Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications
Abstract
:1. Introduction
2. Relevant Features of Staphylococci
| Classification | Relevant Features | Groups/Subclasses | Examples |
|---|---|---|---|
| Class I (lantibiotics) | Small, heat-stable peptides (<5 kDa), containing modified amino acids (lanthionine, 3-methyl-lanthionine, dehydrated amino acids, S-aminovinyl-cystein, among others) | Type A (linear) | Nisin, Pep5, Epidermin, |
| Type B (globular) | Mersacidin | ||
| Type C (two components) | Lacticin 3147 | ||
| Type D (reduced antimicrobial activity) | SapT | ||
| Class II | Small, heat-stable peptides (<10 kDa), containing no modified amino acids | IIa (linear; pediocin-like) | Pediocin PA-1 |
| IIb (linear; two components) | Lactacin F | ||
| IIc (cyclic peptides) | Enterocin AS-48 | ||
| IId (linear)* | Aureocin A53 | ||
| IIe (linear; more than two components) | Aureocin A70 | ||
| Class III | Large, heat-labile proteins | Type IIIa (bacteriolysins) | Lysostaphin |
| Type IIIb (non-lytic) | Helveticin J |
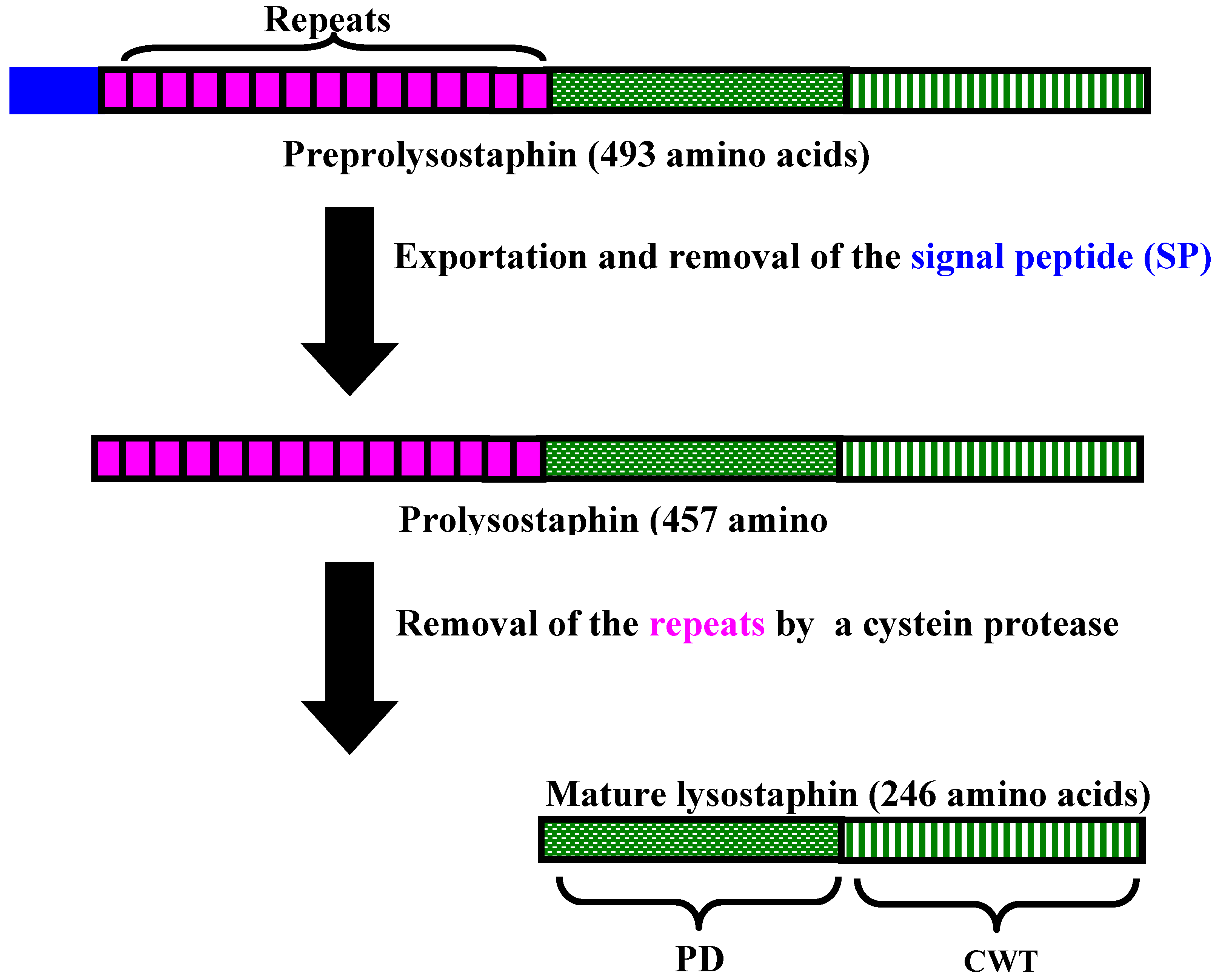
3. Lysostaphin General Features
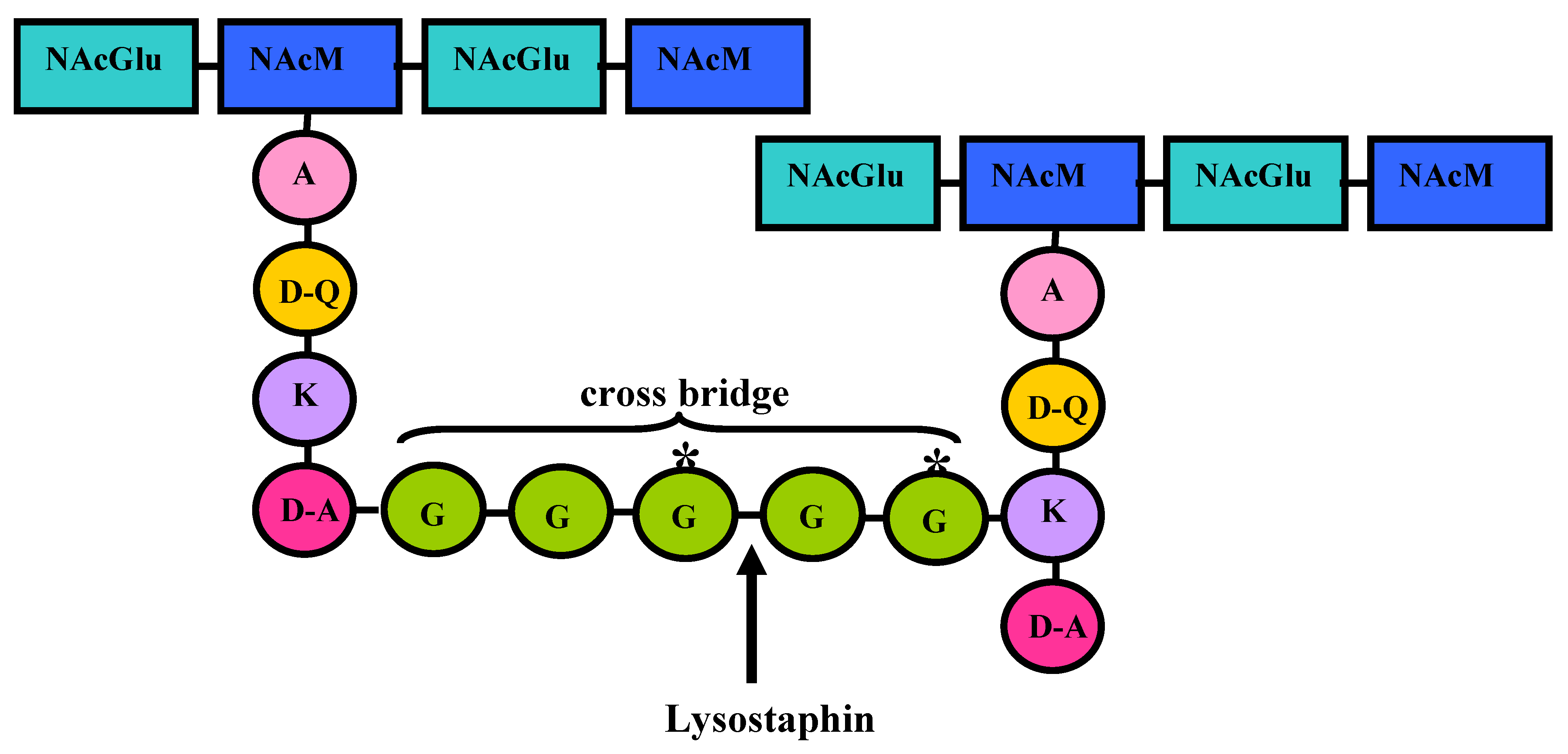
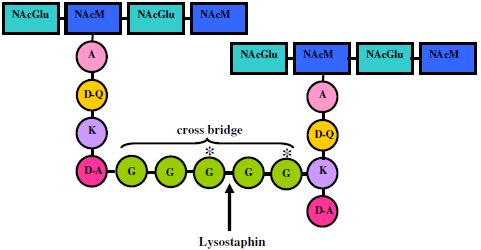
4. Lysostaphin mode of action
| Indicator Strains | Origin | Inhibition Zones |
|---|---|---|
| S. aureus | ||
| MB269, MB274, MB276, MB277, MB288, MB289, MB295, MB302, MB303 | ||
| Bovine mastitis; Brazil | +++ | |
| 2, 3, 7, 9, 10 | Bovine mastitis; Argentina | +++ |
| 3H1, 13H1 | Salad | +++ |
| 6H4, 13S2 | Salad | ++ |
| LI1, A70, A53 | Pasteurized milk | +++ |
| LF2, LIN4 | Sausage | +++ |
| Q2, QRFH1 | Cheese | +++ |
| S. carnosus* CN83 | Meat fermentation product | ++ |
| S. epidermidis* | ||
| CN69 | Blood | ++ (t) |
| CN72 | Blood | +++ |
| S. haemolyticus* | ||
| CN61 | Blood | ++ |
| CN68 | Blood | - |
| S. saprophyticus* | ||
| CN86 | Urine | ++ |
| CN88 | Fistula | ++ |
| S. hominis CN70* | Blood | ++ |
| S. simulans CN87* | Blood | +++ |
| S. xylosus CN93* | Skin | +++ |
| S. hyicus ATCC 11249* | - | +++ |
| S. intermedius ATCC 29663 | - | ++ |

5. Lysostaphin Potential Biotechnological Applications
5.1. Research Applications
5.2. Human Medical Use
5.2.1. In vitro studies
5.2.2. In vivo studies
5.2.3. Reduction of nasal carriage of staphylococci
5.3. Veterinary Use
6. Development of Resistance to Lysostaphin: A Possibility
7. Conclusions
Acknowledgements
References
- Heng, N.C.K.; Wescombe, P.A.; Burton, J.P.; Jack, R.W.; Tagg, J.R. The diversity of bacteriocins in Gram-positive bacteria. In Bacteriocins: Ecology and Evolution; Riley, M.A., Chavan, M.A., Eds.; Springer: New York, NY, USA, 2007; pp. 45–92. [Google Scholar]
- Bierbaum, G.; Sahl, H.-G. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr. Pharm. Biotechnol. 2009, 10, 2–18. [Google Scholar]
- Nissen-Meyer, J.; Rogne, P.; Oppegård, C.; Haugen, H.S.; Kristiansen, P.E. Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by Gram-positive bacteria. Curr. Pharm. Biotechnol. 2009, 10, 10–37. [Google Scholar]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar]
- Gálvez, A.; Abriouel, H.; Lopez, R.L; Omar, N.B. Bacteriocin-based strategies for food preservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.C.F.; Ceotto, H.; Coelho, M.L.V.; Nascimento, J.S. Staphylococcal antimicrobial peptides: relevant properties and potential biotechnological applications. Curr. Pharm. Biotechnol. 2009, 10, 38–61. [Google Scholar]
- Euzéby, J.P. List of prokaryotic names with standing in nomenclature—Genus. Staphylococcus. Available online: http://www.bacterio.cict.fr accessed April 2010.
- Bannerman, T.L.; Peacock, S.J. Staphylococcus, Micrococcus, and other catalase-positive cocci. In Manual of Clinical Microbiology; Murray, P.R., Baron, E.J., Jorgensen, J.H., Landry, M.L., Pfaller, M.A., Eds.; ASM Press: Washington D.C., USA, 2007; pp. 384–404. [Google Scholar]
- Casey, A.L.; Lambert, P.A.; Elliott, T.S.J. Staphylococci. J. Antimicrob. Agents 2007, 29 (Suppl. 3), S23–S32. [Google Scholar]
- Evans, H.L.; Saywer, R.G. Cycling chemotherapy: a promising approach to reducing the morbidity and mortality of nosocomial infections. Drugs Today 2003, 39, 733–738. [Google Scholar]
- Rogers, K.L.; Fey, P.D.; Rupp, M.E. Coagulase-negative staphylococcal infections. Infect. Dis. Clin. N. Am. 2009, 23, 73–98. [Google Scholar]
- Schindler, C.A.; Schuhardt, V.T. Lysostaphin: A new bacteriolytic agent for the Staphylococcus. Proc. Natl. Acad. Sci. USA 1964, 51, 414–421. [Google Scholar] [CrossRef]
- Ehlert, K.; Schrodr, W.; Labischinski, H. Specificities of FemA and FemB for different glycine residues: FemB cannot substitute for FemA in staphylococcal peptidoglycan pentaglycine side chain formation. J. Bacteriol. 1997, 179, 7573–7576. [Google Scholar]
- Rohrer, S.; Ehlert, K.; Tschierske, M.; Labischinski, H.; Berger-Bächi, B. The essential Staphylococcus aureus gene fmhB is involved in the first step of peptidoglycan pentaglycine interpeptide formation. Proc. Natl. Acad. Sci. USA. 1999, 96, 9351–9356. [Google Scholar]
- Kumar, J.K. Lysostaphin: an antistaphylococcal agent. Appl. Microbiol. Biotechnol. 2008, 80, 555–561. [Google Scholar]
- Giesbrecht, P.; Wecke, J.; Recnicke, B. On the morphogenesis of the cell wall of staphylococci. Int. Rev. Cytol. 1976, 44, 225–318. [Google Scholar]
- Joris, B.; Englebert, S.; Chu, C.P.; Kariyama, R.; Daneo-Moore, L.; Shockman, G.D.; Ghuysen, J.M. Modular design of the Enterococcus hirae muramidase-2 and Streptococcus autolysis. FEMS Microbiol. Lett. 1992, 91, 257–264. [Google Scholar] [CrossRef]
- Browder, H.P.; Zygmunt, W.A.; Young, J.R.; Tavormina, P.A. Lysostaphin: enzymatic mode of action. Biochem. Biophys. Res. Commun. 1965, 19, 383–389. [Google Scholar]
- Trayer, H.R.; Buckley III, C.E. Molecular properties of lysostaphin, a bacteriolytic agent specific for Staphylococcus aureus. J. Biol. Chem. 1970, 245, 4842–4846. [Google Scholar] [PubMed]
- Heinrich, P.; Rosenstein, R.; Bohmer, M.; Sonner, P.; Götz, F. The molecular organization of the lysostaphin gene and its sequences repeated in tandem. Mol. Gen. Genet. 1987, 209, 563–569. [Google Scholar]
- Thumm, G.; Götz, F. Studies on prolysostaphin processing and characterization of the lysostaphin immunity factor (Lif) of Staphylococcus simulans biovar staphylolyticus. Mol. Microbiol. 1997, 23, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, R.S.; Simpson, W.J.; Tagg, J.R. Cloning and sequence analysis of zooA, a Streptococcus zooepidemicus gene encoding a bacteriocin-like inhibitory substance having a domain structure similar to that of lysostaphin. Gene 1997, 189, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Schneewindt, O. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 1996, 15, 4789–4797. [Google Scholar] [PubMed]
- Gründling, A.; Schneewind, O. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J. Bacteriol. 2006, 188, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Recsei, P.A.; Gruss, A.D.; Novick, R.P. Cloning, sequence, and expression of the lysostaphin gene from Staphylococcus simulans. Proc. Natl. Acad. Sci. USA 1987, 84, 1127–1131. [Google Scholar] [CrossRef]
- Larrimore, S.A.; Clark, S.B.; Robinson, J.M.; Heath, H.E.; Sloan, G.L. Coordinate production of three exoenzymes by Staphylococcus staphylolyticus. J. Gen. Microbiol. 1982, 128, 1529–1535. [Google Scholar] [PubMed]
- Heath, S.L.; Heath, H.E.; Sloan, G.L. Plasmid-encoded lysostaphin endopeptidase gene of Staphylococcus simulans biovar staphylolyticus. FEMS Microbiol. Lett. 1987, 44, 129–133. [Google Scholar] [CrossRef]
- Heath, H.E.; Heath, S.L.; Nitterauer, J.D.; Rose, K.E.; Sloan, G.L. Plasmid-encoded lysostaphin endopeptidase resistance of Staphylococcus simulans biovar staphylolyticus. Biochem. Biophys. Res. Commun. 1989, 160, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.M.; Bramley, A.J.; Lax, A.J. Expression of the lysostaphin gene of Staphylococcus simulans in a eukaryotic system. Appl. Environ. Microbiol. 1994, 60, 771–776. [Google Scholar] [PubMed]
- Mierau, I.; Leij, P.; van Swam, I.; Blommestein, B.; Floris, E.; Mond, J.; Smid, E.J. Industrial scale production and purification of a heterologous protein in Lactococcus lactis using the nisin-controlled gene expression system NICE: The case of lysostaphin. Microb. Cell Fact. 2005, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Mierau, I.; Olieman, C.; Mond, J.; Smid, E.J. Optimization of the Lactococcus lactis nisin-controlled gene expression system NICE for industrial applications. Microb. Cell Fact. 2005, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Sugai, M.; Fujiwara, T.; Akiyama, T.; Ohara, M.; Komatsuzawa, H.; Inoue, S.; Suginaka, H. Purification and molecular characterization of glycylglycine endopeptidase produced by Staphylococcus capitis. J. Bacteriol. 1997, 179, 1193–1202. [Google Scholar] [PubMed]
- Sugai, M.; Fujiwara, T.; Ohta, K.; Komatsuzawa, H.; Ohara, M.; Suginaka, H. epr, which encodes glycylglycine endopeptidase resistance, is homologous to femAB and affects serine content of peptidoglycan cross bridges in Staphylococcus capitis and Staphylococcus aureus. J. Bacteriol. 1997, 179, 4311–4318. [Google Scholar] [PubMed]
- King, B.F.; Biel, M.L.; Wilkinson, B.J. Facile penetration of the Staphylococcus aureus capsule by lysostaphin. Infect. Immun. 1980, 29, 892–896. [Google Scholar] [PubMed]
- Schindler, C.A.; Schuhardt, V.T. Purification and properties of lysostaphin—A lytic agent for Staphylococcus aureus. Biochem. Biophys. Acta 1965, 97, 242–250. [Google Scholar]
- Zygmunt, W.A.; Harrison, E.F.; Browder, H.P.; Tavormina, P.A. Comparative inhibition of methicillin-resistant strains of Staphylococcus aureus by lysostaphin and other antibiotics. Appl. Microbiol. 1968, 16, 1174–1178. [Google Scholar] [PubMed]
- Zygmunt, W.A.; Browder, H.P.; Tavormina, P.A. Susceptibility of coagulase-negative staphylococci to lysostaphin ant other antibiotics. Appl. Microbiol. 1968, 16, 1168–1173. [Google Scholar]
- Grüdling, A.; Schneewind, O. Cross-linked peptidoglycan mediates lysostaphin binding to the cell wall envelope of Staphylococcus aureus. J. Bacteriol. 2006, 188, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar]
- Schleifer, K.H.; Fisher, U. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int. J. Syst. Bacteriol. 1982, 32, 153–156. [Google Scholar] [CrossRef]
- Schneewindt, O.; Fowler, A.; Faull, K.F. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 1995, 268, 103–106. [Google Scholar] [PubMed]
- Francius, G.; Domenech, O.; Mingeot-Leclercq, M.P.; Dufrêne, Y.F. Direct observation of Staphylococcus aureus cell wall digestion by lysostaphin. J. Bacteriol. 2008, 190, 7904–7909. [Google Scholar] [CrossRef] [PubMed]
- Patron, R.L.; Climo, M.W.; Goldstein, B.P.; Archer, G.L. Lysostaphin treatment of experimental aortic valve endocarditis caused by a Staphylococcus aureus isolate with reduced susceptibility to vancomycin. Antimicrob. Agents Chemother. 1999, 43, 1754–1755. [Google Scholar] [PubMed]
- Fimland, G.; Blingsmo, R.; Sletten, K.; Jung, G.; Nes, I.F.; Nissen-Meyer, J. New biologically active hybrid bacteriocins constructed by combining regions from various pediocin-like bacteriocins: the C-terminal region is important for determining specificity. Appl. Environ. Microbiol. 1996, 62, 3313–3318. [Google Scholar]
- Kusuma, C.M.; Kokai-Kun, J.F. Comparison of four methods for determining lysostaphin susceptibility of various strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3256–3263. [Google Scholar] [PubMed]
- DeHart, H.; Heath, H.; Heath, L.; LeBlanc, P.; Sloan, G. The lysostaphin endopeptidase resistance gene (epr) specifies modification of peptidoglycan cross bridges in Staphylococcus simulans and Staphylococcus aureus. Appl. Environ. Microbiol. 1995, 61, 1475–1479. [Google Scholar] [PubMed]
- Ehlert, K.; Tschierske, M.; Mori, C.; Schöder, W.; Berger-Bächi, B. Site-specific serine incorporation by Lif and Epr into positions 3 and 5 of the staphylococcal peptidoglycan interpeptide bridge. J. Bacteriol. 2000, 182, 2635–2638. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.M.; Hardman, J.K.; Sloan, G.L. Relationship between lysostaphin endopeptidase production and cell wall composition of Staphylococcus aureus. J. Bacteriol. 1979, 137, 1158–1164. [Google Scholar] [PubMed]
- Klesius, P.H.; Schuhardt, V.Y. Use of lysostaphin in the isolation of highly polymerized deoxyribonucleic acid and in the taxonomy of aerobic Micrococcaceae. J. Bacteriol. 1968, 95, 739–743. [Google Scholar]
- Geary, C.; Stevens, M. Rapide lysostaphin test to differentiate Staphylococcus and Micrococcus species. J. Clin. Microbiol. 1986, 23, 1044–1045. [Google Scholar] [PubMed]
- Schaffner, W.; Melly, M.A.; Hash, J.H; Koenig, M.G. Lysostaphin: an enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J. Biol. Med. 1967, 39, 215–229. [Google Scholar] [PubMed]
- Walsh, S.; Shah, A; Mood, J. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob. Agents Chemother 2002, 47, 554–558. [Google Scholar]
- Zygmunt, W.A.; Tavormina, P.A. Lysostaphin: model for a specific enzymatic approach to infectious disease. Prog. Drug Res. 1972, 16, 309–333. [Google Scholar]
- Dajcs, J.J.; Thibodeaux, B.A.; Girgis, D.O.; Shaffer, M.D.; Delvisco, S.M.; O’Callaghan, R.J. Immunity to lysostaphin and its therapeutic value for ocular MRSA infections in the rabbit. Invest. Opthalmol. Visual Sci. 2002, 43, 3712–3716. [Google Scholar]
- Harrison, F.E.; Cropp, C.B. Comparative in vitro activities of lysostaphin and other antistaphylococcal antibiotics on clinical isolates of Staphylococcus aureus. Appl. Microbiol. 1965, 13, 212–215. [Google Scholar] [PubMed]
- Zygmunt, W.A.; Harrison, E.F.; Browder, H.P. Microbiological activities of lysostaphin and penicillins against bacteriophage 80/81 strains of Staphylococcus aureus. Appl. Microbiol. 1965, 13, 491–493. [Google Scholar] [PubMed]
- Huber, M.M.; Huber, T.W. Susceptibility of methicillin-resistant Staphylococcus aureus to lysostaphin. J. Clin. Microbiol. 1989, 27, 1122–1124. [Google Scholar] [PubMed]
- von Eiff, C.; Kokai-Kun, J.F.; Becker, K.; Peters, G. In vitro activity of recombinant lysostaphin against Staphylococcus aureus isolates from anterior nares and blood. Antimicrob. Agents Chemother. 2003, 47, 3613–3615. [Google Scholar] [CrossRef] [PubMed]
- Polak, J.; Della Latta, P.; Blackburn, P. In vitro activity of recombinant lysostaphin-antibiotic combinations toward methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 1993, 17, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Coote, P.J. Potent, synergistic inhibition of Staphylococcus aureus upon exposure to a combination of the endopeptidase lysostaphin and the cationic peptide ranalexin. J. Antimicrob. Chemother. 2007, 59, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Sax, H.; Gastmeier, P. The preventable proportion of nosocomial infections: an overview of published reports. J. Hosp. Infect. 2003, 54, 258–266. [Google Scholar]
- Vuong, C.; Otto, M. Staphylococcus epidermidis infections. Microbes Infect. 2002, 4, 481–489. [Google Scholar] [CrossRef]
- Sader, H.S.; Jones, R.N.; Andrade-Baiocchi, S.; Biedenbach, D.J. Four-year evolution of frequency of occurrence and antimicrobial susceptibility patterns of bacteria from bloodstream infections in Latin American medical centers. Diagn. Microbiol. Infect. Dis. 2003, 44, 273–280. [Google Scholar]
- Wu, J.A.; Kusuma, C.; Mond, J.J.; Kokai-Kun, J.F. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob. Agents Chemother. 2003, 47, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Mond, J.; Walsh, S. Lysostaphin-coated catheters eradicate Staphylococcus aureus challenge and block surface colonization. Antimicrob. Agents Chemother. 2004, 48, 2704–2707. [Google Scholar] [CrossRef] [PubMed]
- Schudhardt, V.T; Schindler, C.A. Lysostaphin therapy in mice infected with Staphylococcus aureus. J. Bacteriol 1964, 88, 815–816. [Google Scholar] [PubMed]
- Dixon, R.E.; Goodman, J.S.; Koenig, M.G. Lysostaphin: an enzymatic approach to staphylococcal disease. III. Combined lysostaphin-methicillin therapy of established staphylococcal abscesses in mice. Yale J. Biol. Med. 1968, 41, 62–68. [Google Scholar] [PubMed]
- Harrison, E.F.; Zygmunt, W.A. Lysostaphin in experimental renal infection. J. Bacteriol. 1967, 93, 520–524. [Google Scholar]
- Climo, M.W.; Patron, R.L.; Goldstein, B.P.; Archer, G.L. Lysostaphin treatment of experimental methicillin-resistant Staphylococcus aureus aortic valve endocarditis. Antimicrob. Agents Chemother. 1998, 42, 1355–1360. [Google Scholar] [PubMed]
- Kiri, N.; Archer, G.; Climo, M.W. Combinations of lysostaphin with β-lactams are synergistic against oxacillin-resistant Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2002, 46, 2017–2020. [Google Scholar] [CrossRef] [PubMed]
- Climo, M.W.; Ehlert, K.; Archer, G.L. Mechanism and suppression of lysostaphin resistance in oxacillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Placencia, F.X.; Kong, L.; Weisman, L.E. Treatment of methicillin-resistant Staphylococcus aureus in neonatal mice: lysostaphin vs. vancomycin. Pediatr. Res. 2009, 65, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Oluola, O.; Kong, L.; Fein, M.; Weisman, L.E. Lysostaphin in treatment of neonatal Staphylococcus aureus infection. Antimicrob. Agents Chemother. 2007, 51, 2198–2220. [Google Scholar] [CrossRef] [PubMed]
- Kokai-Kun, J.F.; Chanturiya, T.; Mond, J.J. Lysostaphin as a treatment for systemic Staphylococcus aureus infection in a mouse model. J. Antimicrob. Chemother. 2007, 60, 1051–1059. [Google Scholar] [PubMed]
- Kokai-Kun, J.F.; Chanturiya, T.; Mond, J.J. Lysostaphin eradicates established Staphylococcus aureus biofilms in jugular vein catheterized mice. J. Antimicrob. Chemother. 2009, 64, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kokai-Kun, J.F.; Walsh, S.M.; Chanturiya, T.; Mond, J.J. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob. Agents Chemother. 2003, 47, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Quickel, K.E., Jr.; Selden, R.; Caldwell, J.R.; Nora, N.F.; Schaffner, W. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl. Microbiol. 1971, 22, 446–450. [Google Scholar] [PubMed]
- Zhao, X.; Lacasse, P. Mammary tissue damage during bovine mastitis: causes and control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar]
- Bradley, A.J. Bovine mastitis: an evolving disease. Vet. J. 2002, 164, 116–128. [Google Scholar]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef] [PubMed]
- Sears, P.M.; Smith, B.S.; Pollak, J.; Gusik, S.N.; Blackburn, P. Lysostaphin efficacy for treatment of Staphylococcus aureus intramammary infection. J. Dairy Sci. 1988, 71 (Suppl. 1), 244. [Google Scholar]
- Oldham, E.R.; Daley, M.J. Lysostaphin: use of a recombinant bactericidal enzyme as a mastitis therapeutic. J. Dairy Sci. 1991, 74, 4175–4182. [Google Scholar]
- Daley, M.J.; Oldham, E.R. Lysostaphin immunogenicity of locally administered recombinant protein used in mastitis therapy. Vet. Immunol. Immunopathol. 1992, 31, 301–312. [Google Scholar]
- Wall, R.J.; Powell, A.M.; Paape, M.J.; Kerr, D.E.; Bannerman, D.D.; Pursel, V.G.; Wells, K.D.; Talbot, N; Hawk, H.W. Genetically enhanced cows resist intramammary Staphylococcus aureus infection. Nat. Biotechnol. 2005, 23, 445–451. [Google Scholar] [PubMed]
- Strandén, A.M.; Ehlert, K.; Labischinski, H.; Berger-Bächi, B. Cell wall monoglycine cross-bridges and methicillin hypersusceptibility in a femAB null mutant of methicillin-resistant Staphylococcus aureus. J. Bacteriol. 1997, 179, 9–16. [Google Scholar] [PubMed]
- Rohrer, S.; Berger-Bächi, B. FemABX peptidyl transferases: a link between branched-chain cell wall peptide formation and ß-lactam resistance in Gram-positive cocci. Antimicrob. Agents Chemother. 2003, 47, 837–846. [Google Scholar]
- Guignard, B.; Entenza, J.M.; Moreillon, P. Beta-lactams against methicillin-resistant Staphylococcus aureus. Curr. Opin. Pharmacol. 2005, 5, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Maruyama, A.; Inose, Y.; Higashide, M.; Hayashi, H.; Ohta, T. Overexpression of sigma factor, sigma (B), urges Staphylococcus aureus to thicken the cell wall and to resist beta-lactams. Biochem. Biophys. Res. Commun. 2001, 288, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Murakami, H.; Kuwahara-Arai, K.; Hanaki, H.; Hiramatsu, K. Contribution of a thickened cell wall and its glutamine nonamidated component to the vancomycin resistance expressed by Staphylococcus aureus Mu50. Antimicrob. Agents Chemother. 2000, 44, 2276–2285. [Google Scholar] [CrossRef] [PubMed]
- Koehl, J.L.; Muthaiyan, A.; Jayaswal, R.K.; Ehlert, K.; Labischinski, H.; Wilkinson, B.J. Cell wall composition and decreased autolytic activity and lysostaphin susceptibility of glycopeptide-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 2004, 48, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Gründling, A.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus mutants with increased lysostaphin resistance. J. Bacteriol. 2006, 188, 6286–6297. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, C.M.; Jadanova, A.; Chanturiya, T.; Kokai-Kun, J.F. Lysostaphin-resistant variants of Staphylococcus aureus demonstrate reduced fitness in vitro and in vivo. Antimicrob. Agents Chemother. 2007, 51, 475–482. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bastos, M.d.C.d.F.; Coutinho, B.G.; Coelho, M.L.V. Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals 2010, 3, 1139-1161. https://doi.org/10.3390/ph3041139
Bastos MdCdF, Coutinho BG, Coelho MLV. Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals. 2010; 3(4):1139-1161. https://doi.org/10.3390/ph3041139
Chicago/Turabian StyleBastos, Maria do Carmo de Freire, Bruna Gonçalves Coutinho, and Marcus Lívio Varella Coelho. 2010. "Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications" Pharmaceuticals 3, no. 4: 1139-1161. https://doi.org/10.3390/ph3041139
APA StyleBastos, M. d. C. d. F., Coutinho, B. G., & Coelho, M. L. V. (2010). Lysostaphin: A Staphylococcal Bacteriolysin with Potential Clinical Applications. Pharmaceuticals, 3(4), 1139-1161. https://doi.org/10.3390/ph3041139