Dihydroartemisinin Promotes N1 Polarization of Tumor-Associated Neutrophils and Enhances Their Anti-Tumor Activity via Hub Gene Modulation
Abstract
1. Introduction
2. Results
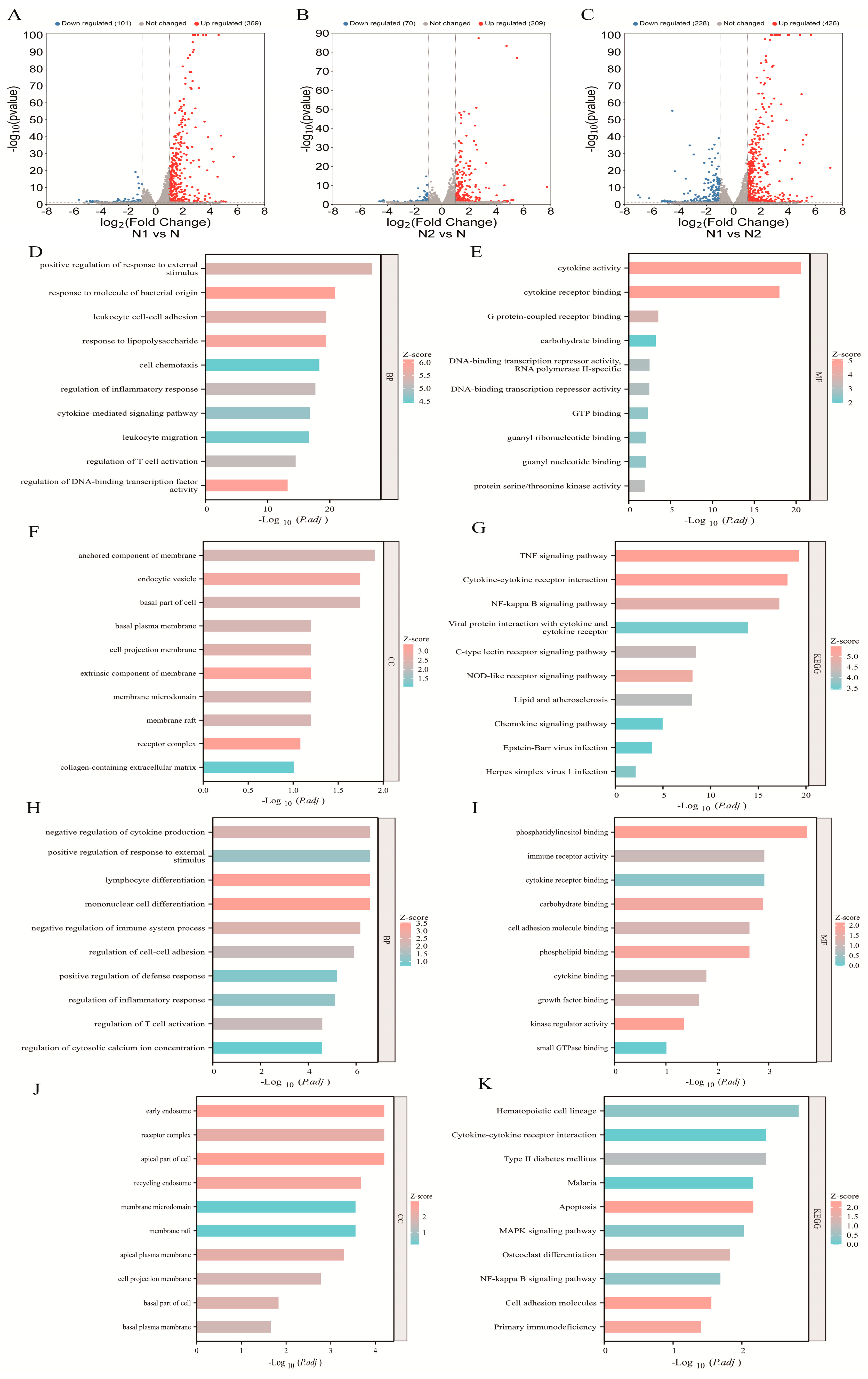
2.1. Transcriptomic Profiling and Functional Enrichment of N1 and N2 TANs
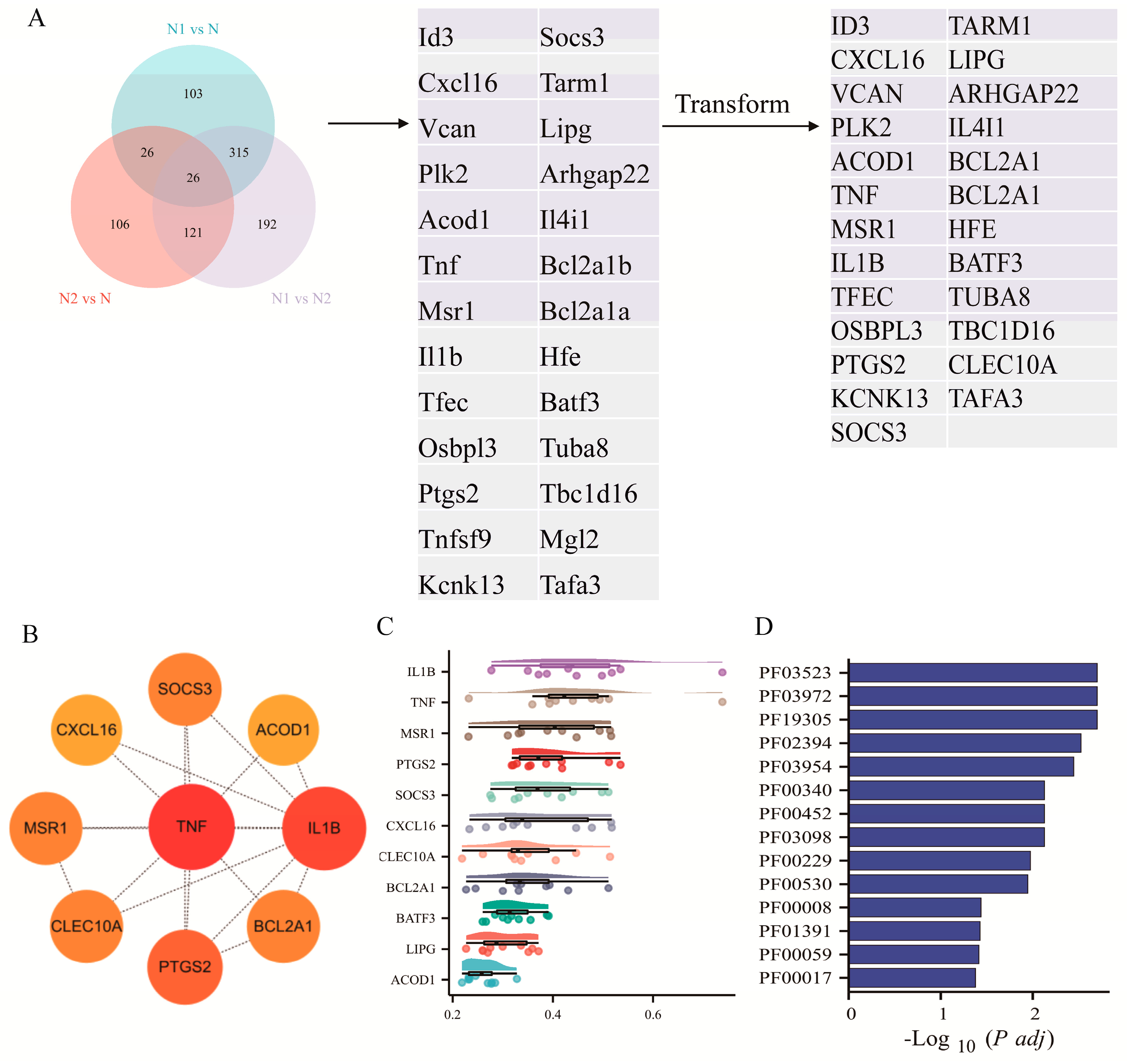
2.2. Network-Based Identification of Hub Genes in TAN Subtypes
2.3. Molecular Docking Analysis of Hub Genes with DHA
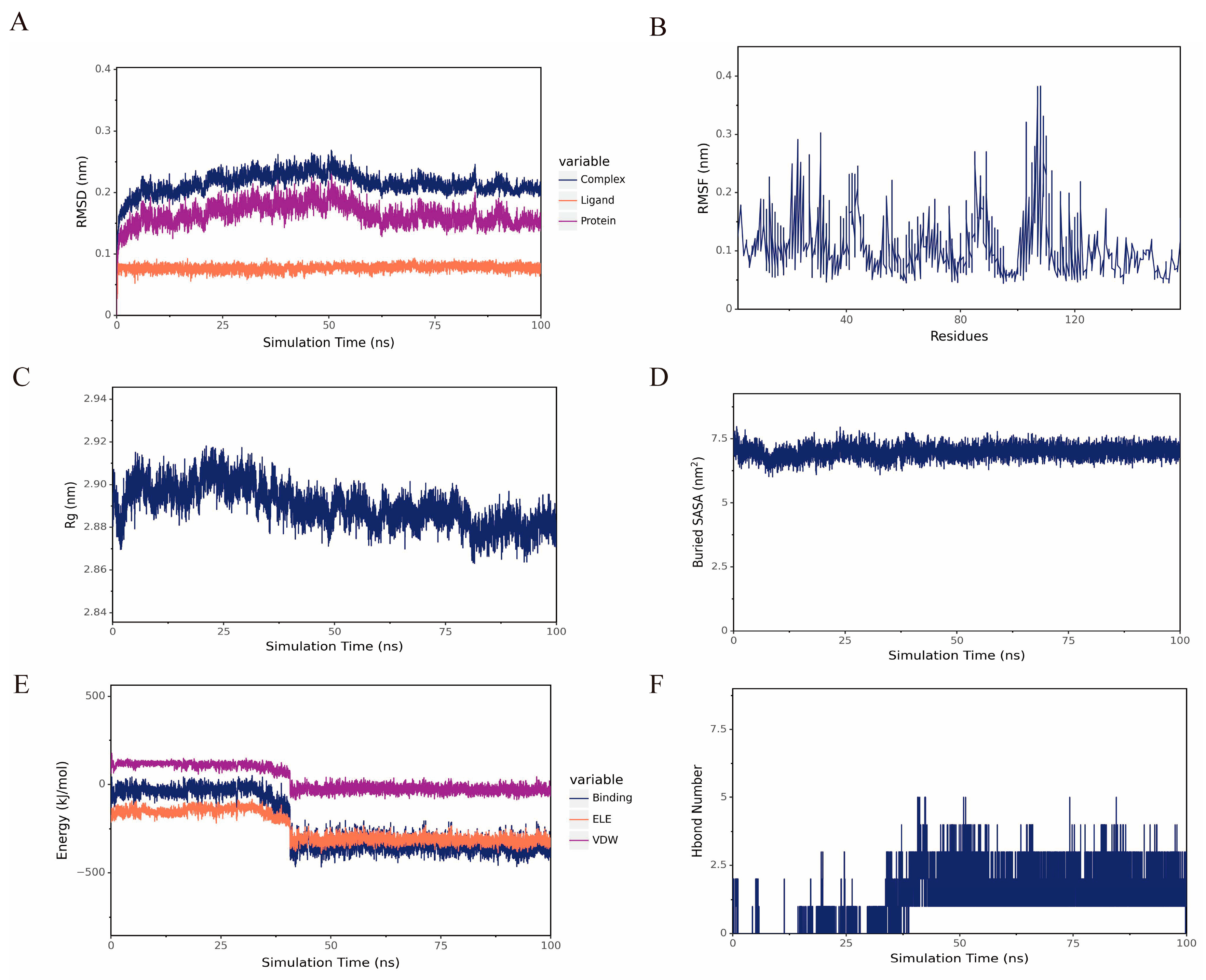
2.4. MD Analysis of TNF with DHA
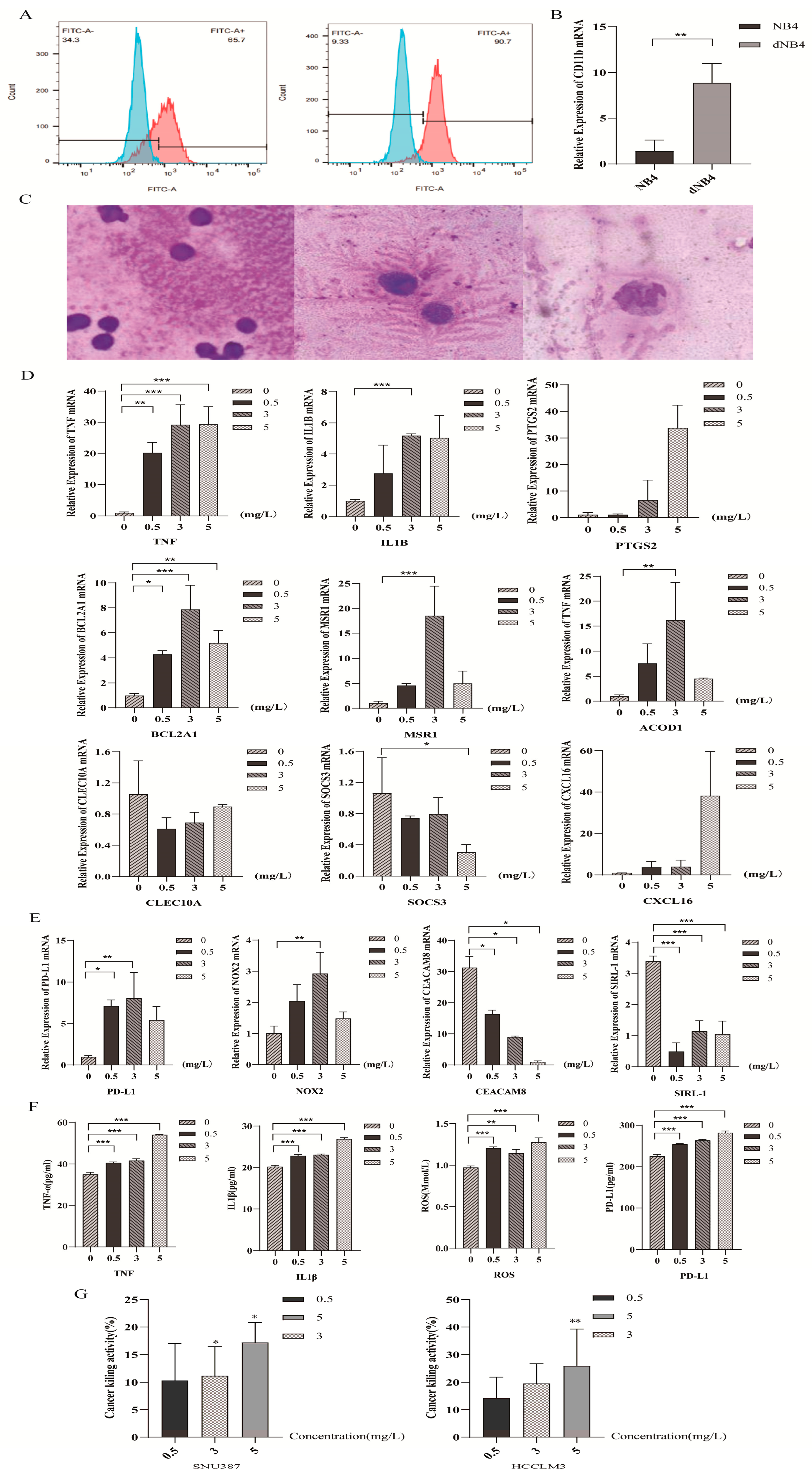
2.5. Dihydroartemisinin Polarized Neutrophil-like Cells Towards N1 Subtype
3. Discussion
4. Materials and Methods
4.1. Bioinformatics Analysis
4.1.1. Transcriptome Dataset Analysis
4.1.2. Differential Gene Expression Analysis and Functional Enrichment Analysis
4.1.3. Construction of a Protein–Protein Interaction Network
4.1.4. Identification of Hub Genes and Molecular Docking Analysis
4.1.5. Molecular Dynamics Simulation of the TNF and DHA Complex
4.1.6. Clustering Analysis of Hub Genes and Protein Domains
4.2. Cell Lines and Reagents
4.3. Neutrophil-like Cell Model
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Leukocyte-Mediated Killing Model (CKA)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.P.; Beirao, B.C.; Pang, L.Y.; Queiroga, F.L.; Argyle, D.J. Inflammation and cancer: Till death tears them apart. Vet. J. 2015, 205, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef]
- Granot, Z.; Jablonska, J. Distinct Functions of Neutrophil in Cancer and Its Regulation. Mediat. Inflamm. 2015, 2015, 701067. [Google Scholar] [CrossRef]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Ohms, M.; Moller, S.; Laskay, T. An Attempt to Polarize Human Neutrophils Toward N1 and N2 Phenotypes in vitro. Front. Immunol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Piccard, H.; Muschel, R.J.; Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. Hematol. 2012, 82, 296–309. [Google Scholar] [CrossRef]
- Ellis, T.N.; Beaman, B.L. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 2004, 112, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Guha, S. Granulocyte colony-stimulating factor/granulocyte colony-stimulating factor receptor biological axis promotes survival and growth of bladder cancer cells. Urology 2007, 69, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Joshita, S.; Nakazawa, K.; Sugiyama, Y.; Kamijo, A.; Matsubayashi, K.; Miyabayashi, H.; Furuta, K.; Kitano, K.; Kawa, S. Granulocyte-colony stimulating factor-producing pancreatic adenosquamous carcinoma showing aggressive clinical course. Intern. Med. 2009, 48, 687–691. [Google Scholar] [CrossRef]
- Kyo, S.; Kanaya, T.; Takakura, M.; Inoue, M. A case of cervical cancer with aggressive tumor growth: Possible autocrine growth stimulation by G-CSF and Il-6. Gynecol. Oncol. 2000, 78, 383–387. [Google Scholar] [CrossRef]
- Shaul, M.E.; Levy, L.; Sun, J.; Mishalian, I.; Singhal, S.; Kapoor, V.; Horng, W.; Fridlender, G.; Albelda, S.M.; Fridlender, Z.G. Tumor-associated neutrophils display a distinct N1 profile following TGFbeta modulation: A transcriptomics analysis of pro- vs. antitumor TANs. Oncoimmunology 2016, 5, e1232221. [Google Scholar] [CrossRef] [PubMed]
- Casbon, A.J.; Reynaud, D.; Park, C.; Khuc, E.; Gan, D.D.; Schepers, K.; Passegue, E.; Werb, Z. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc. Natl. Acad. Sci. USA 2015, 112, E566–E575. [Google Scholar] [CrossRef]
- Andzinski, L.; Kasnitz, N.; Stahnke, S.; Wu, C.F.; Gereke, M.; von Kockritz-Blickwede, M.; Schilling, B.; Brandau, S.; Weiss, S.; Jablonska, J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer 2016, 138, 1982–1993. [Google Scholar] [CrossRef]
- Sun, R.; Luo, J.; Li, D.; Shu, Y.; Luo, C.; Wang, S.S.; Qin, J.; Zhang, G.M.; Feng, Z.H. Neutrophils with protumor potential could efficiently suppress tumor growth after cytokine priming and in presence of normal NK cells. Oncotarget 2014, 5, 12621–12634. [Google Scholar] [CrossRef]
- Galdiero, M.R.; Garlanda, C.; Jaillon, S.; Marone, G.; Mantovani, A. Tumor associated macrophages and neutrophils in tumor progression. J. Cell Physiol. 2013, 228, 1404–1412. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, Y.; Wu, Y.; Qi, Q.; Wang, J.; Chen, L.; Wang, F. The immunosuppressive tumor microenvironment in hepatocellular carcinoma-current situation and outlook. Mol. Immunol. 2022, 151, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Le-Xin, C.; Ming-Jun, L.; Chun-Qi, X.; Jia-Xin, Z.; Jing-Ya, Y.; Li-Xin, N.; Mei-Qi, W.; En-Xin, Z.; Xiao-Jun, Z. Yi Qi Chu Tan Formula (YQCTF) inhibited the progress of lung cancer via regulating tumor-associated neutrophil: An integrated study of network pharmacology, proteomics and pharmacodynamics. J. Ethnopharmacol. 2024, 318, 116943. [Google Scholar] [CrossRef]
- Peng, Q.; Li, S.; Shi, X.; Guo, Y.; Hao, L.; Zhang, Z.; Ji, J.; Zhao, Y.; Li, C.; Xue, Y.; et al. Dihydroartemisinin broke the tumor immunosuppressive microenvironment by inhibiting YAP1 expression to enhance anti-PD-1 efficacy. Phytother. Res. 2023, 37, 1740–1753. [Google Scholar] [CrossRef]
- Wang, C.Z.; Wan, C.; Luo, Y.; Zhang, C.F.; Zhang, Q.H.; Chen, L.; Liu, Z.; Wang, D.H.; Lager, M.; Li, C.H.; et al. Effects of dihydroartemisinin, a metabolite of artemisinin, on colon cancer chemoprevention and adaptive immune regulation. Mol. Biol. Rep. 2022, 49, 2695–2709. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Yu, R.; Jin, L.; Li, F.; Fujimoto, M.; Wei, Q.; Lin, Z.; Ren, X.; Jin, Q.; Li, H.; Meng, F.; et al. Dihydroartemisinin inhibits melanoma by regulating CTL/Treg anti-tumor immunity and STAT3-mediated apoptosis via IL-10 dependent manner. J. Dermatol. Sci. 2020, 99, 193–202. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Wang, Y.; Guo, Z.; Gu, A.D.; Dan, H.C.; Baldwin, A.S.; Hao, W.; Wan, Y.Y. Dihydroartemisinin ameliorates inflammatory disease by its reciprocal effects on Th and regulatory T cell function via modulating the mammalian target of rapamycin pathway. J. Immunol. 2012, 189, 4417–4425. [Google Scholar] [CrossRef]
- Wenisch, C.; Parschalk, B.; Zedwitz-Liebenstein, K.; Wernsdorfer, W.; Graninger, W. The effect of artemisinin on granulocyte function assessed by flow cytometry. J. Antimicrob. Chemother. 1997, 39, 99–101. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qu, Y.; Jin, S.; Zhang, Y.; Qin, L. ALDH3A1 upregulation inhibits neutrophils N2 polarization and halts oral cancer growth. Oral Dis. 2024, 30, 4231–4242. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Peng, W.; Huang, Y.; Cheng, L.; Meng, Y.; Kwantwi, L.B.; Yang, J.; Xu, J.; Xiao, H.; Kzhyshkowska, J.; et al. Tumor-activated neutrophils promote metastasis in breast cancer via the G-CSF-RLN2-MMP-9 axis. J. Leukoc. Biol. 2023, 113, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Shah, H.P.; Malu, K.; Berliner, N.; Gaines, P. Differentiation and characterization of myeloid cells. Curr. Protoc. Immunol. 2014, 104, 22F.5.1–22F.5.28. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, K.; Sui, Y.; Xu, Z.; Cui, H.; Wang, Y.; Zhang, H.; Xu, Z.; Xu, W.; Ding, Q.; et al. Characterization of CD66b and its relationship between immune checkpoints and their synergistic impact in the prognosis of surgically resected lung adenocarcinoma. Lung Cancer 2021, 160, 84–91. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakagawa, T.; Sugihara, T.; Horiuchi, T.; Yoshizaki, U.; Fujimura, T.; Fukuhara, H.; Urano, T.; Takayama, K.; Inoue, S.; et al. Prognostic value of CD66b positive tumor-infiltrating neutrophils in testicular germ cell tumor. BMC Cancer 2016, 16, 898. [Google Scholar] [CrossRef] [PubMed]
- Blanks, M.J.; Stehle, J.R., Jr.; Du, W.; Adams, J.M.; Willingham, M.C.; Allen, G.O.; Hu, J.J.; Lovato, J.; Molnar, I.; Cui, Z. Novel innate cancer killing activity in humans. Cancer Cell Int. 2011, 11, 26. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Bai, Y.; Liu, J.; Wong, Y.K.; Xie, L.; Zhang, Q.; Luo, P.; Gao, P.; Gu, L.; et al. Single-cell transcriptome analysis reveals the regulatory effects of artesunate on splenic immune cells in polymicrobial sepsis. J. Pharm. Anal. 2023, 13, 817–829. [Google Scholar] [CrossRef]
- Houh, Y.K.; Kim, K.E.; Park, S.; Hur, D.Y.; Kim, S.; Kim, D.; Bang, S.I.; Yang, Y.; Park, H.J.; Cho, D. The Effects of Artemisinin on the Cytolytic Activity of Natural Killer (NK) Cells. Int. J. Mol. Sci. 2017, 18, 1600. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.L.; Zhang, G.H.; Gao, Y.F. Artesunate promotes Th1 differentiation from CD4+ T cells to enhance cell apoptosis in ovarian cancer via miR-142. Braz. J. Med. Biol. Res. 2019, 52, e7992. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Macarie, R.D.; Vadana, M.; Cecoltan, S.; Preda, M.B.; Hudita, A.; Gan, A.M.; Jakobsson, G.; Tucureanu, M.M.; et al. Transcriptional Profiling and Functional Analysis of N1/N2 Neutrophils Reveal an Immunomodulatory Effect of S100A9-Blockade on the Pro-Inflammatory N1 Subpopulation. Front. Immunol. 2021, 12, 708770. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- UniProt, C. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, Y.; Chen, B.; Fan, L. Target prediction and potential application of dihydroartemisinin on hepatocarcinoma treatment. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 7711–7724. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Laraib, A.; UrRehman, S.; Jamil, S.; Zia, M.A.; Muhammad, S.; Bibi, S.; Ali, N.; Hassan, M.; Nadeem, M.U.; Bai, F. Aloin as a Potential Anti-Breast Cancer Agent: Insights From Molecular Docking and Density Functional Theory (DFT) Analysis. ChemstrySelect 2025, 10, e202406211. [Google Scholar] [CrossRef]
- Muhammad, S.; Zaid, A.; Bibi, S.; Urrehman, S.; Alshahrani, M.Y.; Kumar, S.; Tousif, M.I.; Al-Sehemi, A.G. Piperine analogues as dual inhibitors for antibacterial and antiarthritic properties through impact of ligands optimization, docking and water solvation. J. Mol. Liq. 2024, 415, 126379. [Google Scholar] [CrossRef]
- Cosconati, S.; Forli, S.; Perryman, A.L.; Harris, R.; Goodsell, D.S.; Olson, A.J. Virtual Screening with AutoDock: Theory and Practice. Expert Opin. Drug Discov. 2010, 5, 597–607. [Google Scholar] [CrossRef]
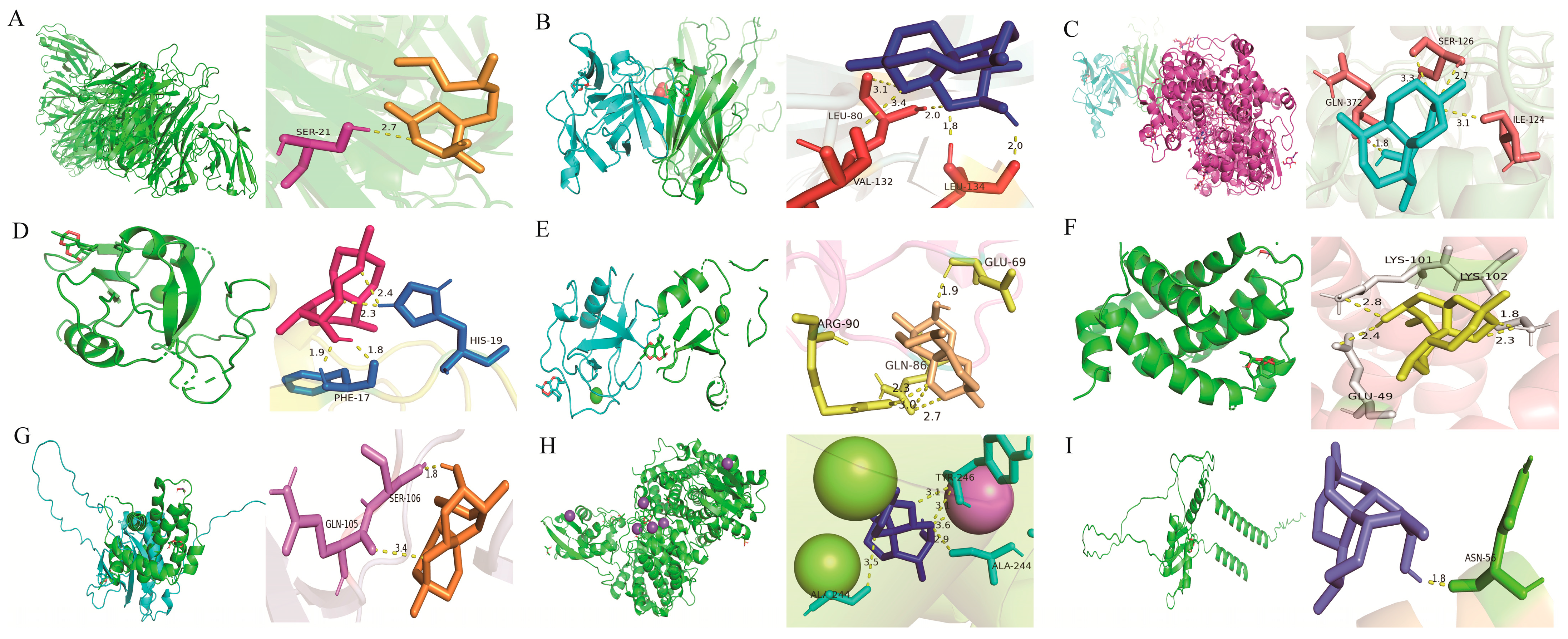
| Protein | PDB ID/Uniprot ID/AlphaFold ID | Binding Energy (kcal/mol) | Root Mean Square Deviation (Å) |
|---|---|---|---|
| TNF | 5M2I | −5.96 | 0.11 |
| IL1B | 8RYS | −7.65 | 0.11 |
| PTGS2 | 5F19 | −6.3 | 0.11 |
| CLEC10A | 6PUV | −6.51 | 0.10 |
| MSR1 | 7DPX | −6.14 | 0.10 |
| BCL2A1 | 5WHI | −6.32 | 0.10 |
| SOCS3 | O14543 | −7.15 | 0.11 |
| ACOD1 | 6R6U | −5.78 | 0.11 |
| CXCL16 | Q9H2A7 | −4.96 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Guo, W.; Liu, Y.; Ma, W.; Wang, J.; Chen, B.; Fan, L. Dihydroartemisinin Promotes N1 Polarization of Tumor-Associated Neutrophils and Enhances Their Anti-Tumor Activity via Hub Gene Modulation. Pharmaceuticals 2026, 19, 88. https://doi.org/10.3390/ph19010088
Guo W, Liu Y, Ma W, Wang J, Chen B, Fan L. Dihydroartemisinin Promotes N1 Polarization of Tumor-Associated Neutrophils and Enhances Their Anti-Tumor Activity via Hub Gene Modulation. Pharmaceuticals. 2026; 19(1):88. https://doi.org/10.3390/ph19010088
Chicago/Turabian StyleGuo, Wenjia, Yu’e Liu, Wencong Ma, Jinghan Wang, Bingdi Chen, and Lieying Fan. 2026. "Dihydroartemisinin Promotes N1 Polarization of Tumor-Associated Neutrophils and Enhances Their Anti-Tumor Activity via Hub Gene Modulation" Pharmaceuticals 19, no. 1: 88. https://doi.org/10.3390/ph19010088
APA StyleGuo, W., Liu, Y., Ma, W., Wang, J., Chen, B., & Fan, L. (2026). Dihydroartemisinin Promotes N1 Polarization of Tumor-Associated Neutrophils and Enhances Their Anti-Tumor Activity via Hub Gene Modulation. Pharmaceuticals, 19(1), 88. https://doi.org/10.3390/ph19010088








