Open-Label Phase II Study of Olokizumab in Adolescent Patients with Polyarticular Juvenile Idiopathic Arthritis: Results of the 24-Week Treatment Period
Abstract
1. Introduction
2. Results
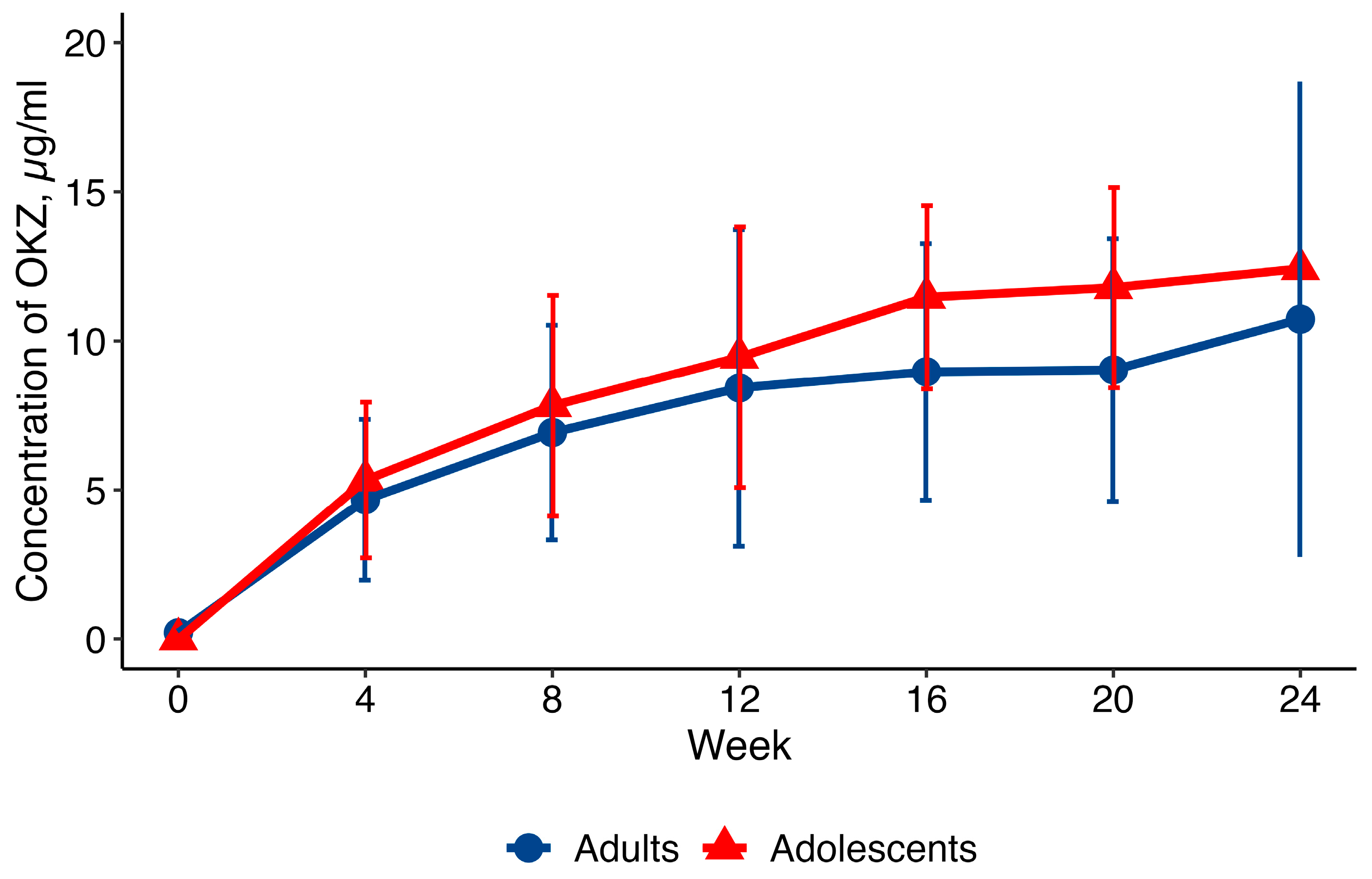
2.1. Pharmacokinetics
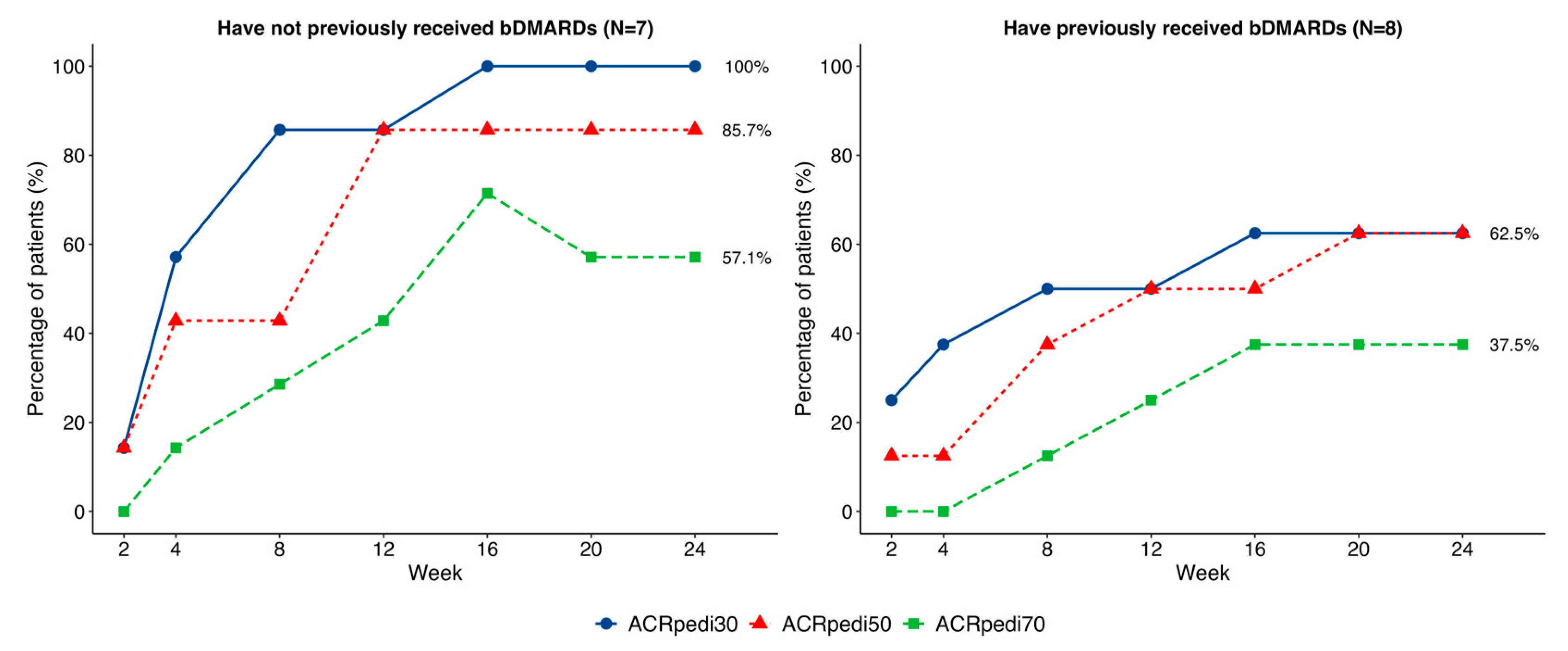
2.2. Effectiveness
2.3. Safety
3. Discussion
4. Materials and Methods
4.1. Eligibility Criteria
4.2. Treatment
4.3. Study Endpoints
- The Proportion of Patients Achieving ACRpedi30/50/70/90 ResponseACRPedi30/50/70/90 is defined as at least 30/50/70/90% improvement from baseline in 3 of 6 variables in the core set, while no more than 1 of the remaining variables can worsen by >30%. The variables in the core set include: physician’s global assessment of disease activity; parent/patient global assessment of overall well-being; functional ability (CHAQ); active joint count; number of joints with limited range of motion; and ESR.
- Change in the JADAS-71 [29]The JADAS-71 includes the physician’s global assessment of disease activity; parent/patient global assessment of overall well-being; ESR, normalized to a 0 to 10 scale; and active joint counts.
- Proportion of Patients Achieving Minimal or Inactive Disease per JADAS Criteria
- Changes in Inflammatory Markers (CRP, ESR)
- Number of Joints with Active Arthritis
- Physicians’ and Parents’ Global Assessments of Disease Activity and Overall Well-Being
- Functional Status Using the CHAQ [30]
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEs | adverse events |
| bDMARDs | biologic disease-modifying antirheumatic drugs |
| CHAQ | Childhood Health Assessment Questionnaire |
| CRP | C-reactive protein |
| ESR | erythrocyte sedimentation rate |
| q4w | every 4 weeks |
| IL-6 | interleukin-6 |
| JADAS-71 | Juvenile Arthritis Disease Activity Score 71 |
| LLOQ | LLOQ lower limit of quantification |
| Cmax | maximum serum concentration |
| MTX | Methotrexate |
| OKZ | Olokizumab |
| PK | Pharmacokinetics |
| pJIA | polyarticular JIA |
| SD | standard deviation |
| SAE | serious adverse event |
| sJIA | systemic JIA |
| Tmax | time to maximum serum concentration |
| TCZ | tocilizumab |
| TNF-α | tumor necrosis factor-α |
| ULN | upper limit of normal |
Appendix A

References
- Ravelli, A.; Martini, A. Juvenile idiopathic arthritis. Lancet 2007, 369, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Zak, M.; Müller, J.; Karup Pedersen, F. Final height, armspan, subischial leg length and body proportions in juvenile chronic arthritis. A long-term follow-up study. Horm. Res. 1999, 52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kriulina, T.Y.; Dvoryakovskaya, T.M.; Surkov, A.G.; Shubina, D.S.; Bairashevskaya, A.V.; Ponomarchuk, M.N.; Alexeeva, E.I.; Shilkrot, I.Y. Situation with medical care for children with rheumatic disease on the example of juvenile arthritis: The view of patients and their parents. Vopr. Prakt. Pediatr. (Clin. Pract. Pediatr.) 2023, 18, 37–55. (In Russian) [Google Scholar] [CrossRef]
- Kriulina, T.Y.; Alexeeva, E.I.; Shilkrot, I.Y.; Dvoryakovskaya, T.M.; Surkov, A.G. Health care for children with juvenile arthritis in the Russian Federation and in the world. Vopr. Prakt. Pediatr. (Clin. Pract. Pediatr.) 2022, 17, 84–103. (In Russian) [Google Scholar] [CrossRef]
- Sevostyanov, V.C.; Zholobova, E.S.; Baranova, O.V.; Poymshina, K.S.; Polukhina, A.I.; Novikov, A.S.; Balashov, S.L. Pediatric rheumatology care in the Central Federal District of the Russian Federation. Vopr. Prakt. Pediatr. (Clin. Pract. Pediatr.) 2019, 14, 90–96. (In Russian) [Google Scholar] [CrossRef]
- Bowyer, S.L.; A Roettcher, P.; Higgins, G.C.; Adams, B.; Myers, L.K.; Wallace, C.; Rennebohm, R.; Moore, T.L.; Pepmueller, P.H.; Spencer, C.; et al. Health status of patients with juvenile rheumatoid arthritis at 1 and 5 years after diagnosis. J. Rheumatol. 2003, 30, 394–400. [Google Scholar]
- Lovell, D.J. Update on treatment of arthritis in children: New treatments, new goals. Bull. NYU Hosp. Jt. Dis. 2006, 64, 72–76. [Google Scholar]
- De Benedetti, F.; Robbioni, P.; Massa, M.; Viola, S.; Albani, S.; Martini, A. Serum interleukin-6 levels and joint involvement in polyarticular and pauciarticular juvenile chronic arthritis. Clin. Exp. Rheumatol. 1992, 10, 493–498. [Google Scholar]
- Saxena, N.; Aggarwal, A.; Misra, R. Elevated concentrations of monocyte derived cytokines in synovial fluid of children with enthesitis related arthritis and polyarticular types of juvenile idiopathic arthritis. J. Rheumatol. 2005, 32, 1349–1353. [Google Scholar]
- Eberhard, B.A.; Laxer, R.M.; Andersson, U.; Silverman, E.D. Local synthesis of both macrophage and T cell cytokines by synovial fluid cells from children with juvenile rheumatoid arthritis. Clin. Exp. Immunol. 1994, 96, 260–266. [Google Scholar] [CrossRef]
- Caiello, I.; Minnone, G.; Holzinger, D.; Vogl, T.; Prencipe, G.; Manzo, A.; De Benedetti, F.; Strippoli, R. IL-6 amplifies TLR mediated cytokine and chemokine production: Implications for the pathogenesis of rheumatic inflammatory diseases. PLoS ONE 2014, 9, e107886. [Google Scholar] [CrossRef]
- Nasonov, E.; Fatenejad, S.; Feist, E.; Ivanova, M.; Korneva, E.; Krechikova, D.G.; Maslyanskiy, A.L.; Samsonov, M.; Stoilov, R.; Zonova, E.V.; et al. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: Efficacy and safety results of a randomised controlled phase III study. Ann. Rheum. Dis. 2022, 81, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Feist, E.; Fatenejad, S.; Grishin, S.A.; Korneva, E.V.; Nasonov, E.L.; Samsonov, M.Y.; Fleischmann, R.M.; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 387, 715–726. [Google Scholar] [CrossRef] [PubMed]
- FFeist, E.; Fatenejad, S.; Grishin, S.; Korneva, E.; E Luggen, M.; Nasonov, E.; Samsonov, M.; Smolen, J.S.; Fleischmann, R.M. Olokizumab, a monoclonal antibody against interleukin-6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: Efficacy and safety results of a randomised controlled phase III study. Ann. Rheum. Dis. 2022, 81, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Feist, E.; Fleischmann, R.M.; Fatenejad, S.; Bukhanova, D.; Grishin, S.; Kuzkina, S.; Luggen, M.; Nasonov, E.; Samsonov, M.; Smolen, J.S. Olokizumab plus methotrexate: Safety and efficacy over 106 weeks of treatment. Ann. Rheum. Dis. 2024, 83, 1454–1464, https://doi.org/10.1136/ard-2023-225473. Erratum in Ann. Rheum. Dis. 2024, 84, 373. [Google Scholar] [CrossRef]
- Petty, R.E.; Southwood, T.R.; Manners, P.; Baum, J.; Glass, D.N.; Goldenberg, J.; He, X.; Maldonado-Cocco, J.; Orozco-Alcala, J.; Prieur, A.-M.; et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004, 31, 390–392. [Google Scholar]
- Trincianti, C.; Van Dijkhuizen, E.H.P.; Alongi, A.; Mazzoni, M.; Swart, J.F.; Nikishina, I.; Lahdenne, P.; Rutkowska-Sak, L.; Avcin, T.; Quartier, P.; et al. Definition and Validation of the American College of Rheumatology 2021 Juvenile Arthritis Disease Activity Score Cutoffs for Disease Activity States in Juvenile Idiopathic Arthritis. Arthritis Rheumatol. 2021, 73, 1966–1975. [Google Scholar] [CrossRef]
- Consolaro, A.; Ruperto, N.; Bracciolini, G.; Frisina, A.; Gallo, M.C.; Pistorio, A.; Verazza, S.; Negro, G.; Gerloni, V.; Goldenstein-Schainberg, C.; et al. Defining criteria for high disease activity in juvenile idiopathic arthritis based on the juvenile arthritis disease activity score. Ann. Rheum. Dis. 2014, 73, 1380–1383. [Google Scholar] [CrossRef]
- Lovell, D.J.; Ruperto, N.; Giannini, E.H.; Martini, A. Advances from clinical trials in juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 2013, 9, 557–563. [Google Scholar] [CrossRef]
- Otten, M.H.; Prince, F.H.M.; Anink, J.; Cate, R.T.; Hoppenreijs, E.P.A.H.; Armbrust, W.; Koopman-Keemink, Y.; A van Pelt, P.; Kamphuis, S.; Gorter, S.L.; et al. Effectiveness and safety of a second and third biological agent after failing etanercept in juvenile idiopathic arthritis: Results from the Dutch National ABC Register. Ann. Rheum. Dis. 2013, 72, 721–727. [Google Scholar] [CrossRef]
- Brunner, H.I.; Klein-Gitelman, M.S.; Miller, M.J.; Barron, A.; Baldwin, N.; Trombley, M.; Johnson, A.L.; Kress, A.; Lovell, D.J.; Giannini, E.H. Minimal clinically important differences of the childhood health assessment questionnaire. J. Rheumatol. 2005, 32, 150–161. [Google Scholar] [PubMed]
- Horneff, G.; Klein, A.; Klotsche, J.; Minden, K.; Huppertz, H.-I.; Weller-Heinemann, F.; Kuemmerle-Deschner, J.; Haas, J.-P.; Hospach, A. Comparison of treatment response, remission rate and drug adherence in polyarticular juvenile idiopathic arthritis patients treated with etanercept, adalimumab or tocilizumab. Arthritis Res. Ther. 2016, 18, 272. [Google Scholar] [CrossRef] [PubMed]
- Alexeeva, E.I.; Dvoryakovskaya, T.M.; Isaeva, K.B.; Sleptsova, T.V.; Denisova, R.V.; Soloshenko, M.A.; Lomakina, O.L.; Fetisova, A.N.; Rudnickaya, M.G.; Vankova, D.D.; et al. Prognostic Factors for the Response to Tocilizumab Therapy in Patients with Juvenile Idiopathic Arthritis without Systemic Manifestations: A Cohort Study. Vopr. Sovrem. Pediatr. (Curr. Pediatr.) 2018, 17, 199–207. [Google Scholar] [CrossRef]
- Gazda, A.; Naishtetik, I.; Kołodziejczyk, B.; Rybak, K.; Mańczak, M.; Wójtowicz, J.; Krasowicz-Towalska, O.; Gietka, P. Clinical outcomes of tocilizumab therapy in polyarticular and systemic juvenile idiopathic arthritis: A single-center analysis (2018–2022). Rheumatol. Int. 2024, 44, 2949–2959. [Google Scholar] [CrossRef]
- Shafran, I.H.; Alasti, F.; Smolen, J.S.; Aletaha, D. Implication of baseline levels and early changes of C-reactive protein for subsequent clinical outcomes of patients with rheumatoid arthritis treated with tocilizumab. Ann. Rheum. Dis. 2020, 79, 874–882. [Google Scholar] [CrossRef]
- Brunner, H.I.; Ruperto, N.; Zuber, Z.; Cuttica, R.; Keltsev, V.; Xavier, R.M.; Burgos-Vargas, R.; Penades, I.C.; Silverman, E.D.; Espada, G.; et al. Efficacy and Safety of Tocilizumab for Polyarticular-Course Juvenile Idiopathic Arthritis in the Open-Label Two-Year Extension of a Phase III Trial. Arthritis Rheumatol. 2021, 73, 530–541. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation; Research (CDER); Center for Biologics Evaluation and Research (CBER). Guidance for Industry Drug-Induced Liver Injury: Premarketing Clinical Evaluation. 2009. Available online: https://www.fda.gov/downloads/Guidances/UCM174090.pdf (accessed on 27 December 2025).
- Brunello, F.; Tirelli, F.; Pegoraro, L.; Dell’APa, F.; Alfisi, A.; Calzamatta, G.; Folisi, C.; Zulian, F. New Insights on Juvenile Psoriatic Arthritis. Front. Pediatr. 2022, 10, 884727. [Google Scholar] [CrossRef]
- Consolaro, A.; Ruperto, N.; Bazso, A.; Pistorio, A.; Magni-Manzoni, S.; Filocamo, G.; Malattia, C.; Viola, S.; Martini, A.; Ravelli, A. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum. 2009, 61, 658–666. [Google Scholar] [CrossRef]
- Kuz’mina, N.N.; Nikishina, J.P.; Shaikov, A.V.; Ruperto, N.; Shelepina, T.A.; Salugina, S.O. The Russian Version of the Childhood Health Assessment Questionnaires (CHAQ) and the Child Health Questionnaires (CAQ). Rheumatol. Sci. Pract. 2002, 40, 40–44. (In Russian) [Google Scholar] [CrossRef]
- Wang, Y.; Jadhav, P.R.; Lala, M.; Gobburu, J.V. Clarification on precision criteria to derive sample size when designing pediatric pharmacokinetic studies. J. Clin. Pharmacol. 2012, 52, 1601–1606. [Google Scholar] [CrossRef]


| Characteristic | Olokizumab (n = 16) | |
|---|---|---|
| Age, years | mean (± SD) | 14.4 ± 1.86 |
| median (Q1; Q3) | 14.0 (13.0; 16.5) | |
| min–max | 12–17 | |
| Sex | Female, n (%) | 9 (56.2%) |
| Male, n (%) | 7 (43.8%) | |
| Body weight, kg | mean (±SD) | 53.8 ± 9.48 |
| median (Q1; Q3) | 51.9 (46.3; 58.5) | |
| min–max | 45.0–79.0 | |
| Disease duration, years | mean (±SD) | 4.7 ± 4.66 |
| median (Q1; Q3) | 3.4 (0.4; 8.9) | |
| min–max | 0–14 | |
| JIA subtype (ILAR 2004) [16] | Systemic * | 1 (6.3%) |
| Oligoarticular extended | 3 (18.8%) | |
| Polyarticular RF-negative | 9 (56.3%) | |
| Polyarticular RF-positive | 3 (18.8%) | |
| Patients on MTX at baseline, n (%) | 9 (56.3%) | |
| Patients on oral glucocorticoids at baseline, n (%) | 3 (18.8%) | |
| Patients with prior bDMARDs, n (%) | 9 (56.3%) | |
| Patients with ≥2 prior bDMARDs, n (%) | 8 (50.0%) | |
| Parameter | Baseline | Week 24 | p-Value |
|---|---|---|---|
| Active joints, median (Q1; Q3) mean (±SD) min-max | 8 (6.5; 17.5) 15.7 ± 15.8 6–61 | 2 (0.5; 10.5) 8.3 ± 11.4 0–35 | 0.002 |
| Physician’s VAS, cm, median (Q1; Q3) mean (±SD) min-max | 6.0 (4.0; 6.8) 5.7 ± 1.8 3.4–8.5 | 1.7 (1.2; 3.5) 2.3 ± 1.6 0.2–5.5 | <0.001 |
| Parents’ VAS, cm, median (Q1; Q3) mean (±SD) min-max | 6.1 (4.8; 7.2) 5.8 ± 1.7 2.3–8.2 | 1.3 (0.9; 4.8) 2.7 ± 2.5 0.0–7.0 | 0.001 |
| JADAS71, median (Q1; Q3) mean (±SD) min-max | 20.8 (18.4; 32.6) 28.4 ± 16.7 14.1–76.1 | 9.5 (2.7; 20.8) 13.6 ± 13.3 0.30–45.70 | <0.001 |
| CHAQ, median (Q1; Q3) mean (± SD) min-max | 1.00 (0.69; 1.44) 1.02 ± 0.49 0.38–2.00 | 0.25 (0.19; 0.75) 0.59 ± 0.64 0.00–1.88 | 0.043 |
| ESR, mm/h, median (Q1; Q3) mean (± SD) min-max | 15.0 (9.5; 33.5) 25.3 ± 24.3 2.0–88.0 | 5.0 (2.0; 7.0) 5.9 ± 4.7 2.00–15.00 | 0.004 |
| CRP, mg/L, median (Q1; Q3) mean (± SD) min-max | 7.3 (1.3; 24.6) 21.0 ± 29.1 0.4- 82.2 | 1.0 (0.2; 2.0) 3.8 ± 10.0 0.2–36.8 | 0.003 |
| System Organ Class Preferred Term | Olokizumab N = 16 | |
|---|---|---|
| n (%) | Total AEs | |
| Number of patients with at least one AE | 12 (75.0) | 23 |
| Blood and lymphatic system disorders | 1 (6.2) | 2 |
| Leukopenia | 1 (6.2) | 1 |
| Neutropenia | 1 (6.2) | 1 |
| Cardiac disorders | 2 (12.5) | 2 |
| Bundle branch block right * | 1 (6.2) | 1 |
| Sinus arrhythmia | 1 (6.2) | 1 |
| General disorders and administration site conditions | 1 (6.2) | 1 |
| Injection site hypersensitivity | 1 (6.2) | 1 |
| Hepatobiliary disorders | 1 (6.2) | 1 |
| Hepatotoxicity | 1 (6.2) | 1 |
| Infections and infestations | 6 (37.5) | 10 |
| Gastrointestinal viral infection | 1 (6.2) | 1 |
| Nasopharyngitis | 1 (6.2) | 1 |
| Otitis media acute | 2 (12.5) | 2 |
| Paronychia | 1 (6.2) | 1 |
| Pharyngitis | 1 (6.2) | 1 |
| Respiratory tract infection | 2 (12.5) | 2 |
| Rhinitis | 1 (6.2) | 1 |
| Varicella | 1 (6.2) | 1 |
| Investigations | 3 (18.8) | 3 |
| Blood alkaline phosphatase increased | 1 (6.2) | 1 |
| Blood bilirubin increased | 1 (6.2) | 1 |
| Blood pressure increased ** | 1 (6.2) | 1 |
| Nervous system disorders | 1 (6.2) | 1 |
| Headache | 1 (6.2) | 1 |
| Skin and subcutaneous tissue disorders | 2 (12.5) | 2 |
| Psoriasis | 1 (6.2) | 1 |
| Urticaria | 1 (6.2) | 1 |
| Vascular disorders | 1 (6.2) | 1 |
| Hypertensive crisis ** | 1 (6.2) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Alexeeva, E.I.; Dvoryakovskaya, T.M.; Nikishina, I.P.; Zholobova, E.S.; Matkava, V.G.; Krekhova, E.A.; Raupov, R.K.; Bukhanova, D.V.; Egorova, A.N.; Grishin, S.A.; et al. Open-Label Phase II Study of Olokizumab in Adolescent Patients with Polyarticular Juvenile Idiopathic Arthritis: Results of the 24-Week Treatment Period. Pharmaceuticals 2026, 19, 79. https://doi.org/10.3390/ph19010079
Alexeeva EI, Dvoryakovskaya TM, Nikishina IP, Zholobova ES, Matkava VG, Krekhova EA, Raupov RK, Bukhanova DV, Egorova AN, Grishin SA, et al. Open-Label Phase II Study of Olokizumab in Adolescent Patients with Polyarticular Juvenile Idiopathic Arthritis: Results of the 24-Week Treatment Period. Pharmaceuticals. 2026; 19(1):79. https://doi.org/10.3390/ph19010079
Chicago/Turabian StyleAlexeeva, Ekaterina I., Tatiana M. Dvoryakovskaya, Irina P. Nikishina, Elena S. Zholobova, Valeriya G. Matkava, Elizaveta A. Krekhova, Rinat K. Raupov, Daria V. Bukhanova, Alina N. Egorova, Sergey A. Grishin, and et al. 2026. "Open-Label Phase II Study of Olokizumab in Adolescent Patients with Polyarticular Juvenile Idiopathic Arthritis: Results of the 24-Week Treatment Period" Pharmaceuticals 19, no. 1: 79. https://doi.org/10.3390/ph19010079
APA StyleAlexeeva, E. I., Dvoryakovskaya, T. M., Nikishina, I. P., Zholobova, E. S., Matkava, V. G., Krekhova, E. A., Raupov, R. K., Bukhanova, D. V., Egorova, A. N., Grishin, S. A., Samsonov, M. Y., & Kostik, M. M. (2026). Open-Label Phase II Study of Olokizumab in Adolescent Patients with Polyarticular Juvenile Idiopathic Arthritis: Results of the 24-Week Treatment Period. Pharmaceuticals, 19(1), 79. https://doi.org/10.3390/ph19010079






