Topical Delivery of Autochthonous Lactic Acid Bacteria Using Calcium Alginate Microspheres as a Probiotic Carrier System with Enhanced Therapeutic Potential
Abstract
1. Introduction
2. Results and Discussion
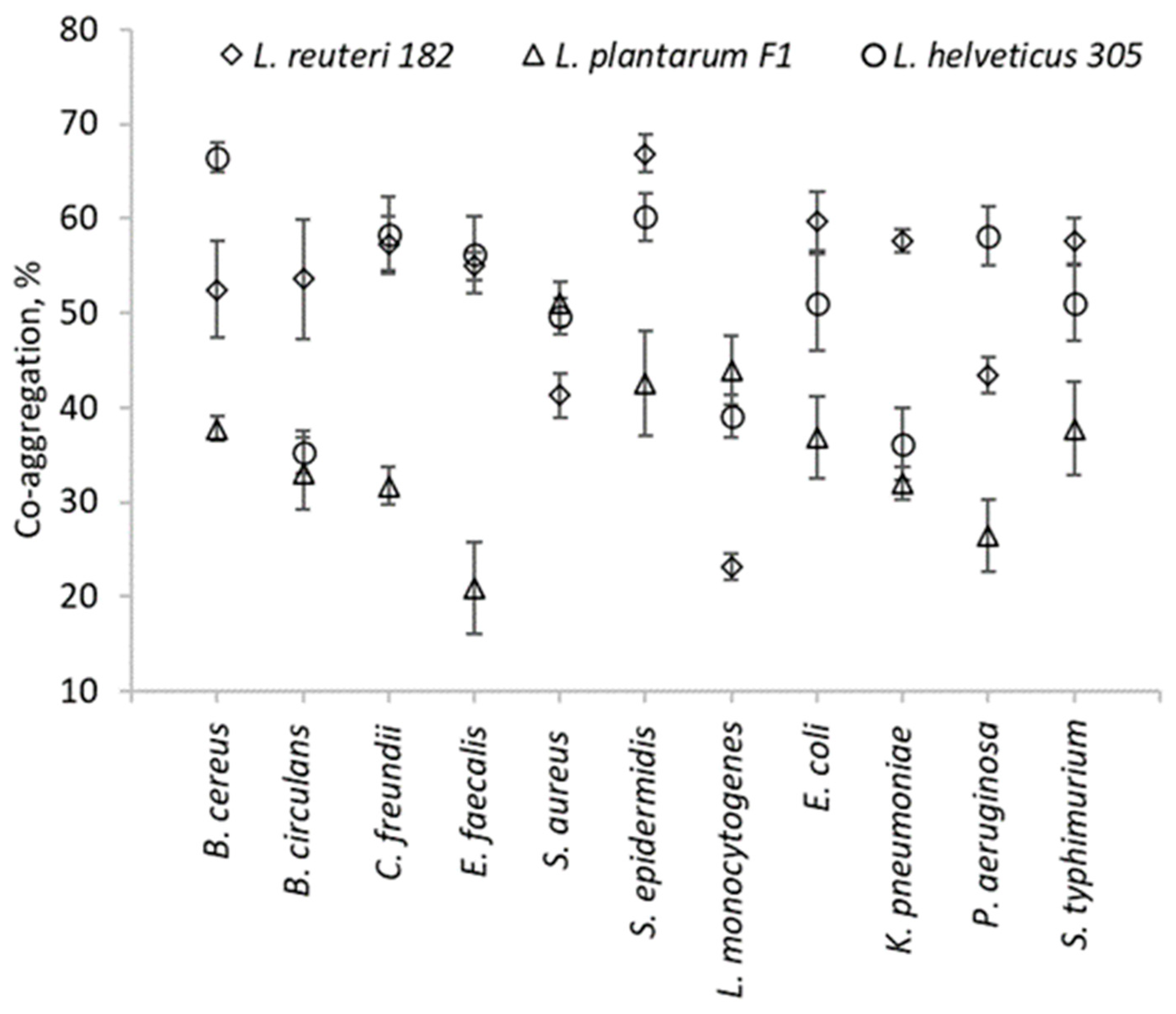
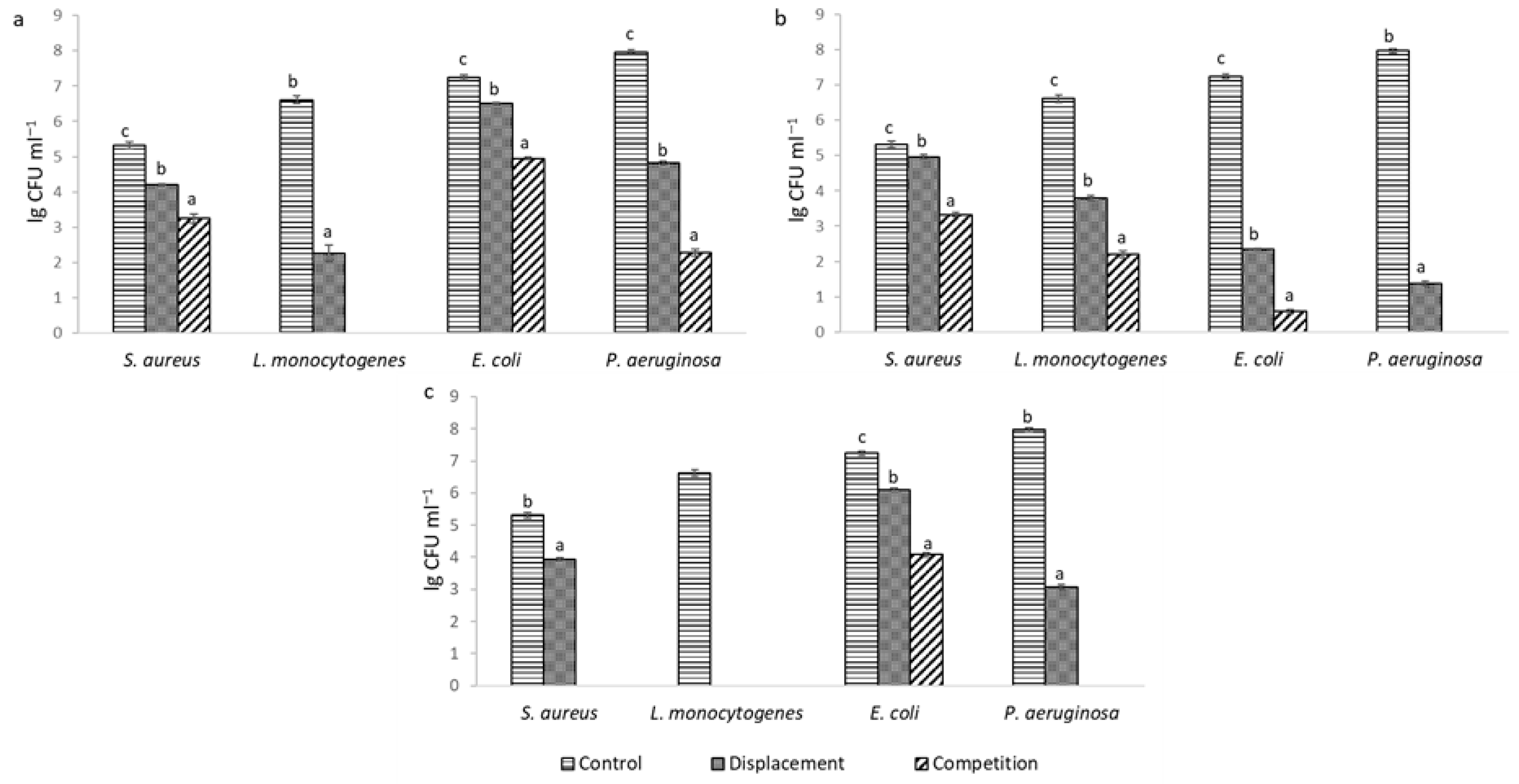
2.1. Antimicrobial Activity and Biological Competition of LAB
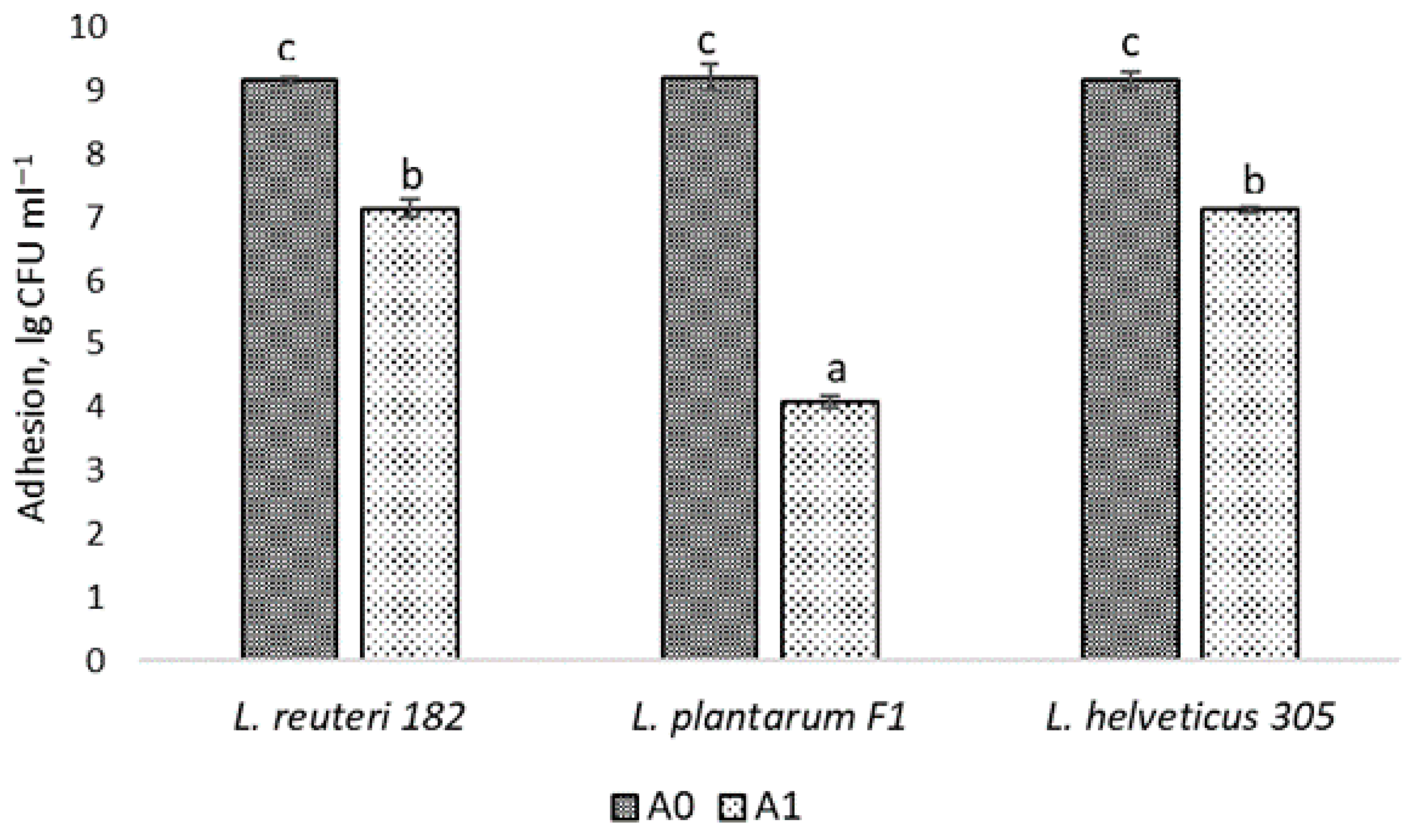
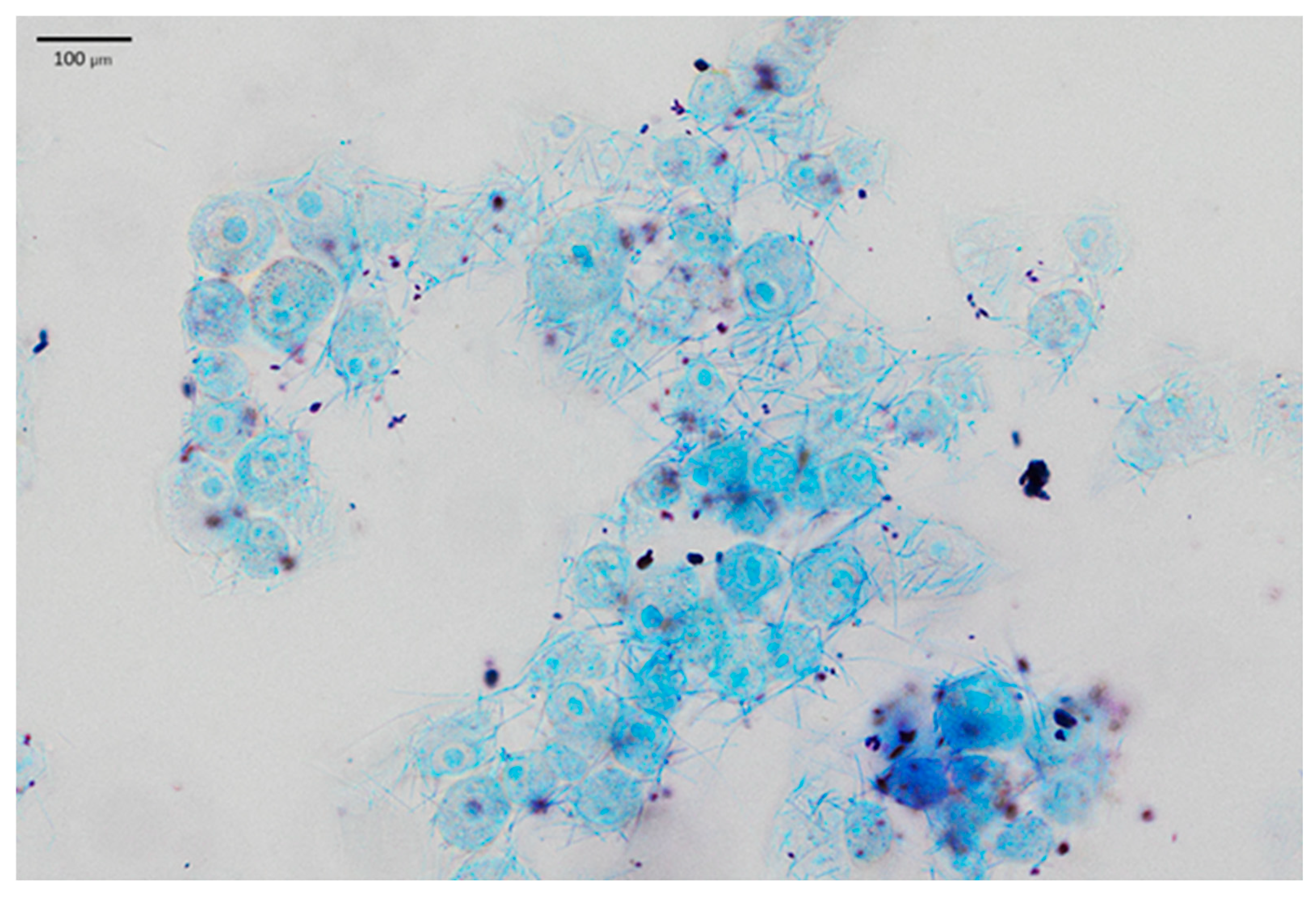
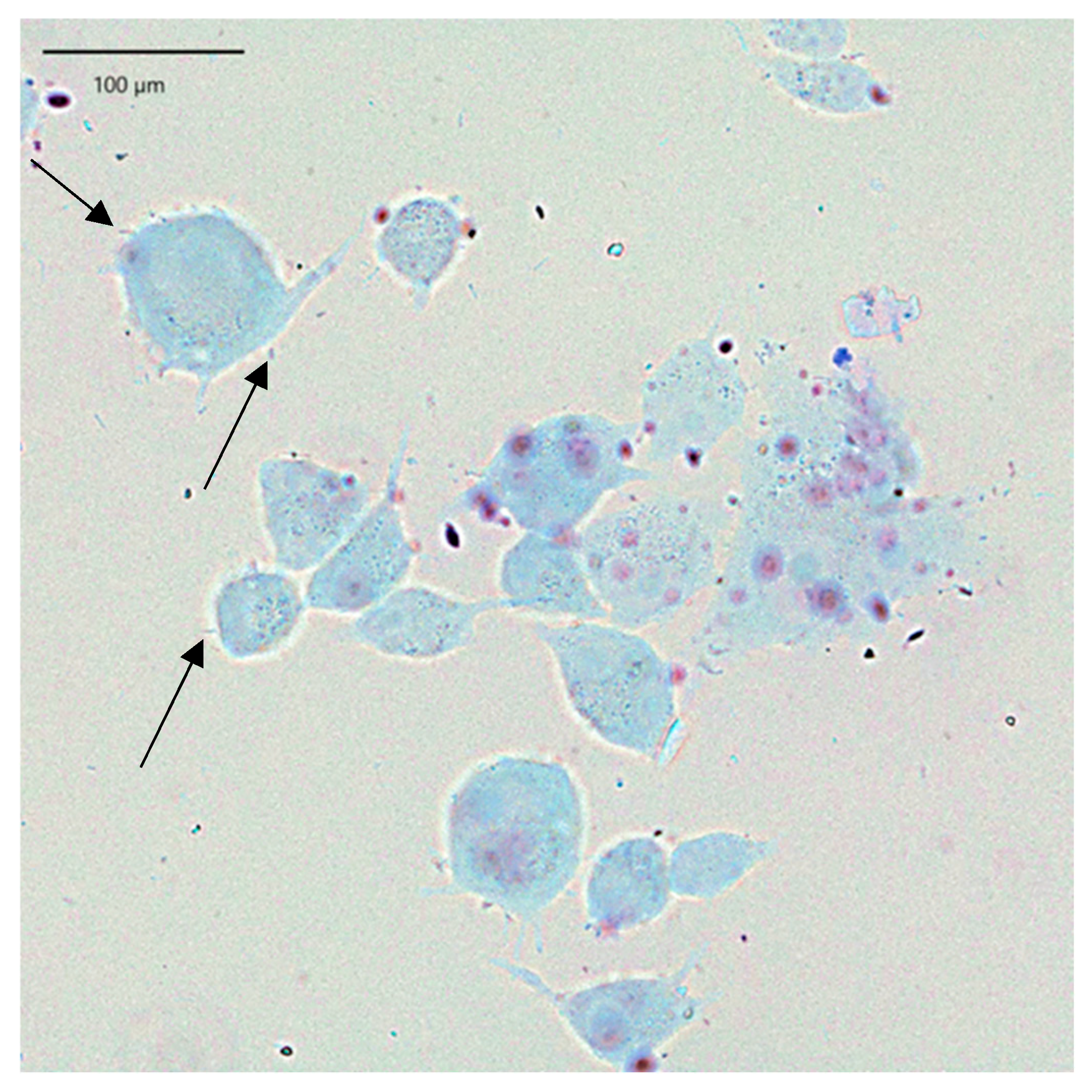
2.2. LAB Adhesion to Human Skin Keratinocytes
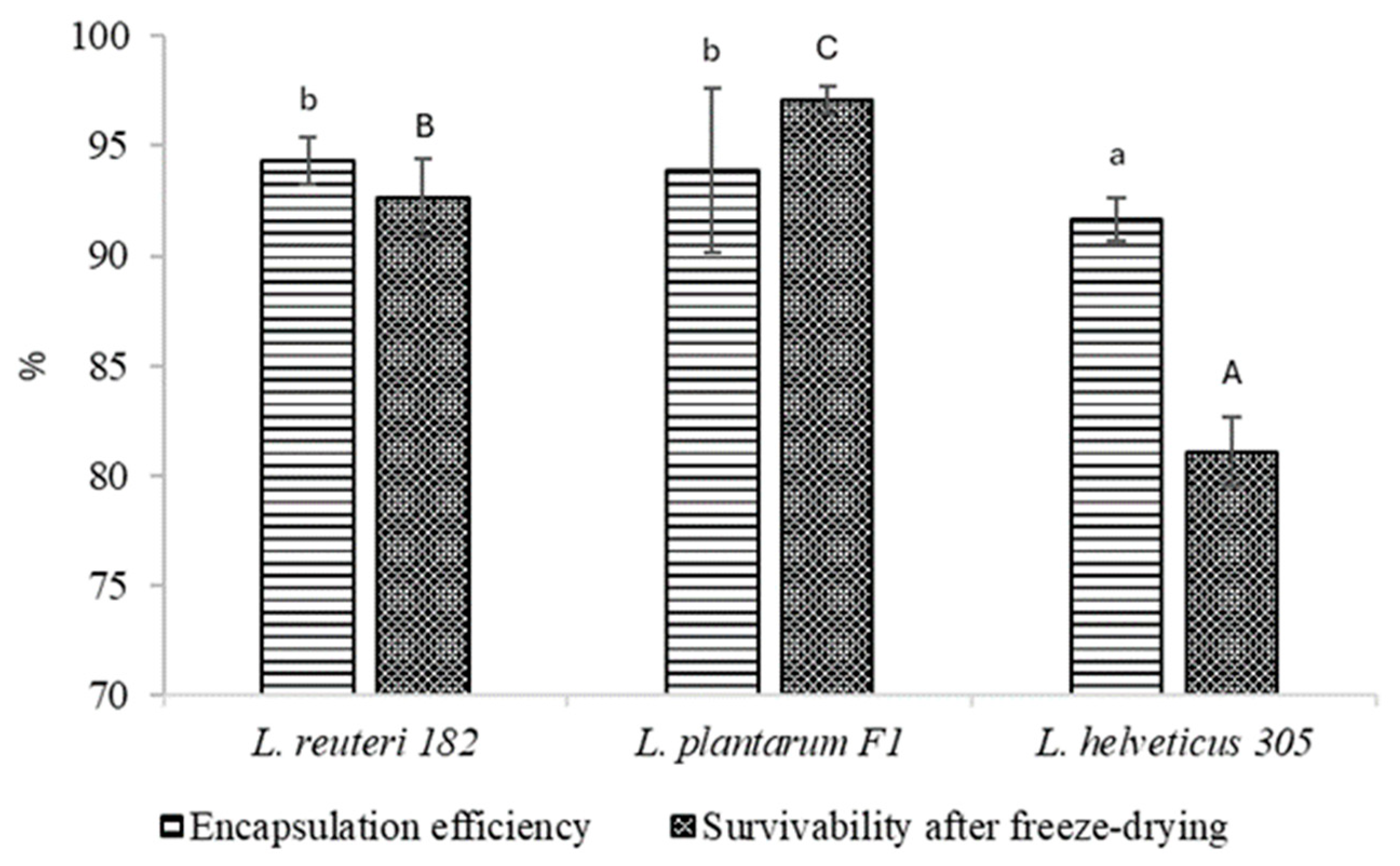
2.3. Encapsulation Efficiency and Survivability During Freeze-Drying of Microspheres with Encapsulated LAB Cells
2.4. The Efficacy of the Preservative of the Topical Probiotic Formulation
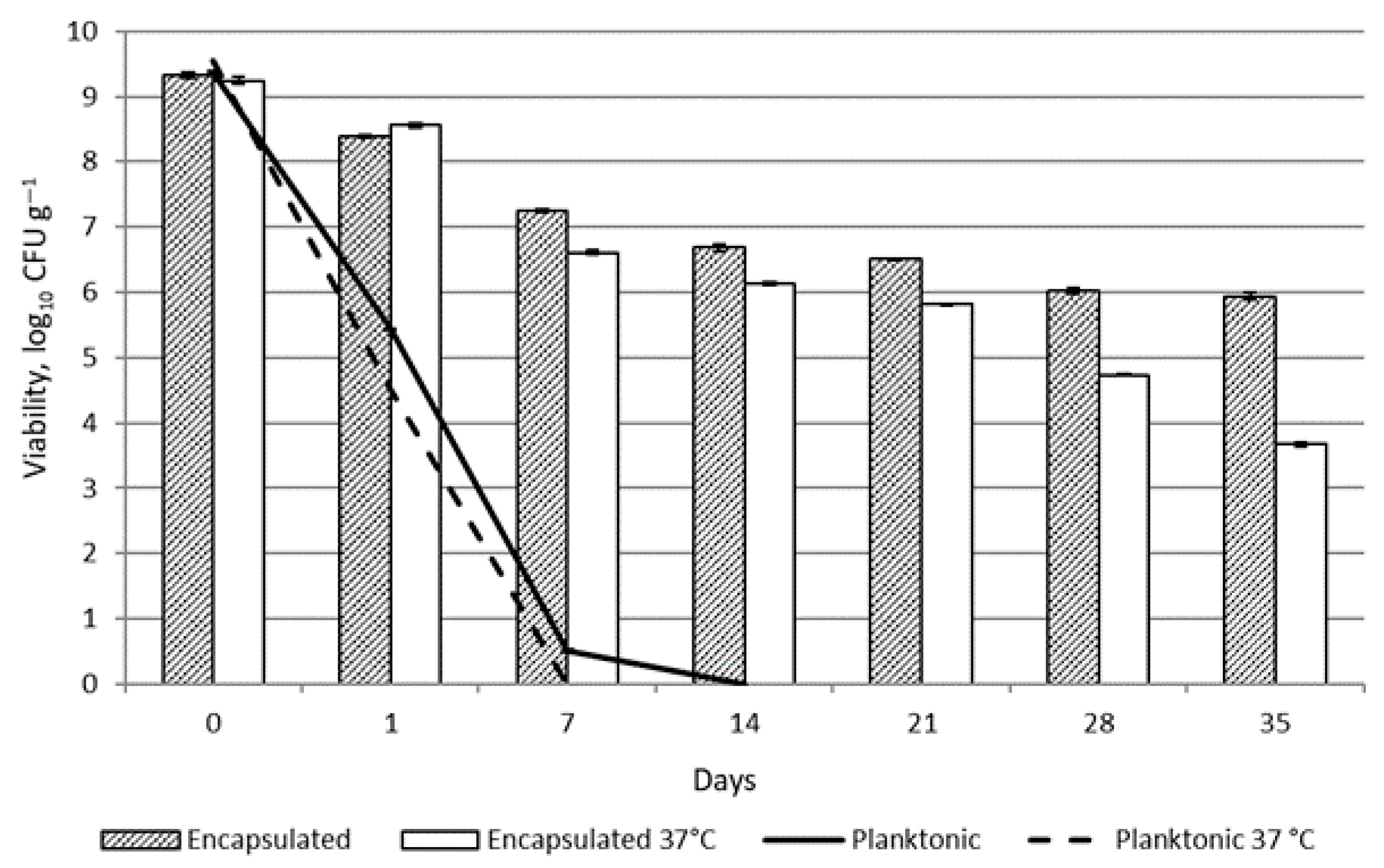
2.5. Survival of Encapsulated LAB Incorporated in the Topical Probiotic Emulsion Matrix
3. Materials and Methods
3.1. Determination of Probiotic Properties of Lactic Acid Bacteria
3.1.1. Microorganism Growth Conditions
3.1.2. Co-Aggregation Assay
3.1.3. Evaluation of the Antimicrobial Activity of LAB Metabolites
3.1.4. Inhibition of Pathogenic Biofilm Assay
3.1.5. LAB Adhesion to Epidermal Cells
3.2. LAB Encapsulation and Application in Topical Formulation Preparation
3.2.1. Microencapsulation Procedure
3.2.2. Enumeration of the Encapsulated Bacteria and Survival Assay
3.2.3. Topical Prebiotic Formulation Preparation
3.2.4. Preservative Challenge Test
3.2.5. Determination of LAB Viability in Topical Formulation Products Under Accelerated Conditions
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallo, R.L. Human skin is the largest epithelial surface for interaction with microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Lizardo, M.; Magalhães, R.M.; Tavaria, F.K. Probiotic adhesion to skin keratinocytes and underlying mechanisms. Biology 2022, 11, 1372. [Google Scholar] [CrossRef]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Khalid Anwer, M.; Fattepur, S. Topical probiotics: More than a skin deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhuo, F.; Guo, Y.; Wang, S.; Zhang, K.; Li, X.; Dai, W.; Dou, X.; Yu, B. Skin microbiota: Pathogenic roles and implications in atopic dermatitis. Front. Cell. Infect. Microbiol. 2025, 14, 1518811. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, C.; Knödlseder, N.; Karoglan, A.; Güell, M.; Paetzold, B. Skin microbiome transplantation and manipulation: Current state of the art. Comput. Struct. Biotechnol. J. 2021, 19, 624–631. [Google Scholar] [CrossRef]
- Petrillo, F.; Buonanno, A.; Fedi, L.; Galdiero, M.; Reibaldi, M.; Tamburini, B.; Galdiero, E. Atopic dermatitis and atopic keratoconjunctivitis: New insights in the analyses of microbiota and probiotic effect. Int. J. Mol. Sci. 2025, 26, 1463. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef]
- Łętocha, A.; Michalczyk, A.; Ostrowska, P.; Miastkowska, M.; Sikora, E. Probiotics-loaded microspheres for cosmetic applications. Appl. Sci. 2024, 14, 1183. [Google Scholar] [CrossRef]
- Butler, É.; Lundqvist, C.; Axelsson, J. Lactobacillus reuteri DSM 17938 as a novel topical cosmetic ingredient: A proof of concept clinical study in adults with atopic dermatitis. Microorganisms 2020, 8, 1026. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Reid, G. Probiotics in cosmetic and personal care products: Trends and challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell. Fact. 2020, 19, 168. [Google Scholar] [CrossRef]
- Silva, D.R.; de Cássia Orlandi Sardi, J.; de Souza Pitangui, N.; Roque, S.M.; da Silva, A.C.B.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta Biomembr. 2016, 1858, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Szafranski, S.P.; Qu, T.; Mukherjee, R.; Stiesch, M.; Wriggers, P.; Junker, P. Numerical and experimental investigation of multi-species bacterial co-aggregation. Sci. Rep. 2023, 13, 11839. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.C.; Botting, J.; Gomes, I.B.; Saavedra, M.J.; Simões, L.C.; Liu, J.; Simões, M. Elucidating bacterial coaggregation through a physicochemical and imaging surface characterization. Sci. Total Environ. 2024, 948, 174872. [Google Scholar] [CrossRef]
- Lee, J.; Lee, N.; Paik, H. Antimicrobial and anti-biofilm effects of probiotic Lactobacillus plantarum KU200656 isolated from kimchi. Food Sci. Biotechnol. 2021, 30, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Kheyrolahzadeh, K.; Khatibi, S.M.H.; Sharifi, S.; Memar, M.Y.; Zununi Vahed, S. The battle of probiotics and their derivatives against biofilms. Infect. Drug Resist. 2020, 13, 659–672. [Google Scholar] [CrossRef]
- Lopes, E.G.; Moreira, D.A.; Gullón, P.; Gullón, B.; Cardelle-Cobas, A.; Tavaria, F.K. Topical application of probiotics in skin: Adhesion, antimicrobial and antibiofilm in vitro assays. J. Appl. Microbiol. 2017, 122, 450–461. [Google Scholar] [CrossRef]
- Reunanen, J.; von Ossowski, I.; Hendrickx, A.P.; Palva, A.; de Vos, W.M. Characterization of the SpaCBA pilus fibers in the probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 2012, 78, 2337–2344. [Google Scholar] [CrossRef]
- Tomás, M.S.J.; de Gregorio, P.R.; Terraf, M.C.L.; Nader-Macías, M.E.F. Encapsulation and subsequent freeze-drying of Lactobacillus reuteri CRL 1324 for its potential inclusion in vaginal probiotic formulations. Eur. J. Pharm. Sci. 2015, 79, 87–95. [Google Scholar] [CrossRef]
- Jeznienė, S.; Bružaitė, I.; Šipailienė, A. Application of biomacromolecules encapsulation systems for the long-term storage of Lactobacillus plantarum F1 and Lactobacillus reuteri 182. Heliyon 2024, 10, e26566. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto-Shinohara, Y.; Sukenobe, J.; Imaizumi, T.; Nakahara, T. Survival of freeze-dried bacteria. J. Gen. Appl. Microbiol. 2008, 54, 9–24. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, F.; Chen, M.; Krystalli, E.; Giatrakou, V.; Zhong, F. Mechanism analysis of calcium-induced low freeze-drying survival of probiotic encapsulated in alginate. Food Hydrocoll. 2023, 145, 109065. [Google Scholar] [CrossRef]
- Tan, L.L.; Sampathkumar, K.; Wong, J.H.; Loo, S.C.J. Divalent cations are antagonistic to survivability of freeze-dried probiotics encapsulated in cross-linked alginate. Food Bioprod. Process. 2020, 124, 369–377. [Google Scholar] [CrossRef]
- Orth, D.S.; Steinberg, D.C. The safety factor in preservative efficacy testing. Cosmet. Toiletr. 2003, 118, 51–58. [Google Scholar]
- Chen, T.; Chang, H. Deciphering trends in replacing preservatives in cosmetics intended for infants and sensitive population. Sci. Rep. 2024, 14, 19053–19058. [Google Scholar] [CrossRef]
- Ezeobiora, C.E.; Adeluola, A.O.; Mendie, U.E. Antimicrobial evaluation of preservative efficacy in formulations of locally sourced kaolin. World J. Pharm. Res. 2020, 9, 156–164. [Google Scholar]
- BS EN ISO 11930:2019+A1:2022; Cosmetics. Microbiology. Evaluation of the Antimicrobial Protection of a Cosmetic Product. BSI: London, UK, 2022.
- Carbol, J.; Tan, P.I.; Varma, Y.; Osborne, D.W. Formulating topical products containing live microorganisms as the active ingredient. Pharm. Technol. Eur. 2018, 30, 24–27. [Google Scholar]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef]
- Woo, J.; Ahn, J. Probiotic-mediated competition, exclusion and displacement in biofilm formation by food-borne pathogens. Lett. Appl. Microbiol. 2013, 56, 307–313. [Google Scholar] [CrossRef]
- Coman, M.M.; Mazzotti, L.; Silvi, S.; Scalise, A.; Orpianesi, C.; Cresci, A.; Verdenelli, M.C. Antimicrobial activity of SYNBIO® probiotic formulation in pathogens isolated from chronic ulcerative lesions: In vitro studies. J. Appl. Microbiol. 2020, 128, 584–597. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Diameter of Inhibition Zone, mm | ||
|---|---|---|---|
| L. reuteri 182 | L. plantarum F1 | L. helveticus 305 | |
| Gram-Positive | |||
| B. cereus | 11.3 ± 1.0 aA | 17.8 ± 0.5 bB | 18.0 ± 0 cB |
| B. circulans | 13.0 ± 0.4 bA | 19.4 ± 0.5 cB | 19.3 ± 0.5 dB |
| C. freundii | 19.1 ± 1.0 cA | 19.3 ± 0.5 cA | 21.3 ± 0.5 eB |
| E. faecalis | no zone | 14.5 ± 0.6 aA | 16.4 ± 0.5 bB |
| S. aureus | no zone | 16.8 ± 0.5 bB | 14.5 ± 0.6 aA |
| S. epidermidis | no zone | 15.5 ± 0.6 aA | 16.5 ± 0.6 bB |
| L. monocytogenes | 19.5 ± 0.6 cA | 19.6 ± 0.5 cdA | 21.3 ± 0.5 eB |
| Gram-Negative | |||
| E. coli | 11.3 ± 0.5 aA | 15.5 ± 0.6 aC | 13.5 ± 0.6 aB |
| K. pneumoniae | 11.1 ± 0.3 aA | 15.0 ± 0 aB | 16.0 ± 0.8 bC |
| P. aeruginosa | 14.5 ± 0.6 dA | 20.6 ± 0.5 dC | 18.3 ± 0.5 cB |
| S. typhimurium | 11.5 ± 0.6 aA | 17.0 ± 0.8 bB | 16.8 ± 0.5 bB |
| Microorganism | Logarithmic Reduction in Time Tx days | Statement of Conformity | ||
|---|---|---|---|---|
| T7 | T14 | T28 | ||
| E. coli | 4.37 | 5.32 | 5.32 | comply with criteria A |
| S. aureus | 5.17 | 5.17 | 5.17 | comply with criteria A |
| P. aeruginosa | 4.47 | 5.61 | 5.61 | comply with criteria A |
| C. albicans | 3.28 | 4.72 | 4.72 | comply with criteria A |
| A. niger | - | 3.63 | 3.63 | comply with criteria A |
| Microorganism | Growth Media | Selective/Differential Media |
|---|---|---|
| Bacillus cereus | Nutrient Broth (NB, Liofilchem, Roseto degli Abruzzi, Italy) | - |
| Bacillus circulans | NB | - |
| Citrobacter freundii | NB | - |
| Enterococcus faecalis | NB | - |
| Escherichia coli | Brain Heart Infusion Broth (BHIB, Liofilchem, Roseto degli Abruzzi, Italy) | Eosin Methylene Blue Agar (Oxoid, Hampshire, UK) |
| Klebsiella pneumoniae | NB | - |
| Listeria monocytogenes | BHIB | Agar Listeria Acc. to Ottaviani and Agosti (Biolife, Monza, Italy) |
| Pseudomonas aeruginosa | BHIB | Pseudomonas Agar Base (Oxoid, Hampshire, UK) |
| Salmonella typhimurium | BHIB | - |
| Staphylococcus aureus | NB | Bair Parker Agar (Liofilchem, Roseto degli Abruzzi, Italy) |
| Staphylococcus epidermidis | NB | - |
| Phase | INCI Name | Concentration, % |
|---|---|---|
| A | Aqua | q.s. to 100 |
| Aloe barbadensis leaf juice powder | 0.1 | |
| Glycerin | 4.0 | |
| Sodium hyaluronate | 0.1 | |
| B | Simmondsia chinensis seed oil | 10.0 |
| Helianthus annuus seed oil | 20.0 | |
| Cetearyl olivate | 1.9 | |
| Sorbitan olivate | 1.9 | |
| Glyceryl stearate | 1.9 | |
| C | Tocopherol | 1.0 |
| Glycerin (and) Aqua (and) Tassmannia lanceolata fruit/leaf extract | 2.0 | |
| Calcium alginate microspheres | 10.0 | |
| Parfum | 1.5 | |
| D | Aqua (and) Lactic acid | 0.1 |
| E | Aqua | 2.0 |
| Sodium benzoate | 0.4 | |
| Potassium sorbate | 0.2 |
| Microorganism | Criterion | Logarithmic Decrease | ||
|---|---|---|---|---|
| 7 d | 14 d | 28 d | ||
| Bacteria | A | ≥3 | ≥3 | ≥3 |
| B | - | ≥3 | ≥3 | |
| C. albicans | A | ≥1 | ≥1 | ≥1 |
| B | - | ≥1 | ≥1 | |
| A. niger | A | - | ≥0 | ≥1 |
| B | - | ≥0 | ≥0 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Jeznienė, S.; Mikalauskienė, E.; Jekabsone, A.; Šipailienė, A. Topical Delivery of Autochthonous Lactic Acid Bacteria Using Calcium Alginate Microspheres as a Probiotic Carrier System with Enhanced Therapeutic Potential. Pharmaceuticals 2026, 19, 66. https://doi.org/10.3390/ph19010066
Jeznienė S, Mikalauskienė E, Jekabsone A, Šipailienė A. Topical Delivery of Autochthonous Lactic Acid Bacteria Using Calcium Alginate Microspheres as a Probiotic Carrier System with Enhanced Therapeutic Potential. Pharmaceuticals. 2026; 19(1):66. https://doi.org/10.3390/ph19010066
Chicago/Turabian StyleJeznienė, Sigita, Emilija Mikalauskienė, Aistė Jekabsone, and Aušra Šipailienė. 2026. "Topical Delivery of Autochthonous Lactic Acid Bacteria Using Calcium Alginate Microspheres as a Probiotic Carrier System with Enhanced Therapeutic Potential" Pharmaceuticals 19, no. 1: 66. https://doi.org/10.3390/ph19010066
APA StyleJeznienė, S., Mikalauskienė, E., Jekabsone, A., & Šipailienė, A. (2026). Topical Delivery of Autochthonous Lactic Acid Bacteria Using Calcium Alginate Microspheres as a Probiotic Carrier System with Enhanced Therapeutic Potential. Pharmaceuticals, 19(1), 66. https://doi.org/10.3390/ph19010066







