The Impact of CYP2D6 Genotyping on Tamoxifen Treatment
Abstract
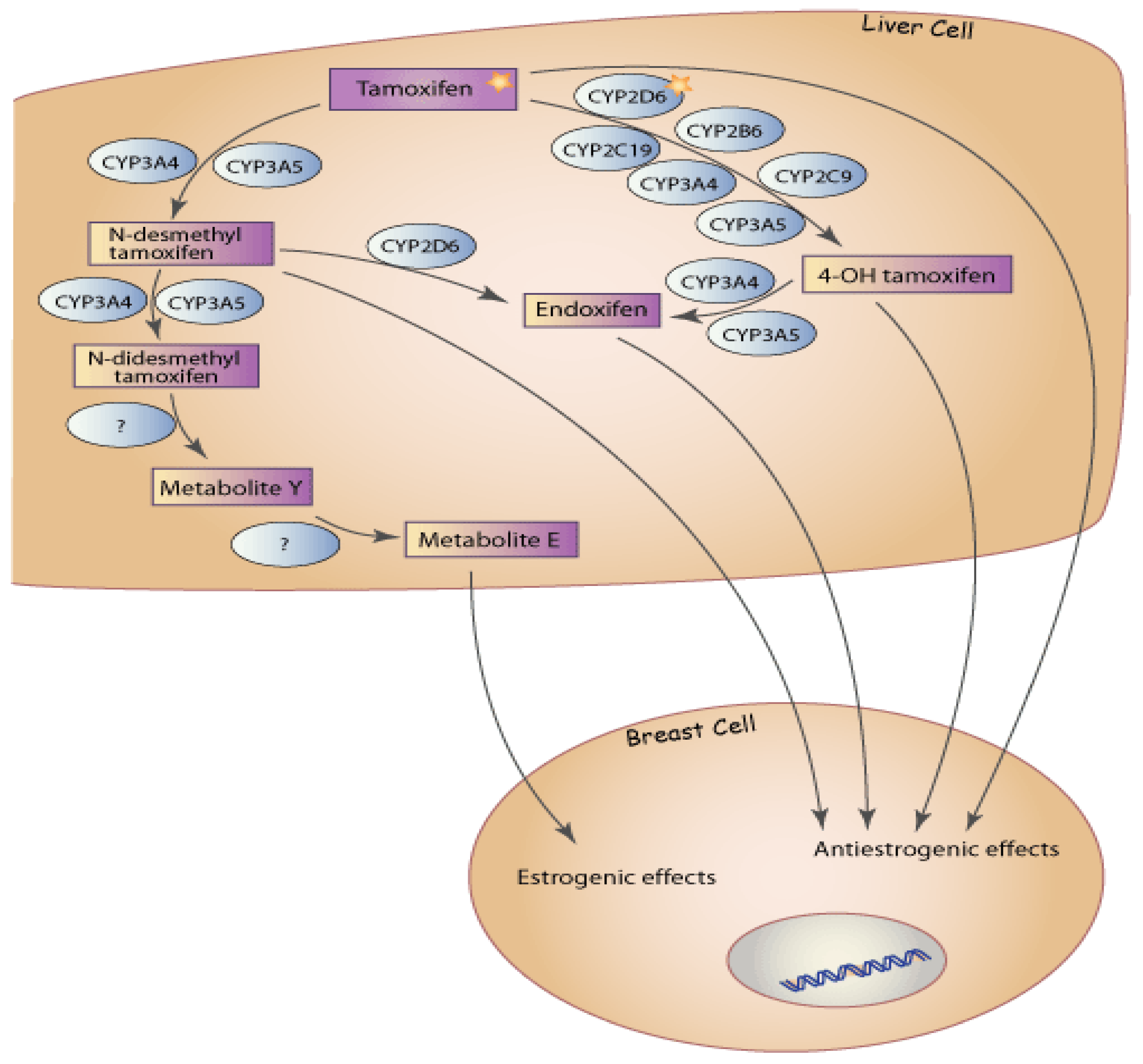
:1. Introduction
2. CYP2D6 Genotype and Tamoxifen Metabolism
| CYP2D6 Alleles | Predicted Enzyme Activity |
|---|---|
| *1, *2, *33 and *35 | Normal |
| *3,*4,*5,*6,*7,*8,*11-*16, *18, *19,*20,*21, *36, *38,*40, *42, *44, *56, *62, 4xn, 6xn and 36xn | None |
| *9,*10, *17, *29, *41, *69, 29xn and 41xn | Reduced |
| *22,*23-*28, *30-*32, *34,*37,*39, *43,*45-*68, *70-*75 | Unknown |
| *1xn, *2xn,*35xn | Increased |
3. CYP2D6 Genotype and Tamoxifen Therapy
4. CYP2D6 Genotype in Non-Caucasian Tamoxifen Treated Breast Cancer Patients
5. Tamoxifen Side Effects and CYP2D6
| Author | Patients | Origin of study | CYP2D6 alleles typed | Median Follow up (years) | CYP2D6 inhibitors in PM definition | Comparison | Main Results |
|---|---|---|---|---|---|---|---|
| Goetz et al. 2005 [21] | N=190 ER+ve Postmenopausal TAM only | American | *4, *6 | 11.4 | No | PM vs. EM + hetEM | DFS HR, 1.86; P = 0.089 RFS HR, 1.85; P= 0.176 OS HR, 1.12; P= 0.780 |
| Goetz et al. 2007 [43] | Same as [21] | American | *4, *6 | 11.4 | Yes | PM vs. EM + IM + hetEM | RFS HR, 1.74; P = 0.017 TTBR HR, 1.91; P=0.034 DFS HR, 1.60; P= 0.027 OS HR, 1.34; P= 0.223 |
| Goetz et al. 2008* [22] | Same as [21] | American | *3, *4, *6, *10, *17, *41 | 14.5 | Yes | PM vs. EM | TTR HR 4.0, P=0.001 DFS HR 2.0, P=0.02 |
| Schroth et al. 2007 [23] | N=206 ER+ve TAM only | German | *4, *5, *10, *41 | 5.9 | No | hetEM + IM+PM vs. EM | RFT HR 2.24; P = 0.02 EFS HR 1.89; P = 0.02 |
| Newman et al. 2008 [24] | N=115 Familial breast cancer ER+ and -ve Adj TAM Some received CT | British | *3,*4,*5, *41 | 10 | Yes | PM vs. EM+ hetEM | TTR HR, 2.1; P = 0.14 OS HR, 2.5; P = 0.17 BRCA2 patients: TTR HR, 3.8; P = 0.083 OS HR, 9.7; P = 0.008 |
| Bijl et al. 2009 [25] | N=85Adj TAM | Dutch | *4 | Not available | Yes | PM vs. EM | BCM HR, 4.0; P = 0.025 |
| Gonzalez-Santiago et al. 2007 * [26] | N=84 Adj TAM | Spanish | *4 | 5.5 | No | hetEM + PM vs. EM | RFS HR, 2.82; P = 0.05 |
| Ramon et al. 2009 [27] | N=91 ER+ve Adj TAM Some received CT | Spanish | Amplichip 33 alleles | 9 | No | *4/*4,*4/*4, *1/*5 and*2/*5 vs. the remaining genotypes | DFS HR not available, P = 0.016 |
| Nowell et al. 2005 [28] | N=162 Adj TAM Some received CT | American | *3,*4,*6 | Not available | No | PM + hetEM vs. EM | OS HR, 0.77; P = 0.51 PFS HR, 0.67; P= 0.19 |
| Wegman et al. 2005 [29] | N=112 ER + and -ve Some received CT Some received TAM | Sweden | *4 | 10.7 | No | Not applicable | Carriers of the CYP2D6*4 allele demonstrated a decreased risk of recurrence when treated with TAM(relative risk, 0.28; P =0.0089) |
| Wegman et al. 2007 [30] | N=677 ER+ve Postmenopausal Some received CT, different dose-different duration of TAM | Sweden | *4 | 7.3 | No | PM vs.. EM + hetEM | RFS HR, <1; P = 0.055 |
| Schroth et al. 2009 [31] | N=1325 TAM only | German / American | *3,*4,*5, *10,*41 | 6.3 | No | hetEM+IM +PM vs. EM PM vs. EM | EFS HR, 1.33; P=0.01 DFS HR, 1.29; P=0.02 TTR HR 1.90; p=0.02 |
| Thompson et al. 2009* [32] | N=618 ER+ve Adj TAM Some received CT | British | Amplichip 33 alleles | 5.6 | No | hetEM+ IM +PM vs. EM | RFS HR 1.52, P=0.06 Postmenopausal, TAM only patients: RFS HR, 1.96; P=0.036 |
| Kitoyani et al. 2008 [34] | N=67 TAM only ER+ve | Japanese | *4, *5, *6, *10, *14, *18, *21, *41 | 8 | No | IM vs. EM | RFS HR 10.04, P = 0.036 |
| Xu et al. 2008 [35] | N=152 Adj TAM Some received CT No eligible women were taking CYP2D6 inhibitors | Chinese | *10 | 5.2 | - | IM vs. EM + hetEM | DFS HR 4.7, P=0.04 |
| Okishiro et al 2009 [36] | N=173 ER+ve Adj TAM Some received CT and goserelin | Japanese | *10 | 4.6 | No | IM vs. EM+ hetEM | RFS HR 0.6, P= 0.39 |
| Kitoyani et al. 2010 [37] | N=282 TAM only 67 patients reported previously [37] | Japanese | *4, *5, *6, *10, *14, *18, *21, *36, *41 | 7.1 | No | IM +PM vs. EM | RFS HR 9.52, P=.000036 |
6. CYP2D6 Inhibitors and Tamoxifen Therapy
7. Complexity of Tamoxifen Metabolism
8. Conclusions and Discussion
Acknowledgements
References
- Seruga, B.; Tannock, I.F. Up-front use of aromatase inhibitors as adjuvant therapy for breast cancer: the emperor has no clothes. J. Clin. Oncol. 2009, 27, 840–842. [Google Scholar]
- Hughes-Davies, L.; Caldas, C.; Wishart, G.C. Tamoxifen: the drug that came in from the cold. Br. J. Cancer 2009, 101, 875–878. [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005, 365, 1687–1717. [CrossRef] [PubMed]
- Jin, Y.; Desta, Z.; Stearns, V.; Ward, B.; Ho, H.; Lee, K.H.; Skaar, T.; Storniolo, A.M.; Li, L.; Araba, A.; Blanchard, R.; et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl. Cancer Inst. 2005, 97, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z.; Ward, B.A.; Soukhova, N.V.; Flockhart, D.A. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J. Pharmacol. Exp. Ther. 2004, 310, 1062–1075. [Google Scholar]
- Jordan, V.C.; Collins, M.M.; Rowsby, L; Prestwich, G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J. Endocrinol. 1977, 75, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Zuo, H.; Lee, K.H.; Trebley, J.P.; Rae, J.M.; Weatherman, R.V.; Desta, Z.; Flockhart, D.A.; Skaar, T.C. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res. Treat. 2004, 85, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.C.; Desta, Z.; Flockhart, D.A.; Skaar, T.C. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother. Pharmacol. 2005, 55, 471–478. [Google Scholar]
- Lim, Y.C.; Li, L.; Desta, Z.; Zhao, Q.; Rae, J.M.; Flockhart, D.A.; Skaar, T.C. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J. Pharmacol. Exp. Ther. 2006, 318, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Stearns, V.; Johnson, M.D.; Rae, J.M.; Morocho, A.; Novielli, A.; Bhargava, P.; Hayes, D.F.; Desta, Z; Flockhart, D.A. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer Inst. 2003, 95, 1758–1764. [Google Scholar] [PubMed]
- Wu, X.; Hawse, J.R.; Subramaniam, M.; Goetz, M.P.; Ingle, J.N.; Spelsberg, T.C. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009, 69, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Zanger, U.M.; Turpeinen, M.; Klein, K.; Schwab, M. Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal. Bioanal. Chem. 2008, 392, 1093–1108. [Google Scholar]
- Brauch, H.; Mürdter, T.E.; Eichelbaum, M.; Schwab, M. Pharmacogenomics of tamoxifen therapy. Clin. Chem. 2009, 55, 1770–1782. [Google Scholar]
- Bradford, L.D. CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants. Pharmacogenomics 2002, 3, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M. Genetic polymorphisms of cytochrome P450 2d6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005, 5, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Antona, C.; Ingelman-Sundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Lim, H.S.; Ju, Lee H.; Seok Lee, K.; Sook Lee, E.; Jang, I.J.; Ro, J. Clinical implications of CYP2D6 genotypes predictive of tamoxifen pharmacokinetics in metastatic breast cancer. J. Clin. Oncol. 2007, 25, 3837–3845. [Google Scholar] [PubMed]
- Borges, S.; Desta, Z.; Li, L.; Skaar, T.C.; Ward, B.A.; Nguyen, A.; Jin, Y.; Storniolo, A.M.; Nikoloff, D.M.; Wu, L.; et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin. Pharmacol. Ther. 2006, 80, 61–74. [Google Scholar] [CrossRef]
- Stearns, V.; Johnson, M.D.; Rae, J.M.; Morocho, A.; Novielli, A.; Bhargava, P.; Hayes, D.F.; Desta, Z.; Flockhart, D.A. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J. Natl. Cancer. Inst. 2003, 95, 1758–1764. [Google Scholar]
- Goetz, M.P.; Rae, J.M.; Suman, V.J.; Safgren, S.L.; Ames, M.M.; Visscher, D.W.; Reynolds, C.; Couch, F.J.; Lingle, W.L.; Flockhart, D.A.; et al. Pharmacogenetics of tamoxifen biotransformation is associated with clinical outcomes of efficacy and hot flashes. J. Clin. Oncol. 2005, 23, 9312–9318. [Google Scholar] [PubMed]
- Goetz, M.P.; Suman, V.; Ames, M.; Black, J.; Safgren, S.; Kuffel, M.; Avula, R.; Moyer, A.; Weinshilboum, R.; Reynolds, C.; Perez, E.; Ingle, J. Tamoxifen pharmacogenetics of CYP2D6, CYP2C19, and SULT1A1: long term follow-up of the North Central Cancer Treatment Group 89-30-52 adjuvant trial. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, Texas, USA, 10-14 December 2008. Abstract 6037.
- Schroth, W.; Antoniadou, L.; Fritz, P.; Schwab, M.; Muerdter, T.; Zanger, U.M.; Simon, W.; Eichelbaum, M.; Brauch, H. Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J. Clin. Oncol. 2007, 25, 5187–5193. [Google Scholar]
- Newman, W.G.; Hadfield, K.D.; Latif, A.; Roberts, S.A.; Shenton, A.; McHague, C.; Lalloo, F.; Howell, S.; Evans, D.G. Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin. Cancer Res. 2008, 14, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Bijl, M.J.; van Schaik, R.H.; Lammers, L.A.; Hofman, A.; Vulto, A.G.; van Gelder, T.; Stricker, B.H.; Visser, L.E. The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res. Treat. 2009, 118, 125–130. [Google Scholar]
- Gonzalez-Santiago, S.; Zárate, R.; Haba-Rodríguez, J.; Gómez, A.; Bandrés, E.; Moreno, S.; Borrega, P.; García-Foncillas, J.; Aranda, E. CYP2D6*4 polymorphism as blood predictive biomarker of breast cancer relapse in patients receiving adjuvant tamoxifen. J. Clin. Oncol. 2007, 25 (18S). abstract 590. ASCO Annual Meeting Proceedings Part I.. [Google Scholar]
- Ramón, y Cajal, T.; Altés, A.; Paré, L.; del Rio, E.; Alonso, C.; Barnadas, A.; Baiget, M. Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res. Treat. 2010, 119, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Nowell, S.A.; Ahn, J.; Rae, J.M.; Scheys, J.O.; Trovato, A.; Sweeney, C.; MacLeod, S.L.; Kadlubar, F.F.; Ambrosone, C.B. Association of genetic variation in tamoxifen-metabolizing enzymes with overall survival and recurrence of disease in breast cancer patients. Breast Cancer Res. Treat. 2005, 91, 249–258. [Google Scholar]
- Wegman, P.; Vainikka, L.; Stål, O.; Nordenskjöld, B.; Skoog, L.; Rutqvist, L.E.; Wingren, S. Genotype of metabolic enzymes and the benefit of tamoxifen in postmenopausal breast cancer patients. Breast Cancer Res. 2005, 7, R284–R290. [Google Scholar] [CrossRef] [PubMed]
- Wegman, P.; Elingarami, S.; Carstensen, J.; Stål, O.; Nordenskjöld, B.; Wingren, S. Genetic variants of CYP3A5, CYP2D6, SULT1A1, UGT2B15 and tamoxifen response in postmenopausal patients with breast cancer. Breast Cancer Res. 2007, 9, R7. [Google Scholar] [CrossRef] [PubMed]
- Schroth, W.; Goetz, M.P.; Hamann, U.; Fasching, P.A.; Schmidt, M.; Winter, S.; Fritz, P.; Simon, W.; Suman, V.J.; Ames, M.M.; et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 2009, 302, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Quinlan, P.; Bray, S.; Johnson, A.; Nikoloff, M.; Fontecha, M.; Ferraldeschi, R.; Howell, A.; Lawrence, J.; Newman, W. CYP2D6 genotype affects outcome in postmenopausal breast cancer patients treated with tamoxifen monotherapy. In Proceedings of the ASCO Breast Cancer Symposium, San Francisco, California, USAr, 8-10 October, 2009. Abstract 35.
- Goetz, M.P.; Berry, D.A.; Klein, T.E. Adjuvant Tamoxifen Treatment Outcome According to Cytochrome P450 2D6 (CYP2D6) Phenotype in Early Stage Breast Cancer: Findings from the International Tamoxifen Pharmacogenomics Consortium. In Proceedings of the San Antonio Breast Cancer Symposium; San Antonio, Texas, USA: December 9-13, 2009. Abstract 33. [Google Scholar]
- Kiyotani, K.; Mushiroda, T.; Sasa, M.; Bando, Y.; Sumitomo, I.; Hosono, N.; Kubo, M.; Nakamura, Y.; Zembutsu, H. Impact of CYP2D6*10 on recurrence-free survival in breast cancer patients receiving adjuvant tamoxifen therapy. Cancer Sci. 2008, 99, 995–999. [Google Scholar]
- Xu, Y.; Sun, Y.; Yao, L.; Shi, L.; Wu, Y.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; et al. Association between CYP2D6*10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann. Oncol. 2008, 19, 1423–1429. [Google Scholar] [PubMed]
- Okishiro, M.; Taguchi, T.; Jin Kim, S.; Shimazu, K.; Tamaki, Y.; Noguchi, S. Genetic polymorphisms of CYP2D6 10 and CYP2C19 2, 3 are not associated with prognosis, endometrial thickness, or bone mineral density in Japanese breast cancer patients treated with adjuvant tamoxifen. Cancer 2009, 115, 952–961. [Google Scholar] [PubMed]
- Kitoyani, K.; Mushiroda, T.; Imamura, C.K.; Hosono, N.; Tsunoda, T.; Kubo, M.; Tanigawara, Y.; Flockhart, D.A.; Desta, Z.; Skaar, T.C.; et al. Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J. Clin. Oncol. 2010. [Epub ahead of print]. [Google Scholar]
- Rae, J.M.; Sikora, M.J.; Henry, N.L.; Li, L.; Kim, S.; Oesterreich, S.; Skaar, T.C.; Nguyen, A.T.; Desta, Z.; Storniolo, A.M.; et al. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J. 2009, 9, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Sloan, J.; Stearns, V.; Slack, R.; Iyengar, M.; Diekmann, B.; Kimmick, G.; Lovato, J.; Gordon, P.; Pandya, K.; et al. Newer antidepressants and gabapentin for hot flashes: an individual patient pooled analysis. J. Clin. Oncol. 2009, 27, 2831–2837. [Google Scholar] [PubMed]
- Kimmick, G.G.; Lovato, J.; McQuellon, R.; Robinson, E.; Muss, H.B. Randomized, double-blind, placebo-controlled, crossover study of sertraline (Zoloft) for the treatment of hot flashes in women with early stage breast cancer taking tamoxifen. Breast J. 2006, 12, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Kugler, J.W.; Sloan, J.A.; Mailliard, J.A.; LaVasseur, B.I.; Barton, D.L.; Novotny, P.J.; Dakhil, S.R.; Rodger, K; Rummans, T.A.; Christensen, B.J. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 2000, 356, 2059–2063. [Google Scholar] [PubMed]
- Loprinzi, C.L.; Sloan, J.A.; Perez, E.A.; Quella, S.K.; Stella, P.J.; Mailliard, J.A.; Halyard, M.Y.; Pruthi, S; Novotny, P.J.; Rummans, T.A. Phase III evaluation of fluoxetine for treatment of hot flashes. J. Clin. Oncol. 2002, 20, 1578–1583. [Google Scholar] [PubMed]
- Goetz, M.P.; Knox, S.K.; Suman, V.J.; Rae, J.M.; Safgren, S.L.; Ames, M.M.; Visscher, D.W.; Reynolds, C; Couch, F.J.; Lingle, W.L.; et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res. Treat. 2007, 101, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ahern, T.P.; Pedersen, L.; Cronin-Fenton, D.P.; Sørensen, H.T.; Lash, T.L. No increase in breast cancer recurrence with concurrent use of tamoxifen and some CYP2D6-inhibiting medications. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 2562–2564. [Google Scholar]
- Dezentje, V.; van Blijderveen, N.J.; Gelderblom, H; Putter, H.; Van Herk-Sukel, M.P.; Casparie, M.K.; Egberts, A.C.; Nortier, J.W.; Guchelaar, H.J. Concomitant CYP2D6 inhibitor use and tamoxifen adherence in early-stage breast cancer: A pharmacoepidemiological study. J. Clin. Oncol. 2009, 27 (18S). CRA509. ASCO Annual Meeting Proceedings Part I.. [Google Scholar]
- Holzman, D. Tamoxifen, antidepressants, and CYP2D6: the conundrum continues. J. Natl. Cancer Inst. 2009, 101, 1370–1371. [Google Scholar] [CrossRef] [PubMed]
- Coller, J.K.; Krebsfaenger, N.; Klein, K.; Endrizzi, K.; Wolbold, R.; Lang, T.; Nüssler, A.; Neuhaus, P.; Zanger, U.M.; Eichelbaum, M.; Mürdter, T.E. The influence of CYP2B6, CYP2C9 and CYP2D6 genotypes on the formation of the potent antioestrogen Z-4-hydroxy-tamoxifen in human liver. Br. J. Clin. Pharmacol. 2002, 54, 157–167. [Google Scholar]
- Falany, C.N.; Wheeler, J.; Oh, T.S.; Falany, J.L. Steroid sulfation by expressed human cytosolic sulfotransferases. J. Steroid. Biochem. Mol. Biol. 1994, 48, 369–375. [Google Scholar]
- Nowell, S.; Falany, C.N. Pharmacogenetics of human cytosolic sulfotransferases. Oncogene 2006, 25, 1673–1678. [Google Scholar]
- Ogura, K.; Ishikawa, Y.; Kaku, T.; Nishiyama, T.; Ohnuma, T.; Muro, K.; Hiratsuka, A. Quaternary ammonium-linked glucuronidation of trans-4-hydroxytamoxifen, an active metabolite of tamoxifen, by human liver microsomes and UDP-glucuronosyltransferase 1A4. Biochem. Pharmacol. 2006, 71, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sharma, A.K.; Dellinger, R.W.; Blevins-Primeau, A.S.; Balliet, R.M.; Chen, G.; Boyiri, T.; Amin, S.; Lazarus, P. Glucuronidation of active tamoxifen metabolites by the human UDP glucuronosyltransferases. Drug Metab. Dispos. 2007, 35, 2006–2014. [Google Scholar]
- Nishiyama, T.; Ogura, K.; Nakano, H.; Ohnuma, T.; Kaku, T.; Hiratsuka, A.; Muro, K.; Watabe, T. Reverse geometrical selectivity in glucuronidation and sulfation of cis- and trans-4-hydroxytamoxifens by human liver UDP-glucuronosyltransferases and sulfotransferases. Biochem. Pharmacol. 2002, 63, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.M.; Carey, L.A.; McLeod, H.L. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat. Rev. Cancer 2009, 9, 576–586. [Google Scholar]
- Tan, S.H.; Lee, S.C.; Goh, B.C.; Wong, J. Pharmacogenetics in breast cancer therapy. Clin. Cancer Res. 2008, 14, 8027–8041. [Google Scholar]
- Ntukidem, N.I.; Nguyen, A.T.; Stearns, V.; Rehman, M.; Schott, A.; Skaar, T.; Jin, Y.; Blanche, P.; Li, L.; Lemler, S.; et al. Estrogen receptor genotypes, menopausal status, and the lipid effects of tamoxifen. Clin. Pharmacol. Ther. 2008, 83, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; McCarty, C.A.; Wilke, R.A.; Glurich, I.; Engel, J.M.; Flockhart, D.A.; Nguyen, A.; Li, L; Mi, D; Skaar, T.C.; Jin, Y. Estrogen receptor genotype is associated with risk of venous thromboembolism during tamoxifen therapy. Breast Cancer Res. Treat. 2009, 115, 643–650. [Google Scholar] [PubMed]
- Dezentje, V.O.; Guchelaar, H.J.; Nortier, J.W.; van de Velde, C.J.; Gelderblom, H. Clinical implications of CYP2D6 genotyping in tamoxifen treatment for breast cancer. Clin. Cancer Res. 2009, 15, 15–21. [Google Scholar]
- Lash, T.L.; Lien, E.A.; Sørensen, H.T.; Hamilton-Dutoit, S. Genotype-guided tamoxifen therapy: time to pause for reflection? Lancet Oncol. 2009, 10, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Higgins, M.J.; Rae, J.M.; Flockhart, D.A.; Hayes, D.F.; Stearns, V. Pharmacogenetics of tamoxifen: who should undergo CYP2D6 genetic testing? J. Natl. Comp. Canc. Netw. 2009, 7, 203–213. [Google Scholar]
- Dowsett, M.; Cuzick, J.; Ingle, J.; Coates, A.; Forbes, J.; Bliss, J.; Buyse, M.; Baum, M.; Buzdar, A.; Colleoni, M.; et al. Meta-Analysis of Breast Cancer Outcomes in Adjuvant Trials of Aromatase Inhibitors Versus Tamoxifen. J. Clin. Oncol. 2009, 28, 509–518. [Google Scholar] [PubMed]
- Gibson, L.; Lawrence, D.; Dawson, C.; Bliss, J. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst. Rev. 2009, (4), CD003370. [Google Scholar]
- Punglia, R.S.; Burstein, H.J.; Winer, E.P.; Weeks, J.C. Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. Natl. Cancer Inst. 2008, 100, 642–648. [Google Scholar]
- Goetz, M.; Ames, M.; Gnant, M.; Filpits, M; Jakesz, R.; Greil, R.; Marth, C.; Samonigg, H.; Suman, V.; Safgren, S.; Kuffel, M.; Weinshilboum, R.; Erlander, M.; Ma, X.J.; Ingle, J. Pharmacogenetic (CYP2D6) and gene expression profiles (HOXB13/IL17BR and molecular grade index) for prediction of adjuvant endocrine therapy benefit in the ABCSG 8 trial. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 10-14 December 2008. Abstract 57..
- Aubert, R.E.; Stanek, E.J.; Yao, J.; Teagarden, J.R.; Subar, M.; Epstein, R.S.; Skaar, T.C.; Desta, Z.; Flockhart, D.A. Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors. J. Clin. Oncol. 2009, 27 (18S). CRA508. ASCO Annual Meeting Proceedings Part I.. [Google Scholar]
- Kelly, C.M.; Juurlink, D.N.; Gomes, T.; Duong-Hua, M.; Pritchard, K.I.; Austin, P.C.; Paszat, L.F. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ 2010, 340, c693. [Google Scholar] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ferraldeschi, R.; Newman, W.G. The Impact of CYP2D6 Genotyping on Tamoxifen Treatment. Pharmaceuticals 2010, 3, 1122-1138. https://doi.org/10.3390/ph3041122
Ferraldeschi R, Newman WG. The Impact of CYP2D6 Genotyping on Tamoxifen Treatment. Pharmaceuticals. 2010; 3(4):1122-1138. https://doi.org/10.3390/ph3041122
Chicago/Turabian StyleFerraldeschi, Roberta, and William G. Newman. 2010. "The Impact of CYP2D6 Genotyping on Tamoxifen Treatment" Pharmaceuticals 3, no. 4: 1122-1138. https://doi.org/10.3390/ph3041122
APA StyleFerraldeschi, R., & Newman, W. G. (2010). The Impact of CYP2D6 Genotyping on Tamoxifen Treatment. Pharmaceuticals, 3(4), 1122-1138. https://doi.org/10.3390/ph3041122




