Combined Thermal and Colorimetric Analysis as a Tool for Detecting Counterfeit Viagra® Tablets
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermal Analysis
2.1.1. Thermogravimetric Analysis (TG)
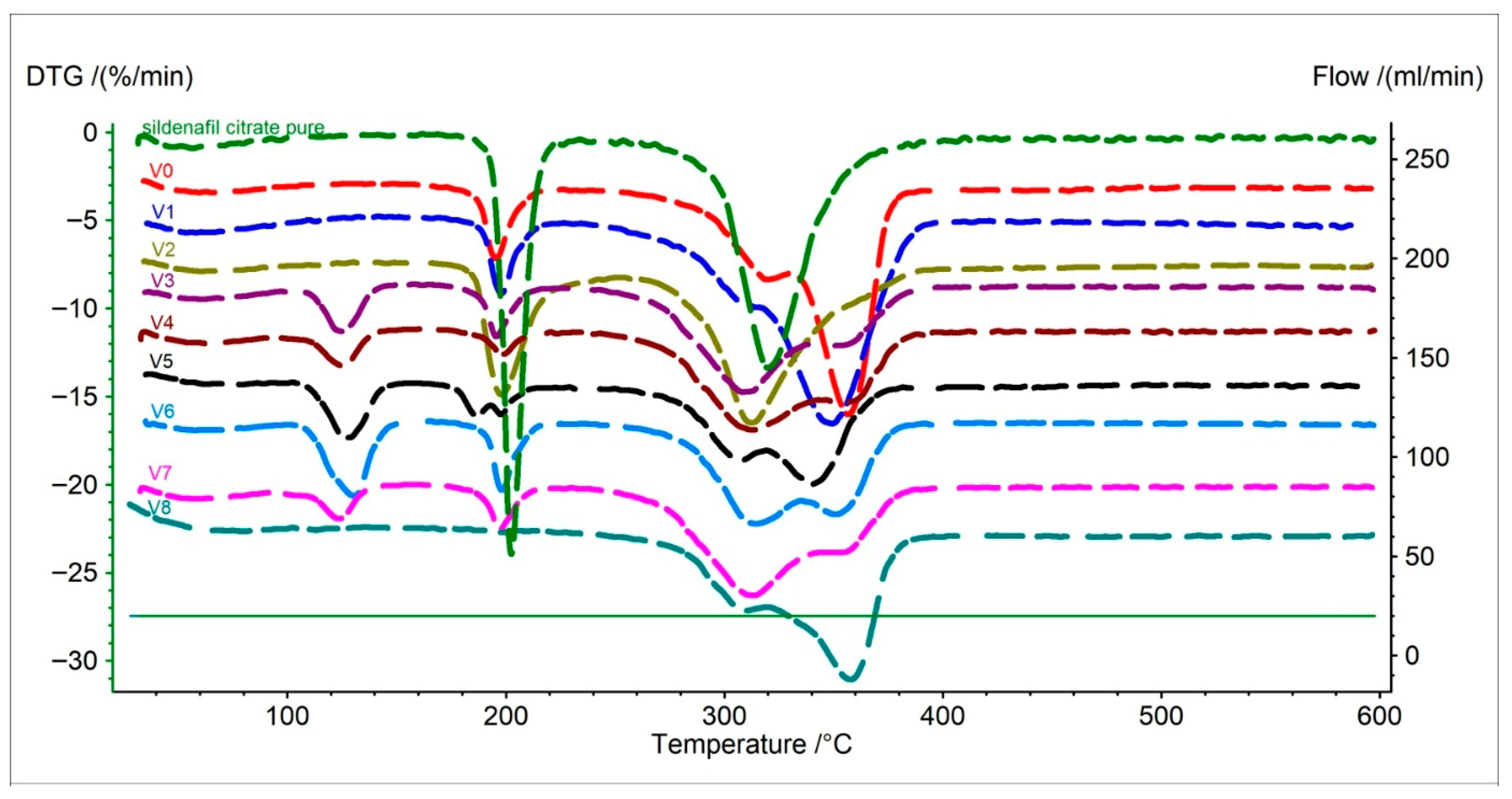
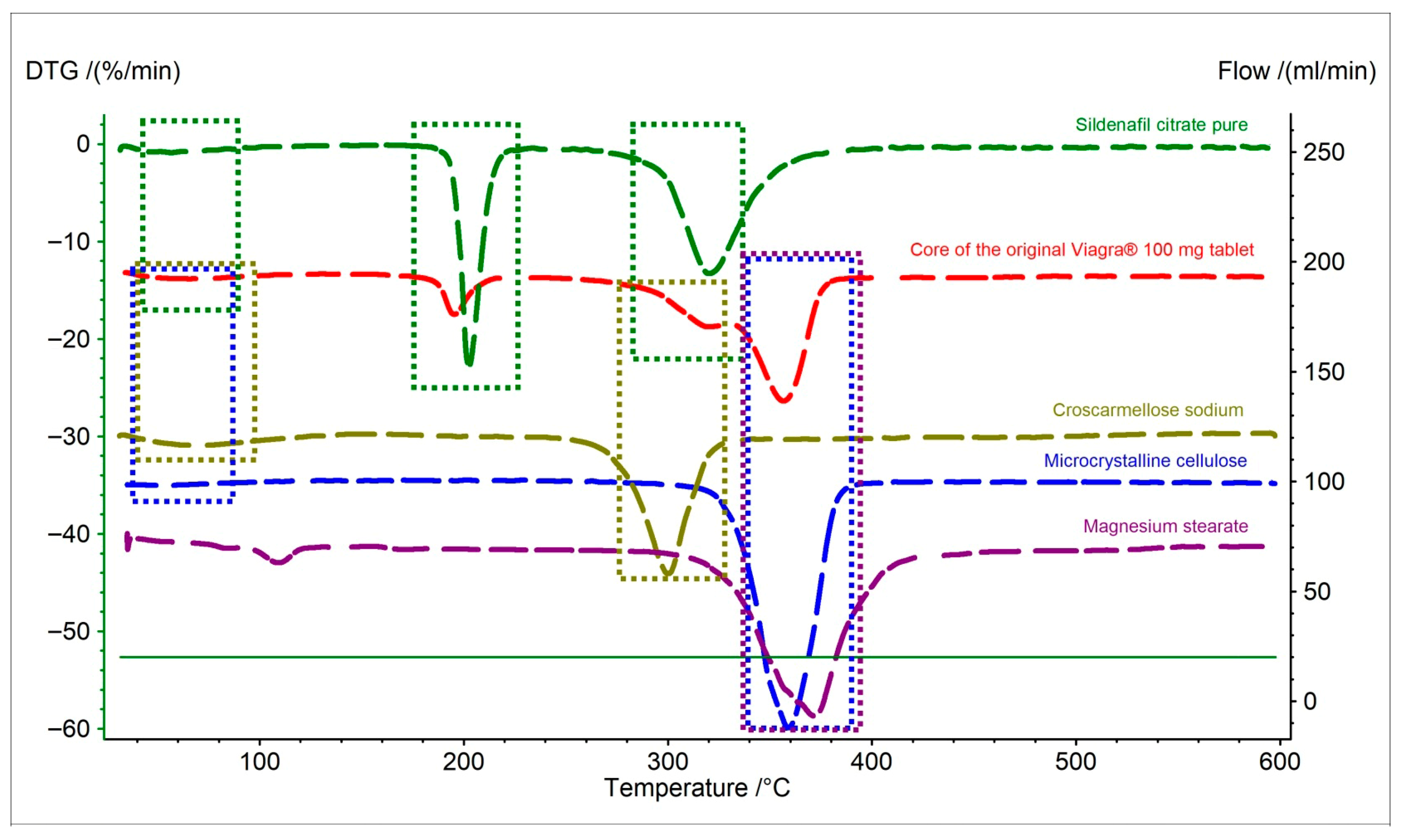
2.1.2. Analysis of the First Derivative of the TG Curve (DTG)
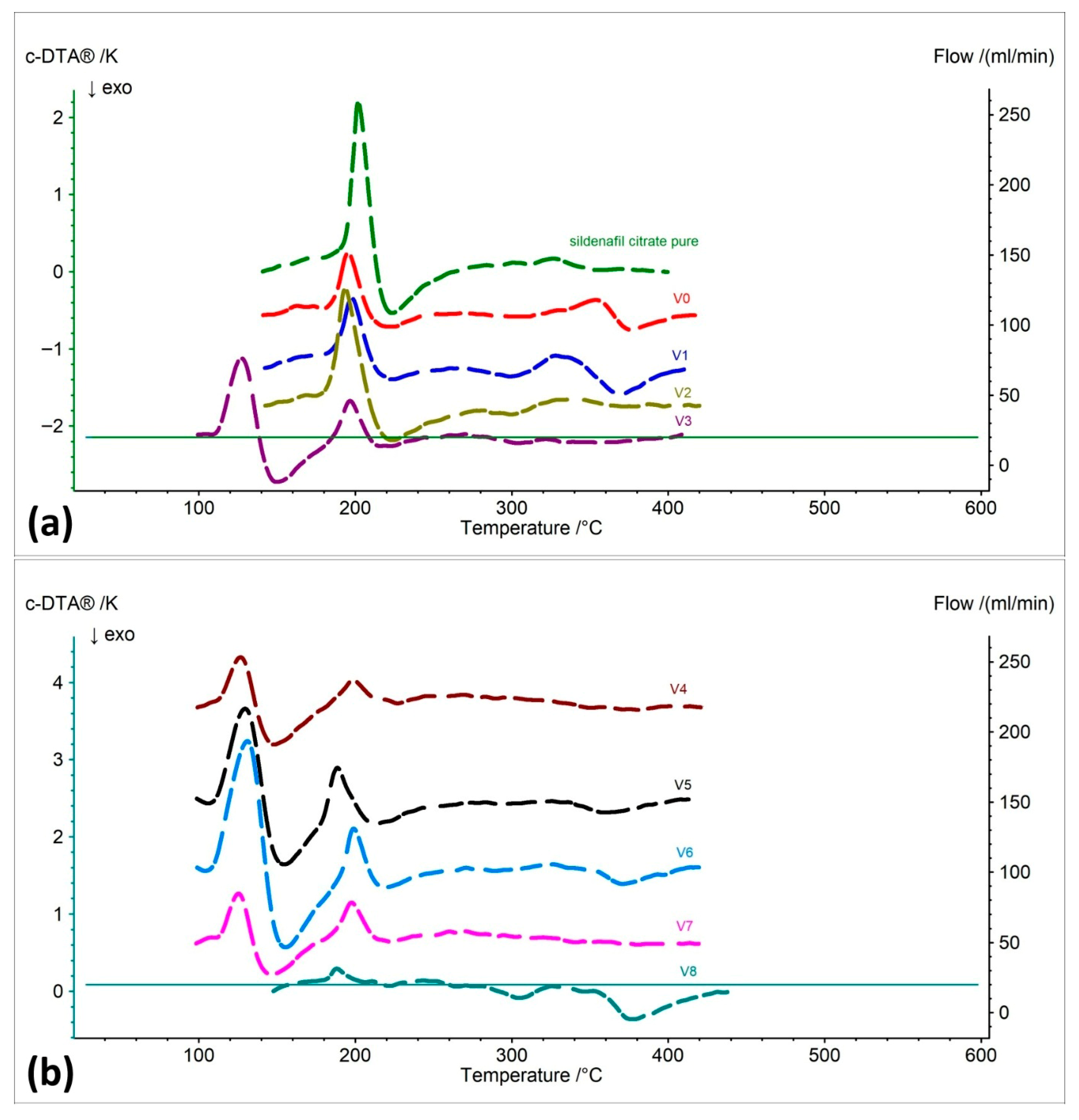
2.1.3. Differential Thermal Analysis (c-DTA)
2.2. Colorimetric Analysis
2.3. Study Limitaions and Future Plans
3. Materials and Methods
3.1. Tested Samples
3.2. Thermal Analyses (TGA, c-DTA)
3.2.1. Sample Preparation for Thermal Testing
3.2.2. Measurement Apparatus
3.2.3. Analysis Procedure
3.3. Colorimetric Analysis
3.3.1. Sample Preparation for Colorimetric Testing
3.3.2. Equipment and Measurement Conditions
3.3.3. Analyzed Color Parameters
3.3.4. Data Processing and Statistical Analysis
- ΔE* < 0.5—difference imperceptible to the human eye,
- 0.5 ≤ ΔE* < 1.5—difference barely visible,
- 1.5 ≤ ΔE* < 3.0—difference poorly visible,
- 3.0 ≤ ΔE* < 6.0—difference visible,
- ΔE* ≥ 6.0—difference very visible.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sansone, A.; Cuzin, B.; Jannini, E.A. Facing Counterfeit Medications in Sexual Medicine. A Systematic Scoping Review on Social Strategies and Technological Solutions. Sex. Med. 2021, 9, 100437. [Google Scholar] [CrossRef]
- Jackson, G.; Arver, S.; Banks, I.; Stecher, V.J. Counterfeit phosphodiesterase type 5 inhibitors pose significant safety risks. J. Clin. Pract. 2010, 64, 497–504. [Google Scholar] [CrossRef]
- Koenraadta, R.; van de Ven, K. The Internet and lifestyle drugs: An analysis of demographic characteristics, methods, and motives of online purchasers of illicit lifestyle drugs in the Netherlands. Drugs Educ. Prev. Policy 2018, 25, 345–355. [Google Scholar] [CrossRef]
- Neil Campbell, N.; Clark, J.P.; Stecher, V.J.; Goldstein, I. Internet-ordered viagra (sildenafil citrate) is rarely genuine. J. Sex. Med. 2012, 9, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- WHO. Substandard and Falsified Medical Products. Fact Sheet. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/substandard-and-falsified-medical-products (accessed on 10 November 2025).
- WHO. Global Surveillance and Monitoring System for Substandard and Falsified Medical Products; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151342-5. [Google Scholar]
- EMA. Falsified Medicines: Overview. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/public-health-threats/falsified-medicines-overview (accessed on 10 November 2025).
- OECD/EUIPO. Trade in Counterfeit Pharmaceutical Products, Illicit Trade; OECD Publishing: Paris, France, 2020; Available online: https://doi.org/10.1787/a7c7e054-en (accessed on 10 November 2025). [CrossRef]
- Park, M.; Ahn, S. Quantitative analysis of sildenafil and tadalafil in various fake drugs recently distributed in Korea. J. Forensic Sci. 2012, 57, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Tama, A.L.; Musa, A.; Usuman, M.A.; Awwalu, S. Evaluation of Sildenafil as an Undeclared Adulterant in Herbal Aphrodisiac Preparations by HPLC. Saudi J. Med. Pharm. Sci. 2020, 6, 168–172. [Google Scholar] [CrossRef]
- Moureaud, C.; Hertig, J.; Dong, Y.; Muraro, I.S.; Alhabash, S. Purchase of prescription medicines via social media: A survey-based study of prevalence, risk perceptions, and motivations. Health Policy 2021, 125, 1421–1429. [Google Scholar] [CrossRef]
- Venhuis, B.J.; de Kaste, D. Towards a decade of detecting new analogues of sildenafil, tadalafil and vardenafil in food supplements: A history, analytical aspects and health risks. J. Pharm. Biomed. Anal. 2012, 69, 196–208. [Google Scholar] [CrossRef]
- Martin, Y.C.; Kofron, J.L.; Traphagen, L.M. Do structurally similar molecules have similar biological activity? J. Med. Chem. 2002, 45, 4350–4358. [Google Scholar] [CrossRef]
- Ortiz, R.S.; Mariotti, K.C.; Limberger, R.P.; Mayorga, P. Physical profile of counterfeit tablets Viagra® and Cialis®. Brazilian J. Pharm. Sci. 2012, 48, 487–495. [Google Scholar] [CrossRef]
- Souza, G.M.S.; Debastiani, R.; Chytry, P.; Knebel, M.; Thomaz, R.; Amaral, L.; Dias, J.F. A simple protocol for the characterization of fraudulent erectile dysfunction medicines using ion beam analytical techniques. Microchem. J. 2025, 213, 113602. [Google Scholar] [CrossRef]
- Ortiz, R.S.; Mariotti, K.C.; Holzschuh, M.H.; Romão, W.; Limberger, R.P.; Mayorga, P. Profiling counterfeit Cialis, Viagra and analogs by UPLC–MS. Forensic Sci. Int. 2013, 229, 13–20. [Google Scholar] [CrossRef]
- Lee, S.; Ji, D.; Park, M.; Chung, K.H. Development of a comprehensive spectral library of sildenafil and related active analogues using LC-QTOF-MS and its application for screening counterfeit pharmaceuticals. Foresic Sci. Int. 2015, 257, 182–188. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H.; Ibrahim, B.; Zyoud, S.H. Screening and Determination of Synthetic PDE-5 Inhibitors in Adulterated Sexual Enhancement Supplements. Molecules 2022, 27, 6737. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.S.; Mariotti, K.C.; Fank, B.; Limberger, R.P.; Anzanello, M.J.; Mayorga, P. Counterfeit Cialis and Viagra fingerprinting by ATR-FTIR spectroscopy with chemometry: Can the same pharmaceutical powder mixture be used to falsify two medicines? Forensic Sci. Int. 2013, 226, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Sacré, P.Y.; Deconinck, E.; Saerens, L.; De Beer, T.; Courselle, P.; Vancauwenberghe, R.; Chiap, P.; Crommen, J.; De Beer, J.O. Detection of counterfeit Viagra® by Raman microspectroscopy imaging and multivariate analysis. J. Pharm. Biomed. Anal. 2011, 56, 454–461. [Google Scholar] [CrossRef]
- Jendrzejewska, I.; Goryczka, T.; Pietrasik, E.; Klimontko, J.; Jampilek, J. Identification of Sildenafil Compound in Selected Drugs Using X-ray Study and Thermal Analysis. Molecules 2023, 28, 2632. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, A.; Pioruńska-Sędłak, K.; Popławska, M.; Maurin, J.K.; Błażewicz, A. Semiquantitative X-ray Powder Diffraction Analysis in Counterfeit Medicines Investigation—The Viagra Example. Crystals 2023, 13, 1485. [Google Scholar] [CrossRef]
- Jung, C.R.; Ortiz, R.S.; Limberger, R.; Mayorga, P. A new methodology for detection of counterfeit Viagra® and Cialis® tablets by image processing and statistical analysis. Forensic Sci. Int. 2012, 216, 92–96. [Google Scholar] [CrossRef]
- Harwacki, J.; Pisklak, D.M.; Szeleszczuk, L. Solid state 13C NMR spectroscopy as a tool for identification of counterfeit Viagra tablets and guide for develop new identification approach of falsified product. Int. J. Pharm. 2023, 636, 122837. [Google Scholar] [CrossRef]
- Ho, H.M.K.; Xiong, Z.; Wong, H.Y.; Buanz, A. The era of fake medicines: Investigating counterfeit medicinal products for erectile dysfunction disguised asherbal supplements. Int. J. Pharm. 2022, 617, 121592. [Google Scholar] [CrossRef]
- Ramos, P.; Raczak, B.K.; Silvestri, D.; Wacławek, S. Application of TGA/c-DTA for distinguishing between two forms of naproxen in pharmaceutical preparations. Pharmaceutics 2023, 15, 1689. [Google Scholar] [CrossRef]
- Echavarría, A.; Pagán, J.; Ibarz, A. Kinetics of color development in glucose/amino acid model systems at different tempeatures. Sci. Agropecu. 2016, 7, 15–21. [Google Scholar] [CrossRef]
- Subert, J.; Cizmárik, J. Application of instrumental colour measurement in development and quality control of drugs and pharmaceutical excipients. Die Pharm. 2008, 63, 331–336. [Google Scholar]
- Canbay, H.S.; Doğantürk, M. Compatibility Studies of Sildenafil with Different Excipients by Using TGA, DSC, XRD and FTIR. Celal Bayar Univ. J. Sci. 2019, 15, 401–407. [Google Scholar]
- Melnikov, P.; Corbi, P.P.; Cuin, A.; Cavicchioli, M.; Guimara, W.R. Physicochemical properties of sildenafil citrate (Viagra) and sildenafil base. J. Pharm. Sci. 2003, 92, 2140–2143. [Google Scholar] [CrossRef]
- Broncel, M.; Juszczak, A.; Szczolko, W.; Silvestri, D.; Białek-Dratwa, A.; Wacławek, S.; Kowalski, O.; Ramos, P. Thermal compatibility of new ACEI derivatives with popular excipients used to produce solid pharmaceutical formulations. Pharmaceuticals 2025, 17, 1323. [Google Scholar] [CrossRef]
- Lopes, M.S.; Catelani, T.A.; Nascimento, A.L.C.S.; Garcia, J.S.; Trevisan, M.G. Ketoconazole: Compatibility with pharmaceutical excipients using DSC and TG techniques. J. Therm. Anal. Calorim. 2020, 141, 1371–1378. [Google Scholar] [CrossRef]
- Rojek, B.; Wesołowski, M. A combined differential scanning calorimetry and thermogravimetry approach for the effectiveassessment of drug substance-excipient compatibility. J. Therm. Anal. Calorim. 2023, 148, 845–858. [Google Scholar] [CrossRef]
- Sznitowska, M. (Ed.) Farmacja Stosowana; PZWL: Warsaw, Poland, 2017. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Qi, S. Analytical Techniques in the Pharmaceutical Sciences. In Thermal Analysis of Pharmaceuticals; Müllertz, A., Perrie, Y., Rades, T., Eds.; Advances in Delivery Science and Technology; Springer: New York, NY, USA, 2016; pp. 363–387. [Google Scholar]
- Nascimento, F.V.B.; Ferreira, A.P.G.; Alarcon, R.T.; Cavalheiro, É.T.G. Studies on the thermal behavior of sildenafil citrate. J. Pharm. Biom. Anal. 2024, 3, 100028. [Google Scholar] [CrossRef]

| Samples | Onset [°C] | Mid [°C] | Infection [°C] | End [°C] | Mass Change [%] |
|---|---|---|---|---|---|
| Sildenafil citrate pure | 197 | 310 | 202.4 | 228.8 | −77.05 |
| V0 | 189.2 | 349.1 | 355.7 | 369.1 | −48.86 |
| V1 | 191.4 | 342.1 | 350.1 | 369.3 | −59.74 |
| V2 | 189 | 305.4 | 311.4 | 354.6 | −64.64 |
| V3 | 190.3 | 318.3 | 308.7 | 352.2 | −37.82 |
| V4 | 190.5 | 323.2 | 313.2 | 362.7 | −40.29 |
| V5 | 179.6 | 332.4 | 339.5 | 360.1 | −27.78 |
| V6 | 192.8 | 332.9 | 312.3 | 360.5 | −33.08 |
| V7 | 192.2 | 319.5 | 314.1 | 357.1 | −40.88 |
| V8 | 313.5 | 335.2 | 359 | 371.4 | −48.71 |
| Samples | STAGE 1 | STAGE 2 | STAGE 3 | STAGE 4 | STAGE 5 | STAGE 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [°C] | %/min | [°C] | %/min | [°C] | %/min | [°C] | %/min | [°C] | %/min | [°C] | %/min | |
| Sildenafil citrate pure | 53.9 | −0.84 | 202.5 | −23.94 | 319.9 | −13.37 | - | - | - | - | - | - |
| V0 | 59.7 | −0.67 | 195.5 | −4.40 | 319.5 | −5.61 | 356.6 | −13.26 | - | - | - | - |
| V1 | 58.1 | −1.23 | 198.0 | −4.68 | 313.1 | −5.42 | 349.4 | −12.08 | - | - | - | - |
| V2 | 60.5 | −0.87 | 198.0 | −7.86 | 312.5 | −9.46 | - | - | - | - | - | - |
| V3 | 61.0 | −1.24 | 125.1 | −3.05 | 195.7 | −3.35 | 308.7 | −6.50 | 353.0 | −3.86 | - | - |
| V4 | 62.9 | −0.95 | 125.1 | −2.19 | 198.6 | −1.56 | 312.9 | −5.86 | 355.0 | −4.32 | - | - |
| V5 | 62.8 | −0.42 | 127.0 | −3.44 | 186.7 | −2.22 | 200.6 | −1.72 | 307.2 | −4.77 | 339.9 | −6.09 |
| V6 | 57.6 | −0.84 | 130.5 | −4.56 | 198.3 | −4.23 | 314.5 | −6.14 | 351.0 | −5.62 | - | - |
| V7 | 58.6 | −0.96 | 123.7 | −2.09 | 197.2 | −2.69 | 313.5 | −6.43 | 353.0 | −4.00 | - | - |
| V8 | 65.9 | −0.38 | 310.3 | −4.93 | 357.9 | −8.83 | - | - | - | - | - | - |
| Samples | Number of Thermal Decomposition Stages | [°C] (%/min) | [°C] (%/min) | [°C] (%/min) | [°C] (%/min) | [°C] (%/min) |
|---|---|---|---|---|---|---|
| Sildenafil citrate pure | 3 | 53.9 (−0.84) | - | 202.5 (−23.94) | 319.9 (−13.37) | - |
| Core of the original Viagra® tablet | 4 | 59.7 (−0.67) | - | 195.5 (−4.40) | 319.5 (−5.61) | 356.6 (−13.26) |
| Microcrystalline cellulose | 2 | 55.4 (−0.76) | - | - | - | 359.0 (−25.65) |
| Croscarmellose sodium | 2 | 70.8 (−1.30) | - | - | 300.2 (−14.47) | - |
| Magnesium stearate | 2 | - | 109.2 (−1.89) | - | - | 371.8 (−17.61) |
| Samples | Stage | Onset [°C] | Peak [°C] | Area [K*s] | Reaction |
|---|---|---|---|---|---|
| Sildenafil citrate pure | 1 | 194.5 | 201.9 | 108.41 | ENDOTHERMIC |
| 2 | - | 223.6 | 138.06 | EXOTHERMIC | |
| 3 | 331.6 | 355.4 | 17.88 | EXOTHERMIC | |
| V0 | 1 | 186.8 | 195.3 | 52.68 | ENDOTHERMIC |
| 2 | - | 222.7 | 19.98 | EXOTHERMIC | |
| 3 | 358.9 | 376.5 | 56.86 | EXOTHERMIC | |
| V1 | 1 | 188.4 | 197.6 | 41.92 | ENDOTHERMIC |
| 2 | - | 223.5 | 31.92 | EXOTHERMIC | |
| 3 | 264.9 | 298.8 | 37.91 | EXOTHERMIC | |
| 4 | 333.1 | 369.8 | 93.12 | EXOTHERMIC | |
| V2 | 1 | 185.1 | 193.5 | 85.94 | ENDOTHERMIC |
| 2 | - | 223.8 | 88.20 | EXOTHERMIC | |
| 3 | 279.6 | 299.5 | 14.29 | EXOTHERMIC | |
| 4 | 349.6 | 360.4 | 7.84 | EXOTHERMIC | |
| V3 | 1 | 113.0 | 127.2 | 102.33 | ENDOTHERMIC |
| 2 | 188.2 | 196.6 | 31.97 | ENDOTHERMIC | |
| 3 | - | 222.3 | 12.72 | EXOTHERMIC | |
| 4 | 277.6 | 304.0 | 7.56 | EXOTHERMIC | |
| 5 | 328.3 | 333.3 | 6.33 | EXOTHERMIC | |
| V4 | 1 | 114.2 | 126.6 | 51.31 | ENDOTHERMIC |
| 2 | 192.9 | 198.6 | 14.63 | ENDOTHERMIC | |
| 3 | - | 227.0 | 6.55 | EXOTHERMIC | |
| 4 | 296.5 | 350.7 | 19.83 | EXOTHERMIC | |
| V5 | 1 | 111.7 | 129.6 | 145.60 | ENDOTHERMIC |
| 2 | 179.8 | 188.5 | 44.05 | ENDOTHERMIC | |
| 3 | - | 212.9 | 21.83 | EXOTHERMIC | |
| 4 | 334.1 | 358.9 | 32.36 | EXOTHERMIC | |
| V6 | 1 | 111.3 | 131.0 | 204.82 | ENDOTHERMIC |
| 2 | 192.4 | 199.0 | 30.50 | ENDOTHERMIC | |
| 3 | - | 219.2 | 19.21 | EXOTHERMIC | |
| 4 | 272.9 | 289.0 | 6.01 | EXOTHERMIC | |
| 5 | 355.5 | 370.9 | 30.87 | EXOTHERMIC | |
| V7 | 1 | 114.2 | 125.3 | 50.90 | ENDOTHERMIC |
| 2 | 188.7 | 197.6 | 26.15 | ENDOTHERMIC | |
| 3 | - | 223.0 | 14.35 | EXOTHERMIC | |
| 4 | 329.3 | 340.9 | 3.30 | EXOTHERMIC | |
| V8 | 1 | 182.6 | 188.2 | 8.30 | ENDOTHERMIC |
| 2 | 275.2 | 304.4 | 20.47 | EXOTHERMIC | |
| 3 | 362.4 | 376.8 | 74.62 | EXOTHERMIC |
| Sample | Color Parameters | ΔE* [±SD] | Picture of Tablets | ||||
|---|---|---|---|---|---|---|---|
| CIE L*a*b* | C* h° | ||||||
| L* [±SD] | a* [±SD] | b* [±SD] | C* [±SD] | h° [±SD] | |||
| V0 | 59.90 [±0.06] | −3.18 [±0.03] | 4.18 [±0.06] | 5.25 [±0.04] | 127.28 [±0.56] | - |  |
| V1 | 60.74 [±0.01] | −2.20 [±0.01] | 5.78 [±0.03] | 6.19 [±0.03] | 110.83 [±0.21] | 2.05 [±0.06] |  |
| V2 | 30.97 [±0.03] | 3.72 [±0.05] | 9.65 [±0.05] | 10.34 [±0.04] | 68.92 [±0.29] | 30.24 [±0.06] |  |
| V3 | 57.60 [±0.01] | −4.28 [±0.03] | 2.35 [±0.01] | 4.88 [±0.03] | 151.20 [±0.17] | 3.14 [±0.06] |  |
| V4 | 57.23 [±0.01] | −3.43 [±0.02] | 2.52 [±0.01] | 4.26 [±0.02] | 143.69 [±0.15] | 3.15 [±0.06] |  |
| V5 | 65.23 [±0.01] | −2.72 [±0.05] | 7.24 [±0.04] | 7.73 [±0.05] | 110.62 [±0.24] | 6.16 [±0.06] |  |
| V6 | 61.57 [±0.01] | −2.96 [±0.04] | 5.28 [±0.03] | 6.05 [±0.02] | 119.28 [±0.30] | 2.01 [±0.06] |  |
| V7 | 46.20 [±0.01] | −5.46 [±0.02] | −7.47 [±0.03] | 9.25 [±0.02] | 233.82 [±0.06] | 18.13 [±0.06] |  |
| V8 | 61.98 [±0.01] | −5.50 [±0.01] | 3.03 [±0.01] | 6.28 [±0.01] | 151.14 [±0.06] | 3.32 [±0.06] |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ramos, P.; Wilczyński, S.; Stocerz, K.; Adamczyk, R.; Stanjek-Cichoracka, A. Combined Thermal and Colorimetric Analysis as a Tool for Detecting Counterfeit Viagra® Tablets. Pharmaceuticals 2026, 19, 78. https://doi.org/10.3390/ph19010078
Ramos P, Wilczyński S, Stocerz K, Adamczyk R, Stanjek-Cichoracka A. Combined Thermal and Colorimetric Analysis as a Tool for Detecting Counterfeit Viagra® Tablets. Pharmaceuticals. 2026; 19(1):78. https://doi.org/10.3390/ph19010078
Chicago/Turabian StyleRamos, Paweł, Sławomir Wilczyński, Klaudia Stocerz, Roman Adamczyk, and Anita Stanjek-Cichoracka. 2026. "Combined Thermal and Colorimetric Analysis as a Tool for Detecting Counterfeit Viagra® Tablets" Pharmaceuticals 19, no. 1: 78. https://doi.org/10.3390/ph19010078
APA StyleRamos, P., Wilczyński, S., Stocerz, K., Adamczyk, R., & Stanjek-Cichoracka, A. (2026). Combined Thermal and Colorimetric Analysis as a Tool for Detecting Counterfeit Viagra® Tablets. Pharmaceuticals, 19(1), 78. https://doi.org/10.3390/ph19010078









