugates: Synthesis and Potential Application in Photodynamic Therapy. Eur. J. Org. Chem. 2003, 8, 1486–1493. [Google Scholar]
- Chaleix, V.; Sol, V.; Guilloton, M.; Granet, R.; Krausz, P. Efficient synthesis of RGD containing cyclic peptide–porphyrin conjugates by ring-closing metathesis on solid support. Tetrahedron Lett. 2004, 45, 5295–5299. [Google Scholar]
- Frochot, C.; Di Stasio, B.; Vanderesse, R.; Belgy, M.J.; Dodeller, M.; Guillemin, F.; Viriot, M.L.; Barberi-Heyob, M. Interest of RGD-containing linear or cyclic peptide targeted tetraphenylchlorin as novel photosensitizers for selective photodynamic activity. Bioorg. Chem. 2007, 35, 205–220. [Google Scholar]
- Conway, C.L.; Walker, I.; Bell, A.; Roberts, D.J.; Brown, S.B.; Vernon, D.I. In vivo and in vitro characterisation of a protoporphyrin IX-cyclic RGD peptide conjugate for use in photodynamic therapy. Photochem. Photobiol. Sci. 2008, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.C.; Clark, R.A.; Cheresh, D.A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994, 264, 569–571. [Google Scholar]
- Haubner, R.; Wester, H.J.; Weber, W.A.; Mang, C.; Ziegler, S.I.; Goodman, S.L.; Senekowitsch-Schmidtke, R.; Kessler, H.; Schwaiger, M. Noninvasive imaging of alpha(v)beta3 integrin expression using 18F-labeled RGD-containing glycopeptide and positron emission tomography. Cancer Res. 2001, 61, 1781–1785. [Google Scholar]
- Assa-Munt, N.; Jia, X.; Laakkonen, P.; Ruoslahti, E. Solution structures and integrin binding activities of an RGD peptide with two isomers. Biochemistry 2001, 40, 2373–2378. [Google Scholar]
- Sheldon, K.; Liu, D.; Ferguson, J.; Gariepy, J. Loligomers: design of de novo peptide-based intracellular vehicles. Proc. Natl. Acad. Sci. USA 1995, 92, 2056–2060. [Google Scholar]
- Singh, D.; Kiarash, R.; Kawamura, K.; LaCasse, E.C.; Gariepy, J. Penetration and intracellular routing of nucleus-directed peptide-based shuttles (loligomers) in eukaryotic cells. Biochemistry 1998, 37, 5798–5809. [Google Scholar]
- Singh, D.; Bisland, S.K.; Kawamura, K.; Gariepy, J. Peptide-based intracellular shuttle able to facilitate gene transfer in mammalian cells. Bioconjug. Chem. 1999, 10, 745–754. [Google Scholar]
- Dozzo, P.; Koo, M.S.; Berger, S.; Forte, T.M.; Kahl, S.B. Synthesis, characterization, and plasma lipoprotein association of a nucleus-targeted boronated porphyrin. J. Med. Chem. 2005, 48, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Maletinska, L.; Blakely, E.A.; Bjornstad, K.A.; Deen, D.F.; Knoff, L.J.; Forte, T.M. Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000, 60, 2300–2303. [Google Scholar]
- van Hell, A.J.; Costa, C.I.; Flesch, F.M.; Sutter, M.; Jiskoot, W.; Crommelin, D.J.; Hennink, W.E.; Mastrobattista, E. Self-assembly of recombinant amphiphilic oligopeptides into vesicles. Biomacromolecules 2007, 8, 2753–2761. [Google Scholar]
- van Hell, A.J.; Fretz, M.M.; Crommelin, D.J.; Hennink, W.E.; Mastrobattista, E. Peptide nanocarriers for intracellular delivery of photosensitizers. J. Control. Release 2009, 141, 347–353. [Google Scholar]
- Tsay, J.M.; Trzoss, M.; Shi, L.; Kong, X.; Selke, M.; Jung, M.E.; Weiss, S. Singlet oxygen production by Peptide-coated quantum dot-photosensitizer conjugates. J. Am. Chem. Soc. 2007, 129, 6865–6871. [Google Scholar]
- Duska, L.R.; Hamblin, M.R.; Bamberg, M.P.; Hasan, T. Biodistribution of charged F(ab')2 photoimmunoconjugates in a xenograft model of ovarian cancer. Br. J. Cancer 1997, 75, 837–844. [Google Scholar]
- Ichikawa, K.; Hikita, T.; Maeda, N.; Takeuchi, Y.; Namba, Y.; Oku, N. PEGylation of liposome decreases the susceptibility of liposomal drug in cancer photodynamic therapy. Biol. Pharm. Bull. 2004, 27, 443–444. [Google Scholar]
- Ishida, T.; Harashima, H.; Kiwada, H. Liposome clearance. Biosci. Rep. 2002, 22, 197–224. [Google Scholar]
- Ferrara, N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog. Horm. Res. 2000, 55, 15–35, discussion 35-16. [Google Scholar] [PubMed]
- Miao, H.Q.; Klagsbrun, M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000, 19, 29–37. [Google Scholar]
- Binetruy-Tournaire, R.; Demangel, C.; Malavaud, B.; Vassy, R.; Rouyre, S.; Kraemer, M.; Plouet, J.; Derbin, C.; Perret, G.; Mazie, J.C. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J 2000, 19, 1525–1533. [Google Scholar]
- Konan, Y.N.; Gurny, R.; Allemann, E. State of the art in the delivery of photosensitizers for photodynamic therapy. J. Photochem. Photobiol. B 2002, 66, 89–106. [Google Scholar]
- Florence, A.T.; Hussain, N. Transcytosis of nanoparticle and dendrimer delivery systems: evolving vistas. Adv. Drug Deliv. Rev. 2001, 50 Suppl. 1, S69–S89. [Google Scholar] [CrossRef] [PubMed]
- Oba, T. Photosensitizer Nanoparticles for Photodynamic Therapy. Current Bioactive Compounds 2007, 3, 239–251. [Google Scholar]
- Lee, S.J.; Park, K.; Oh, Y.K.; Kwon, S.H.; Her, S.; Kim, I.S.; Choi, K.; Kim, H.; Lee, S.G.; Kim, K.; Kwon, I.C. Tumor specificity and therapeutic efficacy of photosensitizer-encapsulated glycol chitosan-based nanoparticles in tumor-bearing mice. Biomaterials 2009, 30, 2929–2939. [Google Scholar]
- Zeisser-Labouebe, M.; Delie, F.; Gurny, R.; Lange, N. Benefits of nanoencapsulation for the hypercin-mediated photodetection of ovarian micrometastases. Eur. J. Pharm. Biopharm. 2009, 71, 207–213. [Google Scholar]
- Roy, I.; Ohulchanskyy, T.Y.; Pudavar, H.E.; Bergey, E.J.; Oseroff, A.R.; Morgan, J.; Dougherty, T.J.; Prasad, P.N. Ceramic-based nanoparticles entrapping water-insoluble photosensitizing anticancer drugs: a novel drug-carrier system for photodynamic therapy. J. Am. Chem. Soc. 2003, 125, 7860–7865. [Google Scholar]
- Wieder, M.E.; Hone, D.C.; Cook, M.J.; Handsley, M.M.; Gavrilovic, J.; Russell, D.A. Intracellular photodynamic therapy with photosensitizer-nanoparticle conjugates: cancer therapy using a 'Trojan horse'. Photochem. Photobiol. Sci. 2006, 5, 727–734. [Google Scholar]
- Yaghini, E.; Seifalian, A.M.; MacRobert, A.J. Quantum dots and their potential biomedical applications in photosensitization for photodynamic therapy. Nanomedicine (Lond) 2009, 4, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Hone, D.C.; Walker, P.I.; Evans-Gowing, R.; FitzGerald, S.; Beeby, A.; Chambrier, I.; Cook, M.J.; Russell, D.A. Generation of cytotoxic singlet oxygen via phthalocyanine-stabilized gold nanoparticles: A potential delivery vehicle for photodynamic therapy. Langmuir 2002, 18, 2985–2987. [Google Scholar]
- Chen, W.; Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar]
- Samia, A.C.; Chen, X.; Burda, C. Semiconductor quantum dots for photodynamic therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar]
- Ma, J.; Chen, J.Y.; Idowu, M.; Nyokong, T. Generation of singlet oxygen via the composites of water-soluble thiol-capped CdTe quantum dots-sulfonated aluminum phthalocyanines. J. Phys. Chem. B 2008, 112, 4465–4469. [Google Scholar]
- Gao, D.; Agayan, R.R.; Xu, H.; Philbert, M.A.; Kopelman, R. Nanoparticles for two-photon photodynamic therapy in living cells. Nano Lett. 2006, 6, 2383–2386. [Google Scholar]
- Ungun, B.; Prud'homme, R.K.; Budijon, S.J.; Shan, J.; Lim, S.F.; Ju, Y.; Austin, R. Nanofabricated upconversion nanoparticles for photodynamic therapy. Opt. Express 2009, 17, 80–86. [Google Scholar]
- Zhang, P.; Steelant, W.; Kumar, M.; Scholfield, M. Versatile photosensitizers for photodynamic therapy at infrared excitation. J. Am. Chem. Soc. 2007, 129, 4526–4527. [Google Scholar]
- Heer, S.; Kompe, K.; Gudel, H.U.; Haase, M. Highly efficient multicolour upconversion emission in transparent colloids of lanthanide doped NaYF4 nanocrystals. Adv. Mater. 2004, 16, 2102–2105. [Google Scholar]
- Siemann, D.W.; Bibby, M.C.; Dark, G.G.; Dicker, A.P.; Eskens, F.A.; Horsman, M.R.; Marme, D.; Lorusso, P.M. Differentiation and definition of vascular-targeted therapies. Clin. Cancer Res. 2005, 11, 416–420. [Google Scholar]
- Trachtenberg, J.; Weersink, R.A.; Davidson, S.R.; Haider, M.A.; Bogaards, A.; Gertner, M.R.; Evans, A.; Scherz, A.; Savard, J.; Chin, J.L.; Wilson, B.C.; Elhilali, M. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: a study of escalating light doses. BJU Int. 2008, 102, 556–562. [Google Scholar]
- Chen, B.; Pogue, B.W.; Zhou, X.; O'Hara, J.A.; Solban, N.; Demidenko, E.; Hoopes, P.J.; Hasan, T. Effect of tumor host microenvironment on photodynamic therapy in a rat prostate tumor model. Clin. Cancer Res. 2005, 11, 720–727. [Google Scholar]
- Krammer, B. Vascular effects of photodynamic therapy. Anticancer Res. 2001, 21, 4271–4277. [Google Scholar]
- Chen, B.; Pogue, B.W.; Hoopes, P.J.; Hasan, T. Vascular and cellular targeting for photodynamic therapy. Crit. Rev. Eukaryot. Gene Expr. 2006, 16, 279–305. [Google Scholar]
- Star, W.M.; Marijnissen, H.P.; van den Berg-Blok, A.E.; Versteeg, J.A.; Franken, K.A.; Reinhold, H.S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986, 46, 2532–2540. [Google Scholar]
- Chaudhuri, K.; Keck, R.W.; Selman, S.H. Morphological changes of tumor microvasculature following hematoporphyrin derivative sensitized photodynamic therapy. Photochem. Photobiol. 1987, 46, 823–827. [Google Scholar]
- Chen, B.; Crane, C.; He, C.; Gondek, D.; Agharkar, P.; Savellano, M.D.; Hoopes, P.J.; Pogue, B.W. Disparity between prostate tumor interior versus peripheral vasculature in response to verteporfin-mediated vascular-targeting therapy. Int. J. Cancer 2008, 123, 695–701. [Google Scholar]
- Chen, B.; Pogue, B.W.; Luna, J.M.; Hardman, R.L.; Hoopes, P.J.; Hasan, T. Tumor vascular permeabilization by vascular-targeting photosensitization: effects, mechanism, and therapeutic implications. Clin. Cancer Res. 2006, 12, 917–923. [Google Scholar]
- Fingar, V.H.; Wieman, T.J.; Wiehle, S.A.; Cerrito, P.B. The role of microvascular damage in photodynamic therapy: the effect of treatment on vessel constriction, permeability, and leukocyte adhesion. Cancer Res. 1992, 52, 4914–4921. [Google Scholar] [PubMed]
- Fingar, V.H.; Wieman, T.J.; Haydon, P.S. The effects of thrombocytopenia on vessel stasis and macromolecular leakage after photodynamic therapy using photofrin. Photochem. Photobiol. 1997, 66, 513–517. [Google Scholar]
- Fingar, V.H.; Wieman, T.J.; Karavolos, P.S.; Doak, K.W.; Ouellet, R.; van Lier, J.E. The effects of photodynamic therapy using differently substituted zinc phthalocyanines on vessel constriction, vessel leakage and tumor response. Photochem. Photobiol. 1993, 58, 251–258. [Google Scholar]
- He, C.; Babasola, F.; Chen, B. Combination of vascular targeting PDT with combretastatin A4 phosphate. Proc. SPIE 2009, 7380, 738032. [Google Scholar]
- Byrne, A.T.; O'Connor, A.E.; Hall, M.; Murtagh, J.; O'Neill, K.; Curran, K.M.; Mongrain, K.; Rousseau, J.A.; Lecomte, R.; McGee, S.; Callanan, J.J.; O'Shea, D.F.; Gallagher, W.M. Vascular-targeted photodynamic therapy with BF2-chelated Tetraaryl-Azadipyrromethene agents: a multi-modality molecular imaging approach to therapeutic assessment. Br. J. Cancer 2009, 101, 1565–1573. [Google Scholar]
- Seshadri, M.; Spernyak, J.A.; Mazurchuk, R.; Camacho, S.H.; Oseroff, A.R.; Cheney, R.T.; Bellnier, D.A. Tumor vascular response to photodynamic therapy and the antivascular agent 5,6-dimethylxanthenone-4-acetic acid: implications for combination therapy. Clin. Cancer Res. 2005, 11, 4241–4250. [Google Scholar]
- Dolmans, D.E.; Kadambi, A.; Hill, J.S.; Waters, C.A.; Robinson, B.C.; Walker, J.P.; Fukumura, D.; Jain, R.K. Vascular accumulation of a novel photosensitizer, MV6401, causes selective thrombosis in tumor vessels after photodynamic therapy. Cancer Res. 2002, 62, 2151–2156. [Google Scholar] [PubMed]
- Chen, B.; Roskams, T.; de Witte, P.A. Antivascular tumor eradication by hypericin-mediated photodynamic therapy. Photochem. Photobiol. 2002, 76, 509–513. [Google Scholar]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The effect of photodynamic therapy on tumor angiogenesis. Cell. Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar]
- Ferrario, A.; Gomer, C.J. Avastin enhances photodynamic therapy treatment of Kaposi's sarcoma in a mouse tumor model. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 251–259. [Google Scholar]
- Bhuvaneswari, R.; Yuen, G.Y.; Chee, S.K.; Olivo, M. Hypericin-mediated photodynamic therapy in combination with Avastin (bevacizumab) improves tumor response by downregulating angiogenic proteins. Photochem. Photobiol. Sci. 2007, 6, 1275–1283. [Google Scholar]
- Kosharskyy, B.; Solban, N.; Chang, S.K.; Rizvi, I.; Chang, Y.; Hasan, T. A mechanism-based combination therapy reduces local tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Res. 2006, 66, 10953–10958. [Google Scholar]
- Bhuvaneswari, R.; Gan, Y.Y.; Lucky, S.S.; Chin, W.W.; Ali, S.M.; Soo, K.C.; Olivo, M. Molecular profiling of angiogenesis in hypericin mediated photodynamic therapy. Mol. Cancer 2008, 7, 56. [Google Scholar]
- Zwick, E.; Bange, J.; Ullrich, A. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr. Relat. Cancer 2001, 8, 161–173. [Google Scholar]
- Zhou, Q.; Olivo, M.; Lye, K.Y.; Moore, S.; Sharma, A.; Chowbay, B. Enhancing the therapeutic responsiveness of photodynamic therapy with the antiangiogenic agents SU5416 and SU6668 in murine nasopharyngeal carcinoma models. Cancer Chemother. Pharmacol. 2005, 56, 569–577. [Google Scholar]
- Dimitroff, C.J.; Klohs, W.; Sharma, A.; Pera, P.; Driscoll, D.; Veith, J.; Steinkampf, R.; Schroeder, M.; Klutchko, S.; Sumlin, A.; Henderson, B.; Dougherty, T.J.; Bernacki, R.J. Anti-angiogenic activity of selected receptor tyrosine kinase inhibitors, PD166285 and PD173074: implications for combination treatment with photodynamic therapy. Invest. New Drugs 1999, 17, 121–135. [Google Scholar]
- Ferrario, A.; Fisher, A.M.; Rucker, N.; Gomer, C.J. Celecoxib and NS-398 enhance photodynamic therapy by increasing N vitro apoptosis and decreasing in vivo inflammatory and angiogenic factors. Cancer Res. 2005, 65, 9473–9478. [Google Scholar] [CrossRef] [PubMed]
- Makowski, M.; Grzela, T.; Niderla, J.; M, L.A.; Mroz, P.; Kopee, M.; Legat, M.; Strusinska, K.; Koziak, K.; Nowis, D.; Mrowka, P.; Wasik, M.; Jakobisiak, M.; Golab, J. Inhibition of cyclooxygenase-2 indirectly potentiates antitumor effects of photodynamic therapy in mice. Clin. Cancer Res. 2003, 9, 5417–5422. [Google Scholar]
- Yee, K.K.; Soo, K.C.; Olivo, M. Anti-angiogenic effects of Hypericin-photodynamic therapy in combination with Celebrex in the treatment of human nasopharyngeal carcinoma. Int. J. Mol. Med. 2005, 16, 993–1002. [Google Scholar]
- Harvey, E.H.; Webber, J.; Kessel, D.; Fromm, D. Killing tumor cells: the effect of photodynamic therapy using mono-L-aspartyl chlorine and NS-398. Am. J. Surg. 2005, 189, 302–305. [Google Scholar]
- Hendrickx, N.; Volanti, C.; Moens, U.; Seternes, O.M.; de Witte, P.; Vandenheede, J.R.; Piette, J.; Agostinis, P. Up-regulation of cyclooxygenase-2 and apoptosis resistance by p38 MAPK in hypericin-mediated photodynamic therapy of human cancer cells. J. Biol. Chem. 2003, 278, 52231–52239. [Google Scholar]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and cancer prognosis. Eur. J. Cancer 2001, 37 Suppl 4, S9–S15. [Google Scholar]
- Koon, H.K.; Chan, P.S.; Wong, R.N.; Wu, Z.G.; Lung, M.L.; Chang, C.K.; Mak, N.K. Targeted inhibition of the EGFR pathways enhances Zn-BC-AM PDT-induced apoptosis in well-differentiated nasopharyngeal carcinoma cells. J. Cell. Biochem. 2009, 108, 1356–1363. [Google Scholar]
- Weyergang, A.; Selbo, P.K.; Berg, K. Y1068 phosphorylation is the most sensitive target of disulfonated tetraphenylporphyrin-based photodynamic therapy on epidermal growth factor receptor. Biochem. Pharmacol. 2007, 74, 226–235. [Google Scholar]
- Soukos, N.S.; Hamblin, M.R.; Keel, S.; Fabian, R.L.; Deutsch, T.F.; Hasan, T. Epidermal growth factor receptor-targeted immunophotodiagnosis and photoimmunotherapy of oral precancer in vivo. Cancer Res. 2001, 61, 4490–4496. [Google Scholar] [PubMed]
- del Carmen, M.G.; Rizvi, I.; Chang, Y.; Moor, A.C.; Oliva, E.; Sherwood, M.; Pogue, B.; Hasan, T. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. J. Natl. Cancer Inst. 2005, 97, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Kalka, K.; Mukhtar, H. In vitro and in vivo inhibition of epidermal growth factor receptor-tyrosine kinase pathway by photodynamic therapy. Oncogene 2001, 20, 2314–2317. [Google Scholar] [CrossRef] [PubMed]
- Korbelik, M.; Krosl, G.; Krosl, J.; Dougherty, G.J. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996, 56, 5647–5652. [Google Scholar]
- Korbelik, M.; Dougherty, G.J. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999, 59, 1941–1946. [Google Scholar]
- Gollnick, S.O.; Owczarczak, B.; Maier, P. Photodynamic therapy and anti-tumor immunity. Lasers Surg. Med. 2006, 38, 509–515. [Google Scholar]
- Kabingu, E.; Vaughan, L.; Owczarczak, B.; Ramsey, K.D.; Gollnick, S.O. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer 2007, 96, 1839–1848. [Google Scholar]
- Gollnick, S.O.; Vaughan, L.; Henderson, B.W. Generation of effective antitumor vaccines using photodynamic therapy. Cancer Res. 2002, 62, 1604–1608. [Google Scholar]
- Korbelik, M.; Stott, B.; Sun, J. Photodynamic therapy-generated vaccines: relevance of tumour cell death expression. Br. J. Cancer 2007, 97, 1381–1387. [Google Scholar]
- Abdel-Hady, E.S.; Martin-Hirsch, P.; Duggan-Keen, M.; Stern, P.L.; Moore, J.V.; Corbitt, G.; Kitchener, H.C.; Hampson, I.N. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001, 61, 192–196. [Google Scholar]
- Thong, P.S.; Ong, K.W.; Goh, N.S.; Kho, K.W.; Manivasager, V.; Bhuvaneswari, R.; Olivo, M.; Soo, K.C. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007, 8, 950–952. [Google Scholar]
- Kabingu, E.; Oseroff, A.R.; Wilding, G.E.; Gollnick, S.O. Enhanced systemic immune reactivity to a Basal cell carcinoma associated antigen following photodynamic therapy. Clin. Cancer Res. 2009, 15, 4460–4466. [Google Scholar]
- Berrahmoune, S.; Fotinos, N.; Bezdetnaya, L.; Lange, N.; Guedenet, J.C.; Guillemin, F.; D'Hallewin, M.A. Analysis of differential PDT effect in rat bladder tumor models according to concentrations of intravesical hexyl-aminolevulinate. Photochem. Photobiol. Sci. 2008, 7, 1018–1024. [Google Scholar]
- Mackenzie, G.D.; Jamieson, N.F.; Novelli, M.R.; Mosse, C.A.; Clark, B.R.; Thorpe, S.M.; Bown, S.G.; Lovat, L.B. How light dosimetry influences the efficacy of photodynamic therapy with 5-aminolaevulinic acid for ablation of high-grade dysplasia in Barrett's esophagus. Lasers Med. Sci. 2008, 23, 203–210. [Google Scholar]
- Thong, P.S.; Watt, F.; Ren, M.Q.; Tan, P.H.; Soo, K.C.; Olivo, M. Hypericin-photodynamic therapy (PDT) using an alternative treatment regime suitable for multi-fraction PDT. J. Photochem. Photobiol. B 2006, 82, 1–8. [Google Scholar]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence rate as a modulator of PDT mechanisms. Lasers Surg. Med. 2006, 38, 489–493. [Google Scholar]
- Thong, P.S.; Olivo, M.; Kho, K.W.; Bhuvaneswari, R.; Chin, W.W.; Ong, K.W.; Soo, K.C. Immune response against angiosarcoma following lower fluence rate clinical photodynamic therapy. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 35–42. [Google Scholar]
- Busch, T.M.; Xing, X.; Yu, G.; Yodh, A.; Wileyto, E.P.; Wang, H.W.; Durduran, T.; Zhu, T.C.; Wang, K.K. Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem. Photobiol. Sci. 2009, 8, 1683–1693. [Google Scholar]
- Seshadri, M.; Bellnier, D.A.; Vaughan, L.A.; Spernyak, J.A.; Mazurchuk, R.; Foster, T.H.; Henderson, B.W. Light delivery over extended time periods enhances the effectiveness of photodynamic therapy. Clin. Cancer Res. 2008, 14, 2796–2805. [Google Scholar]
- Kousis, P.C.; Henderson, B.W.; Maier, P.G.; Gollnick, S.O. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007, 67, 10501–10510. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Olivo, M.; Bhuvaneswari, R.; Lucky, S.S.; Dendukuri, N.; Soo-Ping Thong, P. Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities. Pharmaceuticals 2010, 3, 1507-1529. https://doi.org/10.3390/ph3051507
Olivo M, Bhuvaneswari R, Lucky SS, Dendukuri N, Soo-Ping Thong P. Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities. Pharmaceuticals. 2010; 3(5):1507-1529. https://doi.org/10.3390/ph3051507
Chicago/Turabian StyleOlivo, Malini, Ramaswamy Bhuvaneswari, Sasidharan Swarnalatha Lucky, Nagamani Dendukuri, and Patricia Soo-Ping Thong. 2010. "Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities" Pharmaceuticals 3, no. 5: 1507-1529. https://doi.org/10.3390/ph3051507
APA StyleOlivo, M., Bhuvaneswari, R., Lucky, S. S., Dendukuri, N., & Soo-Ping Thong, P. (2010). Targeted Therapy of Cancer Using Photodynamic Therapy in Combination with Multi-faceted Anti-Tumor Modalities. Pharmaceuticals, 3(5), 1507-1529. https://doi.org/10.3390/ph3051507



