Benzotriazole in Cancer: A Systematic Review on Preclinical Evidence and Structure–Activity Relationship
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy and Selection of Studies
2.2. Data Extraction
2.3. Risk of Bias Assessment
3. Results and Discussions
3.1. In Vitro Evidence
3.1.1. N-Substituted Benzotriazole Derivatives
Benzotriazole-Aryl Derivatives
Benzotriazole-Alkyl-Aryl Derivatives
Bis-Benzotriazole Hybrids
3.1.2. C-Substituted Derivatives of Benzotriazole
3.1.3. Fused Benzotriazole Derivatives
3.1.4. Organometallic Compounds Containing Benzotriazole
3.1.5. Risk of Bias Assessment (QUIN Tool)
3.2. In Vivo Evidence
3.2.1. Qualitative Synthesis
3.2.2. Risk of Bias Assessment (SYRCLE Tool)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cirillo, N. Global Epidemiological Trends in the Incidence and Mortality for Melanoma. Ski. Health Dis. 2025, 5, 84–86. [Google Scholar] [CrossRef]
- World Health Organization. Mortality and Global Health Estimates. Global Health Observatory. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 1 October 2025).
- Qiu, Y. Exploring the Synergy of Immunotherapy and Conventional Treatments in Cancer Therapy. MedScien 2024, 2, 1–7. [Google Scholar] [CrossRef]
- Syed, B.M. Cancer Immunotherapy: Advances and Challenges. LIAQUAT Med. Res. J. 2024, 6, 1–3. [Google Scholar] [CrossRef]
- Pibiri, I. Recent Advances: Heterocycles in Drugs and Drug Discovery. Int. J. Mol. Sci. 2024, 25, 9503. [Google Scholar] [CrossRef] [PubMed]
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in Synthetic Approach to and Antifungal Activity of Triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.; Woo, L.W.L.; Trusselle, M.N.; Chander, S.K.; Purohit, A.; Reed, M.J.; Potter, B.V.L. Dual Aromatase–Sulfatase Inhibitors Based on the Anastrozole Template: Synthesis, in Vitro SAR, Molecular Modelling and in Vivo Activity. Org. Biomol. Chem. 2007, 5, 2940. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.M.; Woo, L.W.L.; Humphreys, A.; Chander, S.K.; Purohit, A.; Reed, M.J.; Potter, B.V.L. A Letrozole-Based Dual Aromatase–Sulphatase Inhibitor with in Vivo Activity. J. Steroid Biochem. Mol. Biol. 2005, 94, 123–130. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhao, L.-J.; Wang, Y.-T. Synthesis and Clinical Application of Small-Molecule Drugs Approved to Treat Prostatic Cancer. Eur. J. Med. Chem. 2023, 262, 115925. [Google Scholar] [CrossRef]
- dos Santos, G.C.; Martins, L.M.; Bregadiolli, B.A.; Moreno, V.F.; da Silva-Filho, L.C.; da Silva, B.H.S.T. Heterocyclic Compounds as Antiviral Drugs: Synthesis, Structure–Activity Relationship and Traditional Applications. J. Heterocycl. Chem. 2021, 58, 2226–2260. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, H.; Lin, K.; Wang, B.; Zhou, W. A Novel and Efficient Asymmetric Synthesis of Anti-HIV Drug Maraviroc. Synth. Commun. 2019, 49, 1721–1728. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Smith, R.B.; Kroboth, P.D.; Vanderlugt, J.T.; Phillips, J.P.; Juhl, R.P. Pharmacokinetics and Pharmacodynamics of Alprazolam after Oral and IV Administration. Psychopharmacology 1984, 84, 452–456. [Google Scholar] [CrossRef]
- Hansen, K.B.; Hsiao, Y.; Xu, F.; Rivera, N.; Clausen, A.; Kubryk, M.; Krska, S.; Rosner, T.; Simmons, B.; Balsells, J.; et al. Highly Efficient Asymmetric Synthesis of Sitagliptin. J. Am. Chem. Soc. 2009, 131, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.G.; Fletcher, A.M.; Thomson, J.E. Syntheses of (R)-Sitagliptin. Tetrahedron Asymmetry 2015, 26, 1109–1116. [Google Scholar] [CrossRef]
- Arroyo, S. Rufinamide. Neurotherapeutics 2007, 4, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Kshatriya, M.R.; Gajjar, J.A. Benzotriazole and Its Derivatives: A Comprehensive Review of Its Synthesis, Activities and Applications. Curr. Chem. Lett. 2025, 14, 299–322. [Google Scholar] [CrossRef]
- Okutani, H.; Lo Vecchio, S.; Arendt-Nielsen, L. Mechanisms and Treatment of Opioid-induced Pruritus: Peripheral and Central Pathways. Eur. J. Pain 2024, 28, 214–230. [Google Scholar] [CrossRef]
- Saini, P.; Sharma, S.; Rani, D.; Singh, V.; Sharma, S.; Thakur, S. An overview on innovative sunscreens transform skin cancer protection. World J. Pharm. Res. 2025, 14, 182–201. [Google Scholar] [CrossRef]
- Goss, P.E. Pre-Clinical and Clinical Review of Vorozole, a New Third Generation Aromatase Inhibitor. Breast Cancer Res. Treat. 1998, 49, S59–S65. [Google Scholar] [CrossRef]
- Sayyad, N.B.; Sabale, P.M.; Umare, M.D.; Bajaj, K.K. Aromatase Inhibitors: Development and Current Perspectives. Indian J. Pharm. Educ. Res. 2022, 56, 311–320. [Google Scholar] [CrossRef]
- Bollikolla, H.B.; Boddapati, S.N.M.; Thangamani, S.; Mutchu, B.R.; Alam, M.M.; Hussien, M.; Jonnalagadda, S.B. Advances in Synthesis and Biological Activities of Benzotriazole Analogues: A Micro Review. J. Heterocycl. Chem. 2023, 60, 705–742. [Google Scholar] [CrossRef]
- Briguglio, I.; Piras, S.; Corona, P.; Gavini, E.; Nieddu, M.; Boatto, G.; Carta, A. Benzotriazole: An Overview on Its Versatile Biological Behavior. Eur. J. Med. Chem. 2015, 97, 612–648. [Google Scholar] [CrossRef]
- Kassab, A.E. Benzotriazole Scaffold: An Overview of Antiproliferative Potential, Mechanisms of Action, and Structure–Activity Relationships. Arch. Pharm. 2023, 356, 2300102. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 15 May 2025).
- Elsevier. Scopus. Available online: https://www.scopus.com/ (accessed on 15 May 2025).
- Clarivate. Web of Science. Available online: https://www.webofscience.com/ (accessed on 15 May 2025).
- Mardale, G.; Prodea, A.; Milan, A.M.; Jorgovan, M.; Mardale, S.; Dumitrascu, V.C.; Soica, C. Review Protocol: Benzotriazole in Cancer: Preclinical Evidence and Structure–Activity Relationship Insights (Systematic Review). Available online: https://zenodo.org/records/17950716 (accessed on 16 December 2025). [CrossRef]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and Validation of a Risk-of-Bias Tool for Assessing in Vitro Studies Conducted in Dentistry: The QUIN. J. Prosthet. Dent. 2024, 131, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Servier. SMART—Servier Medicinal and Academic Research Tools. Available online: https://smart.servier.com/ (accessed on 12 September 2025).
- Creative Commons. Creative Commons Attribution 4.0 International License. Available online: https://creativecommons.org/licenses/by/4.0/ (accessed on 12 September 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dixit, P.P.; Nair, P.S.; Patil, V.J.; Jain, S.; Arora, S.K.; Sinha, N. Synthesis and Antibacterial Activity of Novel (Un)Substituted Benzotriazolyl Oxazolidinone Derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 3002–3005. [Google Scholar] [CrossRef] [PubMed]
- Loddo, R.; Novelli, F.; Sparatore, A.; Tasso, B.; Tonelli, M.; Boido, V.; Sparatore, F.; Collu, G.; Delogu, I.; Giliberti, G.; et al. Antiviral Activity of Benzotriazole Derivatives. 5-[4-(Benzotriazol-2-Yl)Phenoxy]-2,2-Dimethylpentanoic Acids Potently and Selectively Inhibit Coxsackie Virus B5. Bioorg. Med. Chem. 2015, 23, 7024–7034. [Google Scholar] [CrossRef]
- Sudhir, M.S.; Venkata Nadh, R.; Radhika, S. Antifungal Activities of Novel 1,2,3-Benzotriazole Derivatives Synthesized by Ultrasonic and Solvent-Free Conditions. Drug Invent. Today 2013, 5, 126–132. [Google Scholar] [CrossRef]
- Khan, I.; Rehman, W.; Rahim, F.; Hussain, R.; Khan, S.; Fazil, S.; Rasheed, L.; Taha, M.; Shah, S.A.A.; Abdellattif, M.H.; et al. Synthesis, In Vitro α-Glucosidase Inhibitory Activity and Molecular Docking Study of New Benzotriazole-Based Bis-Schiff Base Derivatives. Pharmaceuticals 2022, 16, 17. [Google Scholar] [CrossRef]
- Singh, V.K.; Rishishwar, P.; Bharadwaj, P.; Alok, S. Synthesis and Anticonvulsant Activity of Some Novel Benzotriazole Derivatives. Int. J. Pharm. Sci. Res. 2020, 11, 352–357. [Google Scholar] [CrossRef]
- Fabitha, K.; Kallingal, A.; Maciejewska, N.; Arya, C.G.; Chandrakanth, M.; Thomas, N.M.; Li, Y.; Gondru, R.; Munikumar, M.; Banothu, J. Novel Fused Pyran Derivatives Induce Apoptosis and Target Cell Cycle Progression in Anticancer Efficacy against Multiple Cell Lines. New J. Chem. 2024, 48, 8038–8054. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Mohamed, K.O.; Malebari, A.M.; El-malah, A. Substituted Imidazole-Thione Linked. Molecules 2021, 26, 5983. [Google Scholar] [CrossRef] [PubMed]
- Korcz, M.; Saczewski, F.; Bednarski, P.J.; Kornicka, A. Synthesis, Structure, Chemical Stability, and in Vitro Cytotoxic Properties of Novel Quinoline-3-Carbaldehyde Hydrazones Bearing a 1,2,4-Triazole or Benzotriazole Moiety. Molecules 2018, 23, 1497. [Google Scholar] [CrossRef] [PubMed]
- Pogaku, V.; Krishna, V.S.; Balachandran, C.; Rangan, K.; Sriram, D.; Aoki, S.; Basavoju, S. The Design and Green Synthesis of Novel Benzotriazoloquinolinyl Spirooxindolopyrrolizidines: Antimycobacterial and Antiproliferative Studies. New J. Chem. 2019, 43, 17511–17520. [Google Scholar] [CrossRef]
- Mioc, M.; Mioc, A.; Prodea, A.; Milan, A.; Balan-Porcarasu, M.; Racoviceanu, R.; Ghiulai, R.; Iovanescu, G.; Macasoi, I.; Draghici, G.; et al. Novel Triterpenic Acid—Benzotriazole Esters Act as Pro-Apoptotic Antimelanoma Agents. Int. J. Mol. Sci. 2022, 23, 9992. [Google Scholar] [CrossRef]
- Kuran, D.; Flis, S.; Antoszczak, M.; Piskorek, M.; Huczyński, A. Ester Derivatives of Salinomycin Efficiently Eliminate Breast Cancer Cells via ER-Stress-Induced Apoptosis. Eur. J. Pharmacol. 2021, 893, 173824. [Google Scholar] [CrossRef]
- Alraqa, S.Y.; Alharbi, K.; Aljuhani, A.; Rezki, N.; Aouad, M.R.; Ali, I. Design, Click Conventional and Microwave Syntheses, DNA Binding, Docking and Anticancer Studies of Benzotriazole-1,2,3-Triazole Molecular Hybrids with Different Pharmacophores. J. Mol. Struct. 2021, 1225, 129192. [Google Scholar] [CrossRef]
- Mermer, A.; Volkan Bulbul, M.; Mervenur Kalender, S.; Keskin, I.; Tuzun, B.; Emre Eyupoglu, O. Benzotriazole-Oxadiazole Hybrid Compounds: Synthesis, Anticancer Activity, Molecular Docking and ADME Profiling Studies. J. Mol. Liq. 2022, 359, 119264. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, Y.; He, L.-Q.; Liu, Z.-J.; Jiang, A.-Q.; Yang, Y.-H.; Zhu, H.-L. Synthesis, Biological Evaluation, and Molecular Docking Studies of Novel 1,3,4-Oxadiazole Derivatives Possessing Benzotriazole Moiety as FAK Inhibitors with Anticancer Activity. Bioorg. Med. Chem. 2013, 21, 3723–3729. [Google Scholar] [CrossRef]
- Khodair, A.I.; Attia, A.M.; Gendy, E.A.; Elshaier, Y.A.M.M.; El-Magd, M.A. Discovery of New S -Glycosides and N -Glycosides of Pyridine-biphenyl System with Antiviral Activity and Induction of Apoptosis in MCF 7 Cells. J. Heterocycl. Chem. 2019, 56, 1733–1747. [Google Scholar] [CrossRef]
- Garton, C.S.; Derose, N.K.; Dominguez, D.; Turbi-Henderson, M.L.; Lehr, A.L.; Padilla, A.D.; Twining, S.D.; Casas, S.; Alozie, C.O.; Gucwa, A.L.; et al. Synthesis and Antiproliferative Evaluation of 2-Deoxy-n-Glycosylbenzotriazoles/Imidazoles. Molecules 2021, 26, 3742. [Google Scholar] [CrossRef]
- Anusha, D.; Susithra, E. Synthesis and Biological Evaluation of Substituted Mannich Bases of Benzotriazole Derivatives As Anticancer Agents. Eur. Chem. Bull. 2022, 11, 62–71. [Google Scholar]
- Zoroddu, S.; Sanna, L.; Bordoni, V.; Weidong, L.; Gadau, S.D.; Carta, A.; Kelvin, D.J.; Bagella, L. Identification of 3-Aryl-1-Benzotriazole-1-Yl-Acrylonitrile as a Microtubule-Targeting Agent (MTA) in Solid Tumors. Int. J. Mol. Sci. 2024, 25, 5704. [Google Scholar] [CrossRef] [PubMed]
- Kassab, A.E.; Hassan, R.A. Novel Benzotriazole N-Acylarylhydrazone Hybrids: Design, Synthesis, Anticancer Activity, Effects on Cell Cycle Profile, Caspase-3 Mediated Apoptosis and FAK Inhibition. Bioorg. Chem. 2018, 80, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Kumar, M.; Violet Dhayabaran, V.; Sudhapriya, N.; Manikandan, A.; Gideon, D.A.; Annapoorani, S. P-TSA.H2O Mediated One-Pot, Multi-Component Synthesis of Isatin Derived Imidazoles as Dual-Purpose Drugs against Inflammation and Cancer. Bioorg. Chem. 2020, 102, 104046. [Google Scholar] [CrossRef]
- Qadri, T.; Aziz, M.; Channar, P.A.; Ejaz, S.A.; Hussain, M.; Attaullah, H.M.; Ujan, R.; Hussain, Z.; Zehra, T.; Saeed, A.; et al. Synthesis, Biological Evaluation and in Silico Investigations of Benzotriazole Derivatives as Potential Inhibitors of NIMA Related Kinase. RSC Adv. 2023, 13, 33826–33843. [Google Scholar] [CrossRef]
- Aziz, M.; Ejaz, S.A.; Channar, P.A.; Alkhathami, A.G.; Qadri, T.; Hussain, Z.; Hussaain, M.; Ujan, R. Identification of Dimethyl 2,2′-((Methylenebis(2-(2H-Benzo[d][1,2,3]Triazol-2-Yl)-4-(2,4,4-Trimethylpentan-2-Yl)-6,1phenylene))Bis(Oxy))Diacetate (TAJ4) as Antagonist of NEK-Family: A Future for Potential Drug Discovery. BMC Cancer 2024, 24, 1521. [Google Scholar] [CrossRef]
- Pon Matheswari, P.; Ilavarasi Jeyamalar, J.; Iruthayaraj, A.; Ravindran Durai Nayagam, B. Synthesis, Structural, Multitargeted Molecular Docking Analysis of Anti-Cancer, Anti-Tubercular, DNA Interactions of Benzotriazole Based Macrocyclic Ligand. Bioorg. Chem. 2024, 147, 107361. [Google Scholar] [CrossRef]
- Grudzińska, M.; Stachnik, B.; Galanty, A.; Sołtys, A.; Podolak, I. Progress in Antimelanoma Research of Natural Triterpenoids and Their Derivatives: Mechanisms of Action, Bioavailability Enhancement and Structure Modifications. Molecules 2023, 28, 7763. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A View on Drug Resistance in Cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Laconde, G.; Amblard, M.; Martinez, J. A Simple and Versatile Method to Synthesize N-Acyl-Benzotriazoles. Tetrahedron Lett. 2019, 60, 341–343. [Google Scholar] [CrossRef]
- Chuang, H.-H.; Zhen, Y.-Y.; Tsai, Y.-C.; Chuang, C.-H.; Hsiao, M.; Huang, M.-S.; Yang, C.-J. FAK in Cancer: From Mechanisms to Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef]
- Thiruchenthooran, V.; Sánchez-López, E.; Gliszczyńska, A. Perspectives of the Application of Non-Steroidal Anti-Inflammatory Drugs in Cancer Therapy: Attempts to Overcome Their Unfavorable Side Effects. Cancers 2023, 15, 475. [Google Scholar] [CrossRef]
- Lopez-Tapia, F.; Brotherton-Pleiss, C.; Yue, P.; Murakami, H.; Costa Araujo, A.C.; Reis dos Santos, B.; Ichinotsubo, E.; Rabkin, A.; Shah, R.; Lantz, M.; et al. Linker Variation and Structure–Activity Relationship Analyses of Carboxylic Acid-Based Small Molecule STAT3 Inhibitors. ACS Med. Chem. Lett. 2018, 9, 250–255. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The Influence of Lipophilicity in Drug Discovery and Design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- McKeage, M.J.; Berners-Price, S.J.; Galettis, P.; Bowen, R.J.; Brouwer, W.; Ding, L.; Zhuang, L.; Baguley, B.C. Role of Lipophilicity in Determining Cellular Uptake and Antitumour Activity of Gold Phosphine Complexes. Cancer Chemother. Pharmacol. 2000, 46, 343–350. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the Role of ABC Transporters in Multidrug-Resistant Cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Halloran, D.; Pandit, V.; Nohe, A. The Role of Protein Kinase CK2 in Development and Disease Progression: A Critical Review. J. Dev. Biol. 2022, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Trembley, J.H.; Kren, B.T.; Abedin, M.J.; Shaughnessy, D.P.; Li, Y.; Dehm, S.M.; Ahmed, K. CK2 Pro-Survival Role in Prostate Cancer Is Mediated via Maintenance and Promotion of Androgen Receptor and NFκB P65 Expression. Pharmaceuticals 2019, 12, 89. [Google Scholar] [CrossRef]
- Zwicker, F.; Hauswald, H.; Weber, K.J.; Debus, J.; Huber, P.E. In Vivo Evaluation of Combined CK2 Inhibition and Irradiation in Human WiDr Tumours. In Vivo 2021, 35, 111–117. [Google Scholar] [CrossRef]
- Shehata, M.; Schnabl, S.; Demirtas, D.; Hilgarth, M.; Hubmann, R.; Ponath, E.; Badrnya, S.; Lehner, C.; Hoelbl, A.; Duechler, M.; et al. Reconstitution of PTEN Activity by CK2 Inhibitors and Interference with the PI3-K/Akt Cascade Counteract the Antiapoptotic Effect of Human Stromal Cells in Chronic Lymphocytic Leukemia. Blood 2010, 116, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Łukowska-Chojnacka, E.; Wińska, P.; Wielechowska, M.; Bretner, M. Synthesis of Polybrominated Benzimidazole and Benzotriazole Derivatives Containing a Tetrazole Ring and Their Cytotoxic Activity. Monatshefte Chem. 2016, 147, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, K.; Wińska, P.; Skierka, K.; Wielechowska, M.; Bretner, M. Synthesis, in Vitro Antiproliferative Activity and Kinase Profile of New Benzimidazole and Benzotriazole Derivatives. Bioorg. Chem. 2017, 72, 1–10. [Google Scholar] [CrossRef]
- Swider, R.; Masłyk, M.; Zapico, J.M.; Coderch, C.; Panchuk, R.; Skorokhyd, N.; Schnitzler, A.; Niefind, K.; De Pascual-Teresa, B.; Ramos, A. Synthesis, Biological Activity and Structural Study of New Benzotriazole-Based Protein Kinase CK2 Inhibitors. RSC Adv. 2015, 5, 72482–72494. [Google Scholar] [CrossRef]
- Borowiecki, P.; Wińska, P.; Bretner, M.; Gizińska, M.; Koronkiewicz, M.; Staniszewska, M. Synthesis of Novel Proxyphylline Derivatives with Dual Anti-Candida Albicans and Anticancer Activity. Eur. J. Med. Chem. 2018, 150, 307–333. [Google Scholar] [CrossRef]
- El-Kardocy, A.; Mostafa, Y.A.; Mohamed, N.G.; Abo-Zeid, M.N.; Hassan, N.A.; Hetta, H.F.; Abdel-Aal, A.B.M. CK2 Inhibition, Lipophilicity and Anticancer Activity of New: N 1versus N 2-Substituted Tetrabromobenzotriazole Regioisomers. New J. Chem. 2020, 44, 13007–13017. [Google Scholar] [CrossRef]
- Wu, L.Q.; Ma, X.; Liu, Z.P. Design, Synthesis, and Biological Evaluation of 3-(1-Benzotriazole)-nor-β-Lapachones as NQO1-Directed Antitumor Agents. Bioorg. Chem. 2021, 113, 104995. [Google Scholar] [CrossRef]
- Ibba, R.; Piras, S.; Corona, P.; Riu, F.; Loddo, R.; Delogu, I.; Collu, G.; Sanna, G.; Caria, P.; Dettori, T.; et al. Synthesis, Antitumor and Antiviral In Vitro Activities of New Benzotriazole-Dicarboxamide Derivatives. Front. Chem. 2021, 9, 660424. [Google Scholar] [CrossRef]
- Li, Q.; Liu, G.; Wang, N.; Yin, H.; Li, Z. Synthesis and Anticancer Activity of Benzotriazole Derivatives. J. Heterocycl. Chem. 2020, 57, 1220–1227. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Xiong, X.Q.; Yang, H.; Wang, P.; Zhang, K.; Awadasseid, A.; Narva, S.; Wu, Y.L.; Zhang, W. Design, Synthesis and Bioactivity of Novel Naphthalimide-Benzotriazole Conjugates against A549 Cells via Targeting BCL2 G-Quadruplex and Inducing Autophagy. Life Sci. 2022, 302, 120651. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, Z.; Wang, X.; Ma, Y.; Li, Y.; Yang, S.; Shi, Y. Discovery of 12O—A Novel Oral Multi-Kinase Inhibitor for the Treatment of Solid Tumor. Molecules 2020, 25, 5199. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Safari, M.; Hekmati, M.; Hekmat, S.; Azin, A. Modification of Carboxylated Multiwall Nanotubes with Benzotriazole Derivatives and Study of Their Anticancer Activities. Med. Chem. Res. 2014, 23, 487–495. [Google Scholar] [CrossRef]
- He, Y.; Pan, Y.; Zhao, X.; Fan, W.; Cai, Y.; Mou, X. NIR-II Absorptive Dithienopyrrole-Thiadiazolobenzotriazole Conjugated Polymer for Photoacoustic Imaging-Guided Glioblastoma Multiforme Photothermal Therapy. Acta Biomater. 2022, 152, 546–561. [Google Scholar] [CrossRef]
- Parsons, J.L.; Dianova, I.I.; Finch, D.; Tait, P.S.; Ström, C.E.; Helleday, T.; Dianov, G.L. XRCC1 Phosphorylation by CK2 Is Required for Its Stability and Efficient DNA Repair. DNA Repair 2010, 9, 835–841. [Google Scholar] [CrossRef]
- Pellarin, I.; Dall’Acqua, A.; Favero, A.; Segatto, I.; Rossi, V.; Crestan, N.; Karimbayli, J.; Belletti, B.; Baldassarre, G. Cyclin-Dependent Protein Kinases and Cell Cycle Regulation in Biology and Disease. Signal Transduct. Target. Ther. 2025, 10, 11. [Google Scholar] [CrossRef]
- Shibuya, M. Tyrosine Kinase Receptor Flt/VEGFR Family: Its Characterization Related to Angiogenesis and Cancer. Genes Cancer 2010, 1, 1119–1123. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Advances in the Design of Organometallic Anticancer Complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Romani, A.M.P. Cisplatin in Cancer Treatment. Biochem. Pharmacol. 2022, 206, 115323. [Google Scholar] [CrossRef] [PubMed]
- Onar, G.; Gürses, C.; Karataş, M.O.; Balcıoğlu, S.; Akbay, N.; Özdemir, N.; Ateş, B.; Alıcı, B. Palladium(II) and Ruthenium(II) Complexes of Benzotriazole Functionalized N-Heterocyclic Carbenes: Cytotoxicity, Antimicrobial, and DNA Interaction Studies. J. Organomet. Chem. 2019, 886, 48–56. [Google Scholar] [CrossRef]
- Stamou, C.; Gourdoupi, C.; Dechambenoit, P.; Papaioannou, D.; Piperigkou, Z.; Lada, Z.G. Antiproliferative Activity of an Organometallic Sn(IV) Coordination Compound Based on 1-Methylbenzotriazole against Human Cancer Cell Lines. Chemistry 2024, 6, 1189–1200. [Google Scholar] [CrossRef]
- El-Asmy, H.A.; Butler, I.S.; Mouhri, Z.S.; Jean-Claude, B.J.; Emmam, M.S.; Mostafa, S.I. Zinc(II), Ruthenium(II), Rhodium(III), Palladium(II), Silver(I), Platinum(II) and MoO 2 2 + Complexes of 2-(2′-Hydroxy-5′- Methylphenyl)-Benzotriazole as Simple or Primary Ligand and 2,2′- Bipyridyl, 9,10-Phenanthroline or Triphenylphosphine as Secondary. J. Mol. Struct. 2014, 1059, 193–201. [Google Scholar] [CrossRef]
- Mansour, A.M.; Mohamed, M.F. Complexes of N-(2-Thiazolyl)-1H-Benzotriazole-1-Carbothioamide with Pd(II), Pt(II), and Zn(II): Spectral, DFT, Cytotoxicity and Anti-Angiogenic Effect on MCF-7 Cell Line. Inorg. Chim. Acta 2014, 423, 373–383. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, Y.; Hu, J.; Yu, H.; Zhi, S.; Zhang, J. Potential Anticancer Activity of Benzimidazole-Based Mono/Dinuclear Zn(II) Complexes towards Human Carcinoma Cells. Polyhedron 2015, 102, 163–172. [Google Scholar] [CrossRef]
- Zhao, J.A.; Yu, H.B.; Zhi, S.C.; Mao, R.N.; Hu, J.Y.; Wang, X.X. Synthesis, Chemical Nuclease Activity, and in Vitro Cytotoxicity of Benzimidazole-Based Cu(II)/Co(II) Complexes. Chin. Chem. Lett. 2017, 28, 1539–1546. [Google Scholar] [CrossRef]
- Li, R.; Cui, B.; Li, Y.; Zhao, C.; Jia, N.; Wang, C.; Wu, Y.; Wen, A. A New Synthetic Cu(II) Compound, [Cu3(p-3-Bmb)2Cl4·(CH3OH)2], Inhibits Tumor Growth in Vivo and in Vitro. Eur. J. Pharmacol. 2014, 724, 77–85. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Y.; Zhao, J.; Zhang, J. In Vitro Antitumor Activity of Novel Benzimidazole-Based Cu(II) Complexes. Bioorg. Med. Chem. 2017, 25, 5733–5742. [Google Scholar] [CrossRef]
- Titova, S.A.; Kruglova, M.P.; Stupin, V.A.; Manturova, N.E.; Silina, E.V. Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine. Pharmaceuticals 2025, 18, 154. [Google Scholar] [CrossRef]
- Tran, L.; Tam, D.N.H.; Elshafay, A.; Dang, T.; Hirayama, K.; Huy, N.T. Quality Assessment Tools Used in Systematic Reviews of in Vitro Studies: A Systematic Review. BMC Med. Res. Methodol. 2021, 21, 101. [Google Scholar] [CrossRef]
- Covert, E.C.; Fitzpatrick, K.; Mikell, J.; Kaza, R.K.; Millet, J.D.; Barkmeier, D.; Gemmete, J.; Christensen, J.; Schipper, M.J.; Dewaraja, Y.K. Intra- and Inter-Operator Variability in MRI-Based Manual Segmentation of HCC Lesions and Its Impact on Dosimetry. EJNMMI Phys. 2022, 9, 90. [Google Scholar] [CrossRef]
- França, T.F.A.; Monserrat, J.M. Reproducibility Crisis in Science or Unrealistic Expectations? EMBO Rep. 2018, 19, e46008. [Google Scholar] [CrossRef]
- Valizadeh, M.; Gheidari, A.; Daghestani, N.; Mohammadzadeh, Z.; Khorakian, F. Evaluation of Various Root Canal Irrigation Methods in Primary Teeth: A Systematic Review. BMC Oral Health 2024, 24, 1535. [Google Scholar] [CrossRef]
- Di Stasio, D.; Romano, A.; Fiori, F.; Assanti, R.A.; Ruocco, E.; Bottone, M.G.; Lucchese, A. Photodynamic Therapy Effects on Oral Dysplastic Keratinocyte Cell Cultures: A Systematic Review. Appl. Sci. 2023, 13, 9075. [Google Scholar] [CrossRef]
- Jakab, A.; Palkovics, D.; Szabó, V.T.; Szabó, B.; Vincze-Bandi, E.; Braunitzer, G.; Lassila, L.; Vallittu, P.; Garoushi, S.; Fráter, M. Mechanical Performance of Extensive Restorations Made with Short Fiber-Reinforced Composites without Coverage: A Systematic Review of In Vitro Studies. Polymers 2024, 16, 590. [Google Scholar] [CrossRef] [PubMed]
- Mounika Veeraiyan, D.J.S.; Saravanan Poorni, D.S.S.; Praveena Kannabiran, D.D. Efficacy of Nano Hydroxyapatite Derived From Marine Sources on Remineralization of Enamel: A Systematic Review. Nanotechnol. Perceptions 2024, 20, 1366–1378. [Google Scholar] [CrossRef]
- Poondru, S.; Parchment, R.E.; Purohit, V.; LoRusso, P.; Horwitz, J.P.; Hazeldine, S.T.; Polin, L.; Corbett, T.; Jasti, B.R. Lack of in Vitro—In Vivo Correlation of a Novel Investigational Anticancer Agent, SH 30. Investig. New Drugs 2002, 20, 23–33. [Google Scholar] [CrossRef]
- Herdiana, Y. Bridging the Gap: The Role of Advanced Formulation Strategies in the Clinical Translation of Nanoparticle-Based Drug Delivery Systems. Int. J. Nanomed. 2025, 20, 13039–13053. [Google Scholar] [CrossRef] [PubMed]
- Grosset, A.-A.; Ouellet, V.; Caron, C.; Fragoso, G.; Barrès, V.; Delvoye, N.; Latour, M.; Aprikian, A.; Bergeron, A.; Chevalier, S.; et al. Validation of the Prognostic Value of NF-ΚB P65 in Prostate Cancer: A Retrospective Study Using a Large Multi-Institutional Cohort of the Canadian Prostate Cancer Biomarker Network. PLoS Med. 2019, 16, e1002847. [Google Scholar] [CrossRef]
- Dalal, K.; Roshan-Moniri, M.; Sharma, A.; Li, H.; Ban, F.; Hessein, M.; Hsing, M.; Singh, K.; LeBlanc, E.; Dehm, S.; et al. Selectively Targeting the DNA-Binding Domain of the Androgen Receptor as a Prospective Therapy for Prostate Cancer. J. Biol. Chem. 2014, 289, 26417–26429. [Google Scholar] [CrossRef]
- Annese, T.; Errede, M.; d’Amati, A.; De Giorgis, M.; Lorusso, L.; Tamma, R.; Ribatti, D. Differential P-Glycoprotein/CD31 Expression as Markers of Vascular Co-Option in Primary Central Nervous System Tumors. Diagnostics 2022, 12, 3120. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a Proliferation Marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Bebarta, V.; Luyten, D.; Heard, K. Emergency Medicine Animal Research: Does Use of Randomization and Blinding Affect the Results? Acad. Emerg. Med. 2003, 10, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Landis, S.C.; Amara, S.G.; Asadullah, K.; Austin, C.P.; Blumenstein, R.; Bradley, E.W.; Crystal, R.G.; Darnell, R.B.; Ferrante, R.J.; Fillit, H.; et al. A Call for Transparent Reporting to Optimize the Predictive Value of Preclinical Research. Nature 2012, 490, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting Animal Research: Explanation and Elaboration for the ARRIVE Guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.A.; Howick, J.; Aronson, J.K.; Roberts, N.; Perera, R.; Koshiaris, C.; Heneghan, C. The Need for Randomization in Animal Trials: An Overview of Systematic Reviews. PLoS ONE 2014, 9, e98856. [Google Scholar] [CrossRef]
- Sena, E.S.; van der Worp, H.B.; Bath, P.M.W.; Howells, D.W.; Macleod, M.R. Publication Bias in Reports of Animal Stroke Studies Leads to Major Overstatement of Efficacy. PLoS Biol. 2010, 8, e1000344. [Google Scholar] [CrossRef]
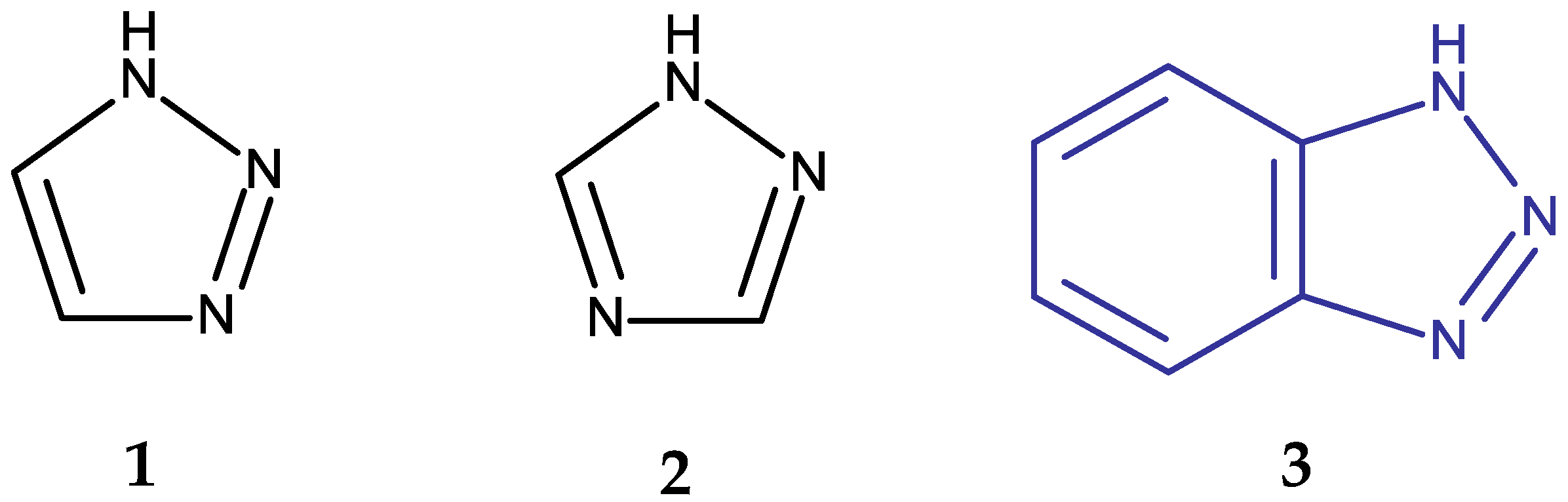




| Chemical Structure | Compound ID | IC50 (μM or μg/mL), Inhibition Rate (%) | Mechanism of Action | Reference | |
|---|---|---|---|---|---|
| Core Structure | Substituents | ||||
| Direct benzotriazole-aryl derivatives | |||||
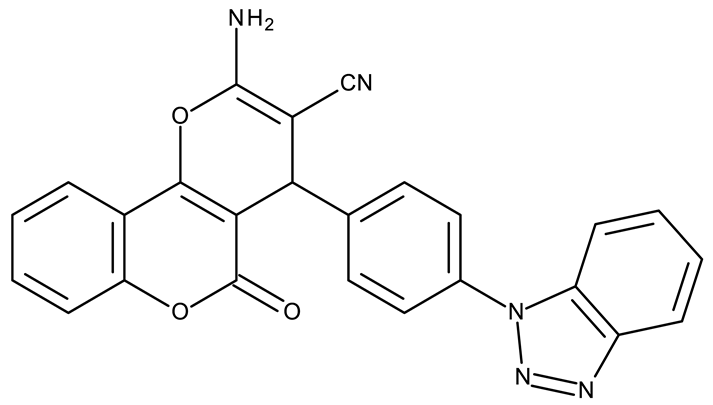 | - | ND 1 (6e) | MCF-7: 12.47 μM A549, HCT116: >50 μM | ↑cell cycle arrest (G0/G1, S and G2/M) | [39] |
 | - | ND 2 (8e) | HCT116: 21.43 μM MCF-7, A549: >50 μM | ||
 | - | ND 3 (10e) | MCF-7, A549, HCT116: >50 μM | ||
 | - | ND 4 (12e) | MCF-7, A549, HCT116: >50 μM | ||
 | - | ND 5 (14e) | MCF-7, A549, HCT116: >50 μM | ||
 | R = H (ND 6) R = 4-methyl (ND 7) R = 4-ethyl (ND 8) R = 4-methoxy (ND 9) R = 4-hydroxyl (ND 10) R = 4-Cl (ND 11) R = 2-Cl (ND 12) R = 3-Cl (ND 13) R = 2,4-(Cl)2 (ND 14) R = 2-F (ND 15) R = 4-Br (ND 16) R = 4-sulphone-amide (ND 17) | ND 6–17 (BI1–12) | MCF-7: 2.29–38.2 μM HL-60: 0.4–37.1 μM HCT-116: 1.51–17.5 μM | ↑apoptosis by ↑PARP cleavage, ↑BAX and ↓Bcl-2; ↑cell cycle arrest (G2/M) | [40] |
 | R1 = H, R2 = H (ND 18) R1 = methyl, R2 = methyl, (ND 19) R1 = H, R2 = phenyl (ND 20) R1 = H, R2 = 2-hydroxyethyl (ND 21) R1 = H, R2 = 2-pyridyl (ND 22) | ND 18–22 (5a–e) | DAN-G: 1.35–6.38 μM LCLC-103H: 1.23–6.29 μM SISO: 1.49–6.23 μM | N/A | [41] |
 | R = phenyl (ND 23) R = 4-methylphenyl (ND 24) R = 4-methoxyphenyl (ND 25) R = 4-chlorophenyl (ND 26) R = 4-fluorophenyl (ND 27) R = 2-furyl (ND 28) R = 2-thienyl (ND 29) R = cyclopentyl (ND 30) | ND 23–30 (7a–h) | DAN-G: 0.6–56.3 μM LCLC-103H: 3.6–51.1 μM SISO: 0.2–53.8 μM | ||
 | R = phenyl (ND 31) R = 4 methylphenyl (ND 32) R = 4-methoxyphenyl (ND 33) R = 4-chlorophenyl (ND 34) R = 4-fluorophenyl (ND 35) R = 2,4,6-trimethylphenyl (ND 36) R = 4-tert-butylphenyl (ND 37) R = 2-naphthyl (ND 38) | ND 31–38 (9a–h) | DAN-G: 26.1–82.1 μM LCLC-103H: 8.1–37.5 μM SISO: 0.6–34.4 μM | ||
 | R1 = H, R2 = H (ND 39) R1 = H, R2 = methyl (ND 40) R1 = H, R2 = ethyl (ND 41) R1 = H, R2 = methoxy (ND 42) R1 = H, R2 = F (ND 43) R1 = H, R2 = Cl (ND 44) R1 = H, R2 = Br (ND 45) R1 = Cl, R2 = H (ND 46) R1 = Cl, R2 = methyl (ND 47) R1 = Cl, R2 = ethyl (ND 48) R1 = Cl, R2 = methoxy (ND 49) R1 = Cl, R2 = F (ND 50) R1 = Cl, R2 = Cl (ND 51) R1 = Cl, R2 = Br (ND 52) R1 = Br, R2 = H (ND 53) R1 = Br, R2 = methoxy (ND 54) | ND 39–54 (4a–p) | A549: 9.81–>100 μM HeLa S3: 20.4–>100 μM | N/A | [42] |
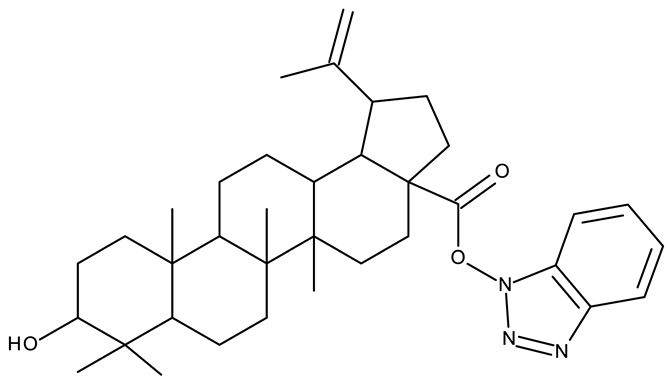 | - | ND 55–57 (1–3) | A375: 12.6–37.5% | ↑apoptosis (intrinsic pathway) | [43] |
 | - | ||||
 | - | ||||
 | - | ND 58 (7) | MCF-7: 4.1 μM MDA-MB-231: 2.6 μM | ↑apoptosis (intrinsic pathway); ↑cell cycle arrest (G2/M); ↑p53; ↑p-eIF2α; ↑IRE1α ↑γH2AX and ↑8-oxoG | [44] |
| Benzotriazole-alkyl-aryl derivatives | |||||
 | R = 4-nitrophenyl (ND 59) R = 3,4-dichlorophenyl (ND 60) R = 4-acetylphenyl (ND 61) R = 4-carboxyphenyl (ND 62) R = 4-ethoxycarbonylphenyl (ND 63) R = benzyl (ND 64) R = phenethoxy (ND 65) R = ethoxycarbonylethyl (ND 66) R = 4-methoxyphenylacetyl (ND 67) R = 4-methylphenylacetyl (ND 68) R = N-benzylacetamide (ND 69) R = N-(4-methoxybenzyl)acetamide (ND 70) R = N-(4-fluorobenzyl)acetamide (ND 71) R = N-(4-chlorobenzyl)acetamide (ND 72) R = N-(4-fluorophenyl)acetamide (ND 73) R = N-(2-fluoro-4-iodophenyl)acetamide (ND 74) R = N-(2,4,5-trifluorophenyl)acetamide (ND 75) R = N-(4-carboxyphenyl)acetamide (ND 76) R = N-(4-nitrophenyl)acetamide (ND 77) R = N-(4-iodophenyl)acetamide (ND 78) | ND 59–78 (4a–e, 6a–e, 8a–j) | A549: 78–89% H-1229: 70–92% | N/A | [45] |
 | R = 3,4-dimethylphenyl (ND 79) R = 3,4-dimethoxyphenyl (ND 80) R = 4-trifluoromethylphenyl (ND 81) R = 3,4-diethoxyphenyl (ND 82) R = 2-methylphenyl (ND 83) R = 3,5-dimethoxyphenyl (ND 84) R = 2-naphthyl (ND 85) R = 3-chloro-4-nitrophenyl (ND 86) R = 6-quinolyl (ND 87) R = 4-hydroxy-3-methoxyphenyl (ND 88) R = 4-hydroxyphenyl (ND 89) R = 4-sulfamoylphenyl (ND 90) R = 4-ethylphenyl (ND 91) | ND 79–91 (4a–m) | PANC-1: 87.82–4650 μg/mL | N/A | [46] |
 | R = 2-F (ND 92) R = 2-Cl (ND 93) R = 2-Br (ND 94) R = 2-methyl (ND 95) R = 2-methoxy (ND 96) R = 2-nitro (ND 97) R = 3-F (ND 98) R = 3-Cl (ND 99) R = 3-Br (ND 100) R = 3-methyl (ND 101) R = 3-methoxy (ND 102) R = 3-nitro (ND 103) R = 4-F (ND 104) R = 4-Br (ND 105) R = 4-methyl (ND 106) R = 4-methoxy (ND 107) R = 4-nitro (ND 108) R = 3,4-dichloro (ND 109) R = 3,4-difluoro (ND 110) | ND 92–110 (4–22) | MCF-7: 5.68–45.16 μM HT-29: 10.21–42.30 μM | ↑apoptosis ↓FAK | [47] |
 | R1 = cyano, R2 = amino, R3 = R5 acetoxy, R4 = H (ND 111) R1 = acetyl, R2 = hydroxyl, R3 = R5 acetoxy, R4 = H (ND 112) R1 = cyano, R2 = hydroxyl R3 = R5 = acetoxy, R4 = H (ND 113) R1 = ethoxycarbonyl, R2 = hydroxyl R3 = R5 = acetoxy, R4 = H (ND 114) R1 = cyano, R2 = amino, R3 = H, R4 = R5 = acetoxy (ND 115) R1 = acetyl, R2 = hydroxyl R3 = H, R4 = R5 = acetoxy (ND 116) R1 = cyano, R2 = hydroxyl R3 = H, R4 = R5 = acetoxy (ND 117) R1 = ethoxycarbonyl, R2 = hydroxyl R3 = H, R4 = R5 = acetoxy (ND 118) R1 = cyano, R2 = amino, R3 = R5 = hydroxyl, R4 = H (ND 118) R1 = acetyl, R2 = R3 = R5 = hydroxyl, R4 = H (ND 120) R1 = cyano, R2 = R3 = R5 = hydroxyl, R4 = H (ND 121) R1 = ethoxycarbonyl, R2 = R3 = R5 = hydroxyl, R4 = H (ND 122) R1 = cyano, R2 = amino, R3 = H, R4 = R5 = hydroxyl (ND 123) R1 = acetyl, R2 = R4 = R5 = hydroxyl, R3 = H (ND 124) R1 = cyano, R2 = R4 = R5 = hydroxyl, R3 = H (ND 125) R1 = ethoxycarbonyl, R2 = R4 = R5 = hydroxyl, R3 = H (ND 126) | ND 111–126 (4a–h, 5a–h) | MCF-7: 32–180 μM | ↑apoptosis by ↑BAX, ↑p53 and ↓Bcl-2 | [48] |
 | R = benzyl (ND 127) R = acetyl (ND 128) | ND 127–128 (5, 6b) | HeLa: 2.9–9.94 μM | N/A | [49] |
 | R1 = H, R2 = 3,5-dimethoxyphenyl (ND 129) R1 = H, R2 = 3,5-dinitrophenyl (ND 130) R1 = H, R2 = 4-aminopyridyl (ND 131) R1 = H, R2 = 3,5-dimethoxy-4-methylphenyl (ND 132) R1 = ethyl, R2 = 4-chlorophenyl (ND 133) R1 = methyl, R2 = 3-chlorophenyl (ND 134) R1 = 3-chlorophenyl, R2 = 4-nitrophenyl (ND 135) R1 = 4-chlorophenyl, R2 = 4-nitrophenyl (ND 136) R1 = 4-(N,N’-dimetzlamino = phenyl, R2 = 4-nitrophenyl (ND 137) R1 = 4-fluorophenyl, R2 = 4-nitrophenyl (ND 138) R1 = H, R2 = phenyl (ND 139) R1 = H, R2 = 4-nitrophenyl (ND 140) R1 = H, R2 = 4-chlorophenyl (ND 141) R1 = H, R2 = 4-metoxyphenyl (ND 142) R1 = H, R2 = 3-chlorophenyl (ND 143) R1 = ethyl, R2 = 3-chlorophenyl (ND 144) | ND 129–144 (6a–p) | MCF-7: 0.012–18.5 μM A549: 0.18–22.9 μM Colo-205: 0.34–12.6 μM A2780: 0.07–13.5 μM | N/A | [50] |
 | - | ND 145 (34) | HeLa: 0.02 μM PC-3: 0.08 μM MCF-7: 0.1 μM SKMEL-28: 0.2 μM SKMES-1: 0.6 μM HepG1: 0.8 μM | ↑apoptosis by ↑p53 signaling pathway; ↑cell cycle arrest (G2/M) | [51] |
 | R = 2-hydroxyphenyl (ND 146) R = 2,3-dihydroxyphenyl (ND 147) R = 2,4-dihydroxyphenyl (ND 148) R = indol-3yl (ND 149) | ND 146–149 (3d–f, 3q) | ND 146–149 OVCAR-3: 0.13–0.037 μM ND 147 HL-60: 0.025 μM) | ↑apoptosis; ↑cell cycle arrest (G2/M); ↓caspase 3 activation | [52] |
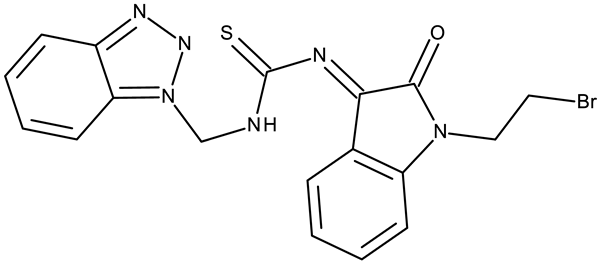 | - | ND 150 (5l) | MCF-7: 2.88 μM | ↓COX; ↓PI3K | [53] |
| Bis-benzotriazole hybrids | |||||
 | - | ND 151 (TAJ1) | MCF-7: 4.04 μM HeLa: 6.08 μM | N/A | [54] |
 | - | ND 152 (TAJ4) | MCF-7: 3.18 ± 0.11 μM HeLa: 8.12 ± 0.43 μM | N/A | [55] |
 | - | ND 153 (BTD) | MCF-7: 83.45 μM | N/A | [56] |
| Chemical Structure | Compound ID | IC50 (μM or μg/mL), Inhibition Rate (%) | Mechanism of Action | Reference | |
|---|---|---|---|---|---|
| Core Structure | Substituents | ||||
| C-substituted derivatives | |||||
 | - | CD 1 (TBB) | C4-2: 28–28.4 μM | ↓CK-2 ↓NFκB p65 | [67] |
| - | CD 1 (TBB) | WiDr: 0.15 μM | ↓CK2; ↓XRCC1 phosphorylation | [68] | |
| - | CD 1 (TBB) | N/A | ↑apoptosis; ↓PTEN and Akt phosphorylation | [69] | |
| - | CD 1 (2) | CCRF-CEM: 7.5–94.3 μM (24–48 h) MCF-7: 55.1–102.1 μM (24–48 h) | N/A | [70] | |
 | - | CD 2 (4) | CCRF-CEM: 76.6–90.6 μM (24–48 h) MCF-7: 53.4–74.1 μM (24–48 h) | does not inhibit CK2 | |
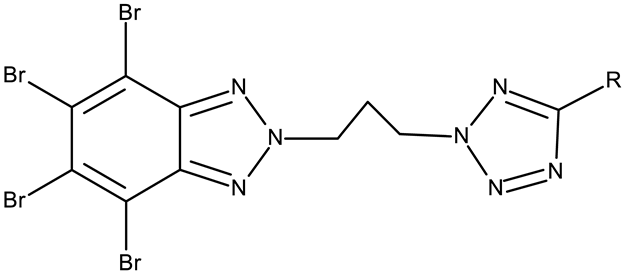 | R = phenyl (CD 3) R = 4-methylphenyl (CD 4) R = 4-chlorophenyl (CD 5) R = 2-chlorophenyl (CD 56) | CD 3–6 (8a–d) | CCRF-CEM: 12.9–94 μM (24–48 h) MCF-7: 1.3–80.3 μM (24–48 h) | ||
 | R = H (CD 7) R = hydroxymethyl (CD 8) R = ethyl ester (CD 9) R = carboxylic acid (CD 10) R = 2-carboxyethyl (CD 11) | CD 7–11 (19–23) | MCF-7: 53–119% CCRF-CEM: 48–93% | N/A | [71] |
 | - | CD 12 (10) | Jurkat T: 14.2 μM L1210: 20.7 μM MDA-MB-231: 17.4 μM MCF-7: 17 μM | ↑apoptosis (intrinsic pathway) | [72] |
 | R = 4-aminobutyl (CD 13) R = 2-aminoethyl (CD 14) | CD 13–14 (14,16) | CD 13 Jurkat T: 8.7 μM L1210: 11.42 μM MDA-MB-231: 9.7 μM MCF-7: 9.0 μM CD 14 Jurkat T: 7.9 μM L1210: 14.3 μM MDA-MB-231: 14 μM MCF-7: 11.6 μM | ||
 | - | CD 15 (42) | CCRF-CEM: 6.5 μM MCF-7: 80 μM | N/A | [73] |
 | - | CD 16 (5) | MCF-7: 9.1 μM A549: 6.3 μM | ↑Bax; ↓CK-2 | [74] |
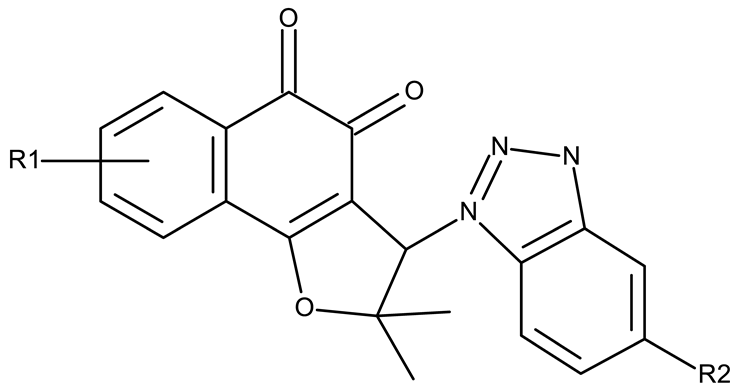 | R1 = R2 = H (CD 17) R1 = 7-methyl, R2 = H (CD 18) R1 = 7-Br, R2 = H (CD 19) R1 = 9-methoxy, R2 = H (CD 20) R1 = H, R2 = n-butyl (CD 21) | CD 17–21 (5a–b, 5f–g, 5k) | CD 17–20 MCF-7: 1.53–2.14 μM HepG2: 1.74–13.79 μM A549: 2.06–14.72 μM CD 21 MCF-7: 2.64 μM HepG2: 2.4 μM A549: 0.49 μM | ↑apoptosis (intrinsic pathway) by ↑NQO1 and ↑ROS; ↑cell cycle arrest (G0/G1) | [75] |
 | R = propane-1,3-diyl (CD 22) R = vinylene (CD 23) | CD 22–23 (3b, 3d) | CCRF-CEM: 0.07–5.5 μM WIL-2NS: 23–>100 μM CCRF-SB: 0.35–8.5 μM SK-MEL28: 2.6–>100 μM SK-MES1: 6.8–>100 μM DU145: 5.1–>100 μM HeLa: 5.4–>100 μM | ↑apoptosis | [76] |
 | - | CD 24 (4d) | |||
 | - | CD 25 (9b) | |||
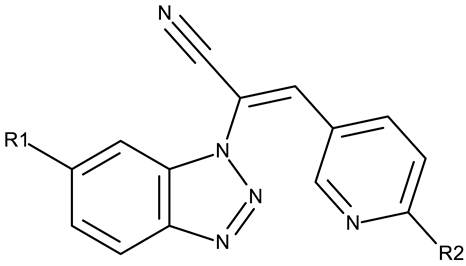 | R1 = H, R2 = Cl (CD 26) R1 = H, R2 = methoxy (CD 27) R1 = Cl, R2 = Cl (CD 28) R1 = Cl, R2 = methoxy (CD 29) | CD 26–35 (1.1–1.4, 2.1–2.6) | VX2: 3.08–56.55 μM A549: 5.47–59.41 μM MGC-803: 3.04–21.77 μM MKN45: 3.04–12.55 μM | N/A | [77] |
 | R1 = H, R2 = Cl (CD 30) R1 = H, R2 = methoxy (CD 31) R1 = Cl, R2 = Cl (CD 32) R1 = Cl, R2 = methoxy (CD 33) | ||||
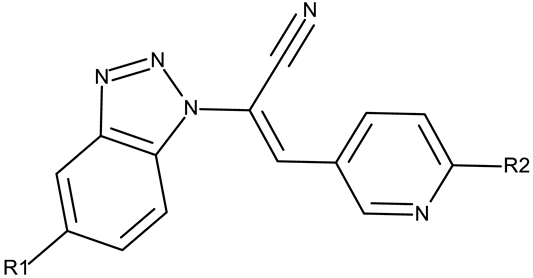 | R1 = Cl, R2 = Cl (CD 34) R1 = Cl, R2 = methoxy (CD 35) | ||||
 | R1 = F, R2 = dimethylamino (CD 36) R1 = H, R2 = piperazin-1-yl (CD 37) R1 = Cl, R2 = piperazin-1-yl (CD 38) | CD 36–38 (1b, 3a, 3c) | A549: 6.73–>20 μM SK-0V-3: 8.94–>20 μM HT-29: 8.34–>20 μM HL-60: 13.03–>60 μM PC-3: 10.85–>20 μM HepG2: 12.12–>20 μM MDA-MB-231: 8.85–>20 μM | ↑apoptosis (intrinsic pathway) by ↓BCL2, ↑Bax, ↑Cyt C, ↑cleaved caspase-9/-3 and ↑cleaved PARP; ↑cell cycle arrest (G0/G1) ↑DNA damage; ↑autophagy by ↑LC3B-II, ↑Beclin1 and ↓p62 | [78] |
 | R1 = F, R2 = H (CD 39) R1 = H, R2 = F (CD 40) R1 = Cl, R2 = H (CD 41) R1 = H, R2 = Cl (CD 42) R1 = Br, R2 = H (CD 43) R1 = H, R2 = Br (CD 44) R1 = methoxy, R2 = H (CD 45) R1 = H, R2 = methoxy (CD 46) R1 = methyl, R2 = H (CD 47) R1 = H, R2 = methyl (CD 48) R1 = cyano, R2 = H (CD 49) R1 = H, R2 = cyano (CD 50) R1 = trifluoromethyl, R2 = H (CD 51) R1 = H, R2 = trifluoromethyl (CD 52) R1 = R2 = H (CD 53) R1 = trifluoromethyl, R2 = Cl (CD 54) | CD 39–54 (12A–P) | CD 39–54 SKOV-3: 0.029–0.915 μM SiHa: 0.009–0.231 μM CD 53 HeLa: 0.024 Μm MCF-7: 0.01 μM 4T1: 0.068 μM OVCAR-5: 0.052 μM A549: 0.035 μM H460: 0.041 μM | ↑apoptosis; ↑cell cycle arrest (G2/M); ↓CDKs and ↓FLTs activity | [79] |
 | R1 = phenyl, R2 = phenyl, R3 = H (CD 55) R1 = nitroso, R2 = phenyl, R3 = phenyl (CD 59) R1 = H, R2 = H, R3 = nitro (CD 57) MWNT = multiwalled carbon nanotube | CD 55–57 (B, C, D) | MKN-45: 0.002–0.06 μg/mL SW742: 0.001 μg/mL | N/A | [80] |
| Fused derivatives | |||||
 | - | FD 1 (from surface-modified NPs ± cRGD peptide) | NPs+ laser: C6: 9.67 μM U87 MG: 28.76 μM GL261: 17.71 μM cRGD NPs + laser: C6: 6.18 μM U87 MG: 21.13 μM GL261: 9.97 μM | N/A | [81] |
| Chemical Structure | Compound ID | IC50 (μM or μg/mL), Inhibition Rate (%) | Mechanism of Action | Reference | |
|---|---|---|---|---|---|
| Core Structure | Substituents | ||||
| Organometallic compounds | |||||
 | R = methyl (OM 1) R = butyl (OM 2) | OM 1–4 (1a–d) | Caco-2: 162–376 μM MCF-7: 192–530 μM | N/A | [87] |
 | R = butyl (OM 3) R = benzyl (OM 4) | ||||
 | - | OM 5 (3a) | Caco-2: 90–201 μM MCF-7: 137–407 μM | ||
 | R = butyl (OM 6) R = benzyl (OM 7) R = 3,4,5-trimethoxybenzyl (OM 8) | OM 6–8 (3b–d) | |||
 mebta | - | OM 9 ([(CH3)2SnCl2(mebta)2]) | MDA-MB-231: 20 μM | N/A | [88] |
 hmbt | - | OM 10–18 ([Zn(hmbt)2(H2O)2]; [Zn(hmbt)(OAc)(H2O)2]; [Pd(bpy)(hmbt)]Cl; [Pt(bpy)(hmbt)]Cl [Pd(phen)(hmbt)]C; [Pt(phen)(hmbt)]Cl; [Ag2(hmbt)2]; [Ag(PPh3)(hmbt)]; [Rh(hmbt)2(H2O)2]Cl) (bpy- 2,2′-bipyridine, phen- 1,10-phenanthroline, PPh3+ triphenylphosphine) | OM 17 MDA-MB231: 1.37 μM OVCAR-8: 1.75 μM OM 14 MDA-MB231: 4.85 μM OVCAR-8: 2.99 μM OM 15 MDA-MB-231: 5.24 μM OVCAR-8: 3.00 μM OM 18 MDA-MB231: 7.52 μM OVCAR-8: 8.50 μM | N/A | [89] |
 L | - | OM 19–21 ([ZnL2]·4EtOH, [PdL(EtOH)2]·Cl, [PtL(EtOH)Cl]) | MCF-7: 3.08–4.28 μg/mL | ↓metastasis; ↓angiogenesis by ↓VEGF | [90] |
 bmb | - | OM 22 (Zn2(p-2-bmb)2(NO3)4) | MCF-7: 33.0 μM QBC939: 37.2 μM SHSY5Y: 30.3 μM EC109: 36.3 μM | ↑apoptosis (intrinsic pathway); ↑cell cycle arrest (G0/G1) | [91] |
| - | OM 23–24 (Cu(p-2-bmb)(OH)(ClO4), OM 3 (Co2(p-2-bmb)2Cl4) | OM 23 SMMC7721: 39.2 μM BGC823: >80 μM HCT116: 43.5 μM | OM 23: ↑apoptosis (intrinsic pathway); ↑cell cycle arrest (G2/M) | [92] | |
 | - | OM 25 (Cu (II)) | HeLa: 24.2–7.18 μM (24–72 h) SGC-7901: 27.64–8.35 μM (24–72 h) | ↑apoptosis (intrinsic pathway) by ↑ROS; ↑cell cycle arrest (G1) by ↓ cyclinD1/cdk4 and ↓pRb/E2F1 | [93] |
 | - | OM 26 (3) | MCF-7: 35.5–54.8 μM EC109: 14.05–28.75 μM SHSY5Y: 34.37–59.78 μM QBC939: 31.71–40.4 μM | N/A | [94] |
 | OM 27 (4) | ||||
| Compound ID | Cancer Model | Treatment | Control | Tumor Weight | Toxicity | Mechanism of Action | Reference |
|---|---|---|---|---|---|---|---|
| CD 1 | 22Rv1 cells (prostate cancer), orthotopic xenograft | i.v., 0.02 mg/kg on days 1, 4 and 7 | TBG-RNAi-F7 | reduced tumor weight | N/A | ↓CK2; ↓AR; ↓NF-κB p65 | [67] |
| CD 1 | WiDr (colon cancer) xenografts, s.c. | i.p., 150 mg/kg, twice daily+ irradiation, 5 days | DMSO | delayed tumor growth | N/A | ↓CK2 | [68] |
| CD 21 | HepG2 (hepatocellular) xenograft, s.c. | i.v., 20 mg/kg, every two days, 19 days | saline | tumor inhibition (52.3%) | none observed | N/A | [75] |
| CD 53 | SiHa cells (squamous cell carcinoma), s.c. | p.o., 5–20 mg/kg, 30 days | cisplatin | tumor inhibition (51.25–79.29%) | none observed | N/A | [79] |
| FD 1 (NPs) | GL261-luc cells (glioblastoma),injected in the right brain and C6 cells (glioma), orthotropic xenograft | NPs (1 mg/Kg) ± laser | PBS | marked tumor reduction (+ laser group) | no weight loss | ↓CD31; ↓ki67 positive cells | [81] |
| OM 25 | sarcoma murine cancer (S180), i.p. | i.p.; 1–10 mg/kg, 7 days | cisplatin, saline | tumor inhibition (42.29–59.86%) | no weight loss; the 10 mg/kg dose induced some hepatotoxicity | ↑apoptosis; ↑cell cycle arrest (G1) by ↓cyclinD1/cdk4 pathway | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Mardale, G.; Prodea, A.; Munteanu, A.; Jorgovan, M.; Mardale, S.; Dumitrascu, V.C.; Șoica, C. Benzotriazole in Cancer: A Systematic Review on Preclinical Evidence and Structure–Activity Relationship. Pharmaceuticals 2026, 19, 77. https://doi.org/10.3390/ph19010077
Mardale G, Prodea A, Munteanu A, Jorgovan M, Mardale S, Dumitrascu VC, Șoica C. Benzotriazole in Cancer: A Systematic Review on Preclinical Evidence and Structure–Activity Relationship. Pharmaceuticals. 2026; 19(1):77. https://doi.org/10.3390/ph19010077
Chicago/Turabian StyleMardale, Gabriel, Alexandra Prodea, Andreea Munteanu, Mihaela Jorgovan, Sabina Mardale, Victor Cristian Dumitrascu, and Codruța Șoica. 2026. "Benzotriazole in Cancer: A Systematic Review on Preclinical Evidence and Structure–Activity Relationship" Pharmaceuticals 19, no. 1: 77. https://doi.org/10.3390/ph19010077
APA StyleMardale, G., Prodea, A., Munteanu, A., Jorgovan, M., Mardale, S., Dumitrascu, V. C., & Șoica, C. (2026). Benzotriazole in Cancer: A Systematic Review on Preclinical Evidence and Structure–Activity Relationship. Pharmaceuticals, 19(1), 77. https://doi.org/10.3390/ph19010077








