Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms
Abstract
:1. Introduction
2. Quercetin
| Food | Quercetin Content |
|---|---|
| Capers | 233 mg/100 g |
| Onions | 22.0 mg/100 g |
| Cocoa powder | 20.0 mg/100 g |
| Cranberries | 14.0 mg/100 g |
| Lingonberries | 7.4 mg/100 g |
| Apples | 4.57 mg/100 g |
| Green tea | 2.69 mg/100g |
| Black tea | 1.99 mg/100g |
| Catsup | 0.86 mg/100 g |
Bioavailability of Quercetin

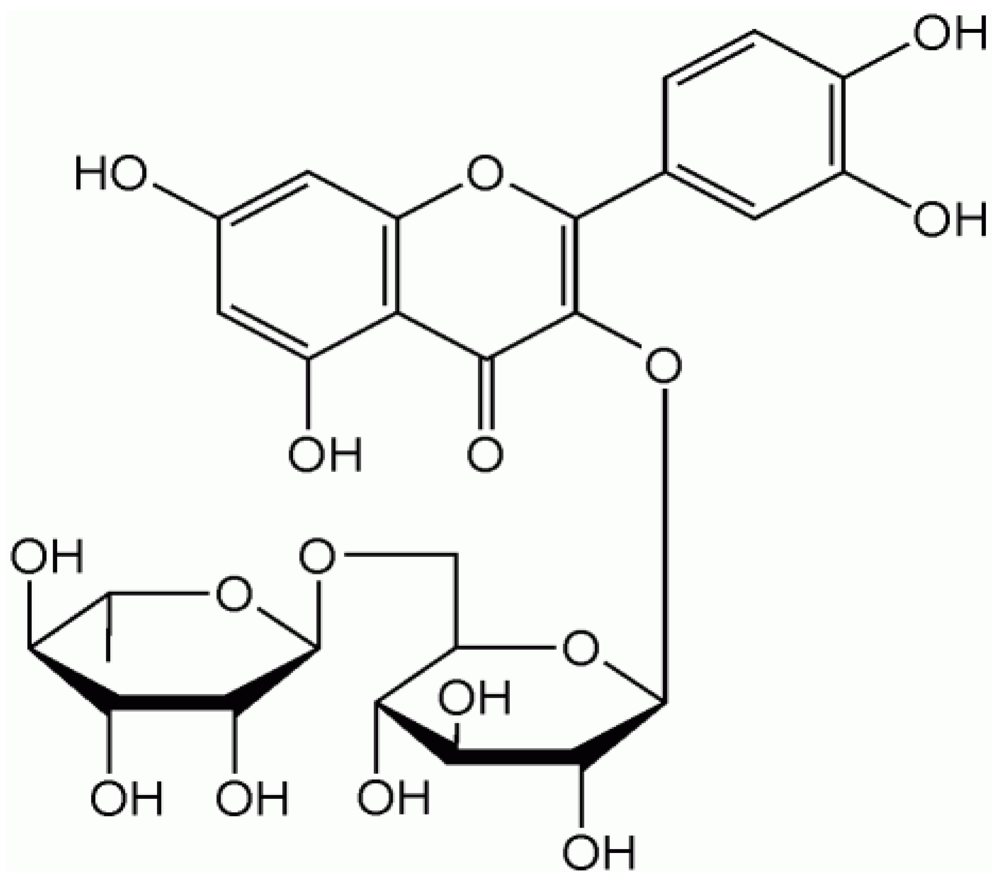
3. Quercetin: A Treatment for Hypertension?
3.1. Effects of Quercetin on BP in Animals
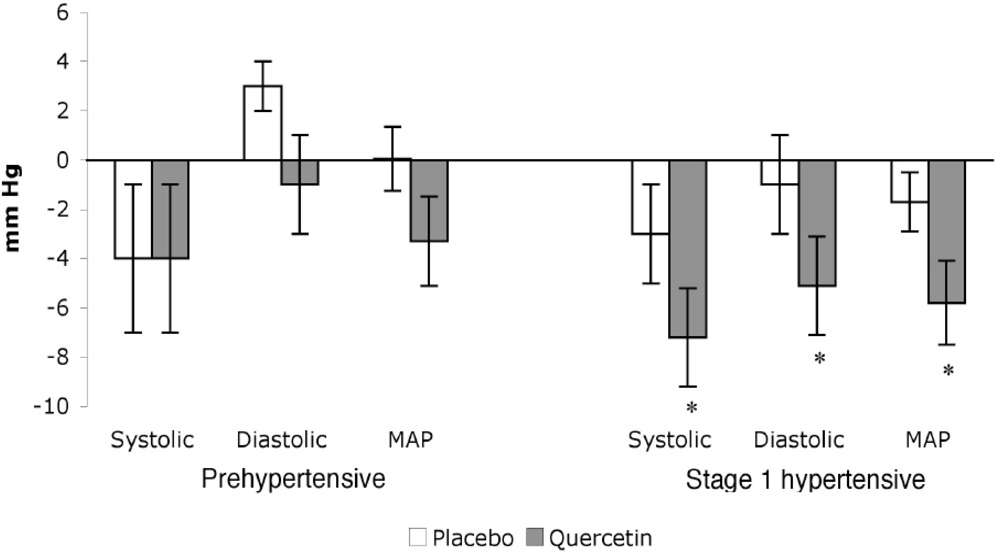
3.2. Effects of Quercetin on BP in Humans
4. Potential Mechanisms for Blood Pressure Reduction
4.1. Oxidative Stress
4.2. Rennin-Angiotensin System

4.3. Vascular Function
4.4. Direct Action on the Vascular Smooth Muscle
5. Safety of Quercetin
6. Conclusions
References
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; Roccella, E.J. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Zago, A.S.; Zanesco, A. Nitric oxide, cardiovascular disease and physical exercise. Arq. Bras. Cardiol. 2006, 87, 264–270. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Andriantsitohaina, R. Endothelial function and cardiovascular disease: Effects of quercetin and wine polyphenols. Free Radic. Res. 2006, 40, 1054–1065. [Google Scholar] [CrossRef] [PubMed]
- Bohm, F.; Pernow, J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc. Res. 2007, 76, 8–18. [Google Scholar] [CrossRef]
- Carretero, O.A. Novel mechanism of action of ACE and its inhibitors. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, 796–1797. [Google Scholar] [CrossRef]
- Parissis, J.T.; Korovesis, S.; Giazitzoglou, E.; Kalivas, P.; Katritsis, D. Plasma profiles of peripheral monocyte-related inflammatory markers in patients with arterial hypertension. Correlations with plasma endothelin-1. Int. J. Cardiol. 2002, 83, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Jakobsen, A.; Heroys, J.; Ralph, A.; Rees, T.; Shaw, M. Targeting cardiovascular protection: The concept of dual renin-angiotensin system control. Medscape J. Med. 2008, 10, 4. [Google Scholar]
- Ziai, S.A.; Seyedhosseini, D.; Taiebi, L.; Salekmoghadam, E.; Mahmoudian, M. Correlation between ACE activity and mean blood pressure in Iranian normotensive subjects after oral administration of a single dose of enalapril. Acta Physiol. Hung 2000, 87, 153–159. [Google Scholar] [PubMed]
- Vikrant, S.; Tiwari, S.C. Essential hypertension - Pathogenesis and pathophysiology. J. Indian Acad. Clin. Med. 2001, 2, 141–161. [Google Scholar]
- Sica, D.A. Angiotensin-converting enzyme inhibitor use in the year 2005. J. Clin. Hypertens. (Greenwich) 2005, 7, 8–11. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P. A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [PubMed]
- Geleijnse, J.M.; Launer, L.J.; Van der Kuip, D.A.; Hofman, A.; Witteman, J.C. Inverse association of tea and flavonoid intakes with incident myocardial infarction: The Rotterdam Study. Am. J. Clin. Nutr. 2002, 75, 880–886. [Google Scholar] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073–2085. [Google Scholar]
- Agriculture, USDA. USDA database for the flavonoid content of selected foods. USDA; Beltsville, 2003. Available online: http://www.nal.usda.gov/fnic/foodcomp/Data/Flav/flav.pdf/ accessed 15 October 2008.
- Chen, X.; Yin, O.Q.; Zuo, Z.; Chow, M.S. Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [PubMed]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Maenpaa, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; vd Gaag, M.; Mengelers, M.J.; van Trijp, J.M.; de Vries, J.H.; Katan, M.B. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic. Biol. Med. 1996, 21, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Lesser, S.; Cermak, R.; Wolffram, S. Bioavailability of quercetin in pigs is influenced by the dietary fat content. J. Nutr. 2004, 134, 1508–1511. [Google Scholar] [PubMed]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials . Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (-)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [PubMed]
- Larson, A.; Bruno, R.; Guo, Y.; Gale, D.; Tanner, J.; Jalili, T.; Symons, J. Acute Quercetin Supplementation Does Not Lower Blood Pressure or Ace Activity in Normotensive Males. J. Am. Diet. Assoc. 2009, 109, 16. [Google Scholar] [CrossRef]
- Carlstrom, J.; Symons, J.D.; Wu, T.C.; Bruno, R.S.; Litwin, S.E.; Jalili, T. A quercetin supplemented diet does not prevent cardiovascular complications in spontaneously hypertensive rats. J. Nutr. 2007, 137, 628–633. [Google Scholar] [PubMed]
- Duarte, J.; Perez-Palencia, R.; Vargas, F.; Ocete, M.A.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Oue, E. Antihypertensive effect of quercetin in rats fed with a high-fat high-sucrose diet. Biosci. Biotechnol. Biochem. 2006, 70, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Jimenez, R.; O'Valle, F.; Galisteo, M.; Perez-Palencia, R.; Vargas, F.; Perez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Protective effects of the flavonoid quercetin in chronic nitric oxide deficient rats. J. Hypertens. 2002, 20, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Hackl, L.P.; Cuttle, G.; Dovichi, S.S.; Lima-Landman, M.T.; Nicolau, M. Inhibition of angiotesin-converting enzyme by quercetin alters the vascular response to brandykinin and angiotensin I. Pharmacology 2002, 65, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Jalili, T.; Carlstrom, J.; Kim, S.; Freeman, D.; Jin, H.; Wu, T.C.; Litwin, S.E.; David Symons, J. Quercetin-supplemented diets lower blood pressure and attenuate cardiac hypertrophy in rats with aortic constriction. J. Cardiovasc. Pharmacol. 2006, 47, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Mackraj, I.; Govender, T.; Ramesar, S. The antihypertensive effects of quercetin in a salt-sensitive model of hypertension. J. Cardiovasc. Pharmacol. 2008, 51, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Perez-Vizcaino, F.; Ibarra, M.; Cogolludo, A. L.; Duarte, J.; Zaragoza-Arnaez, F.; Moreno, L.; Lopez-Lopez, G.; Tamargo, J. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J. Pharmacol. Exp. Ther. 2002, 302, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Pace-Asciak, C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. 1996, 27, 363–366. [Google Scholar] [PubMed]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [PubMed]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [PubMed]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; Lin, P.H.; Karanja, N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kurbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; Wolffram, S.; Muller, M.J. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1–10. [Google Scholar] [CrossRef]
- Wyld, P.J.; Grant, J.; Lippi, A.; Criscuoli, M.; Del Re, G.; Subissi, A. Pharmacokinetics and biochemical efficacy of idrapril calcium, a novel ACE inhibitor, after multiple oral administration in humans. Br. J. Clin. Pharmacol. 1994, 38, 421–425. [Google Scholar] [PubMed]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Sainani, G.S.; Maru, V.G. Role of endothelial cell dysfunction in essential hypertension. J. Assoc. Physicians India 2004, 52, 966–969. [Google Scholar] [PubMed]
- Perticone, F.; Ceravolo, R.; Pujia, A.; Ventura, G.; Iacopino, S.; Scozzafava, A.; Ferraro, A.; Chello, M.; Mastroroberto, P.; Verdecchia, P.; Schillaci, G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation 2001, 104, 191–196. [Google Scholar] [PubMed]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Moens, A.L.; Goovaerts, I.; Claeys, M.J.; Vrints, C.J. Flow-mediated vasodilation: A diagnostic instrument, or an experimental tool? Chest 2005, 127, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Vita, J.A. Polyphenols and cardiovascular disease: Effects on endothelial and platelet function. Am. J. Clin. Nutr. 2005, 81, 292–297. [Google Scholar]
- Nicholson, S.K.; Tucker, G.A.; Brameld, J.M. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc. Nutr. Soc. 2008, 67, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Perez-Vizcaino, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Gu, Z.L. Effects of quercetin on production and release of endothelin and cGMP from cultured endothelial cells. Zhongguo Yao Li Xue Bao 1996, 17, 442–444. [Google Scholar] [PubMed]
- Rendig, S.V.; Symons, J.D.; Longhurst, J.C.; Amsterdam, E.A. Effects of red wine, alcohol, and quercetin on coronary resistance and conductance arteries. J. Cardiovasc. Pharmacol. 2001, 38, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Harwood, M.; Danielewska-Nikiel, B.; Borzellaca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A Critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties . Food Chem. Toxicol. 2007, 45, 2178–2205. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Larson, A.J.; Symons, J.D.; Jalili, T. Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms. Pharmaceuticals 2010, 3, 237-250. https://doi.org/10.3390/ph3010237
Larson AJ, Symons JD, Jalili T. Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms. Pharmaceuticals. 2010; 3(1):237-250. https://doi.org/10.3390/ph3010237
Chicago/Turabian StyleLarson, Abigail J., J. David Symons, and Thunder Jalili. 2010. "Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms" Pharmaceuticals 3, no. 1: 237-250. https://doi.org/10.3390/ph3010237
APA StyleLarson, A. J., Symons, J. D., & Jalili, T. (2010). Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms. Pharmaceuticals, 3(1), 237-250. https://doi.org/10.3390/ph3010237





