A Combined SIRT5 Activation and SIRT3 Inhibition Prevents Breast Cancer Spheroids Growth by Reducing HIF-1α and Mitophagy
Abstract
1. Introduction
2. Results
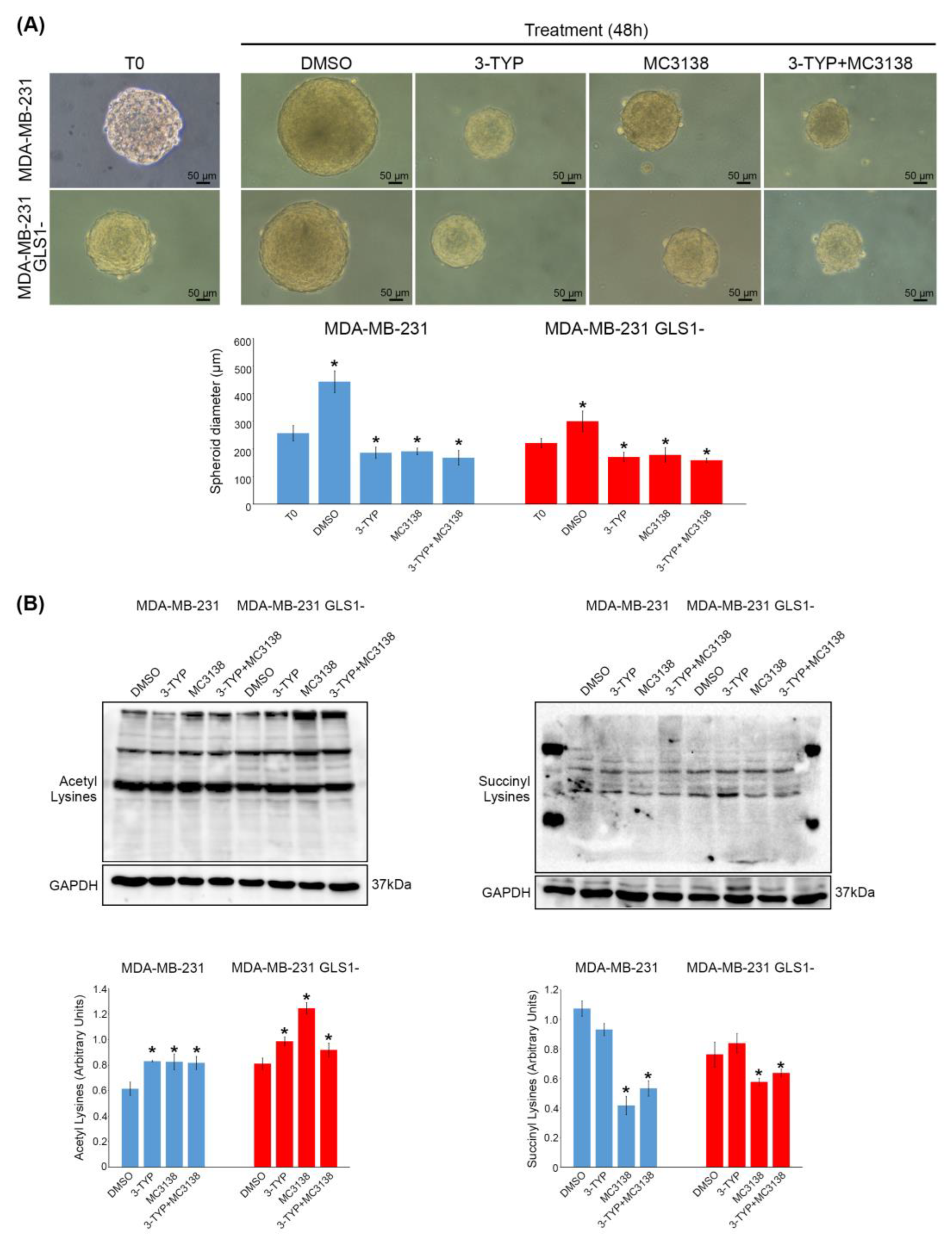
2.1. SIRT3 Inhibition and SIRT5 Activation Reduce the Growth of MDA-MB-231 Spheroids
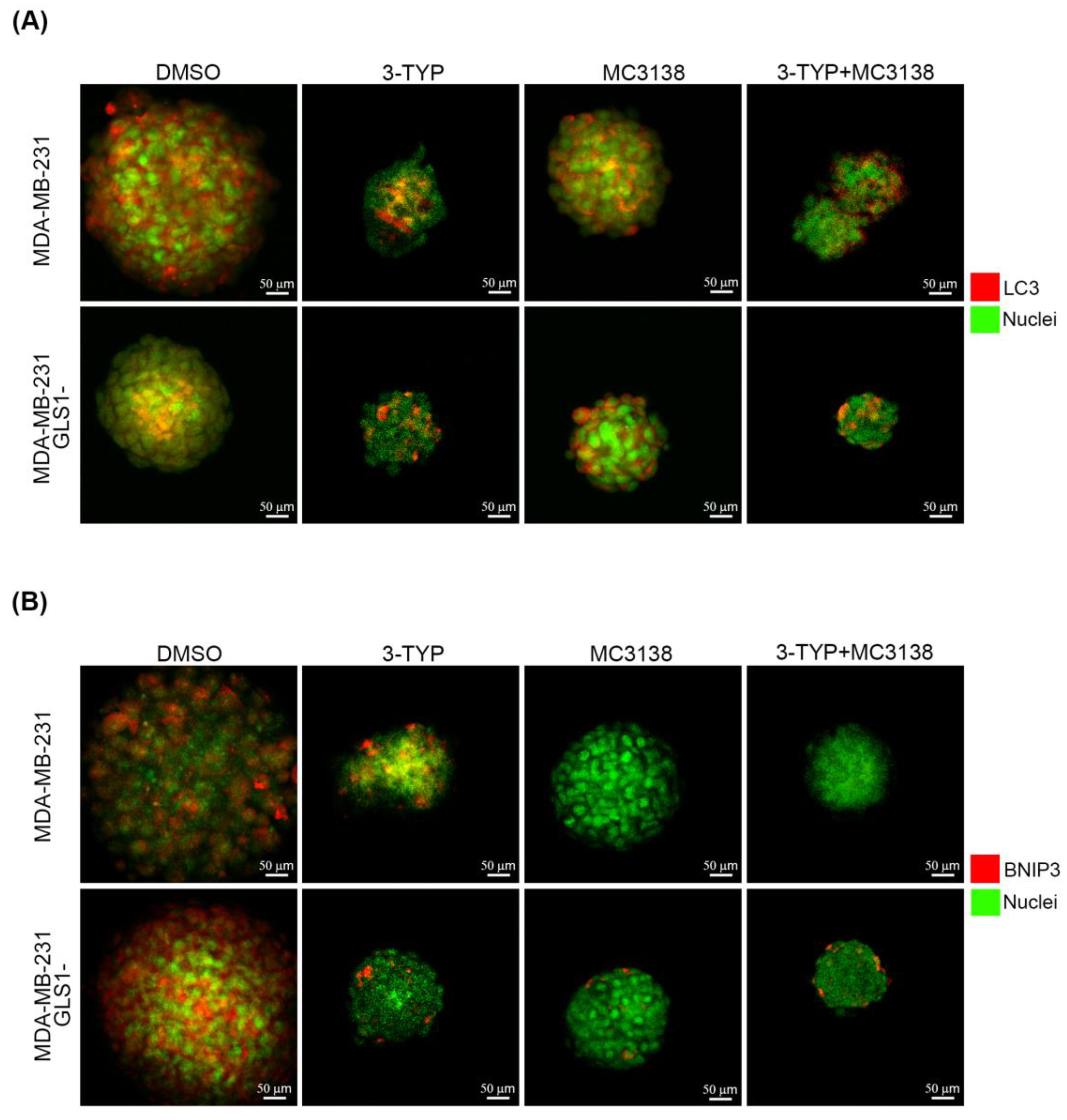
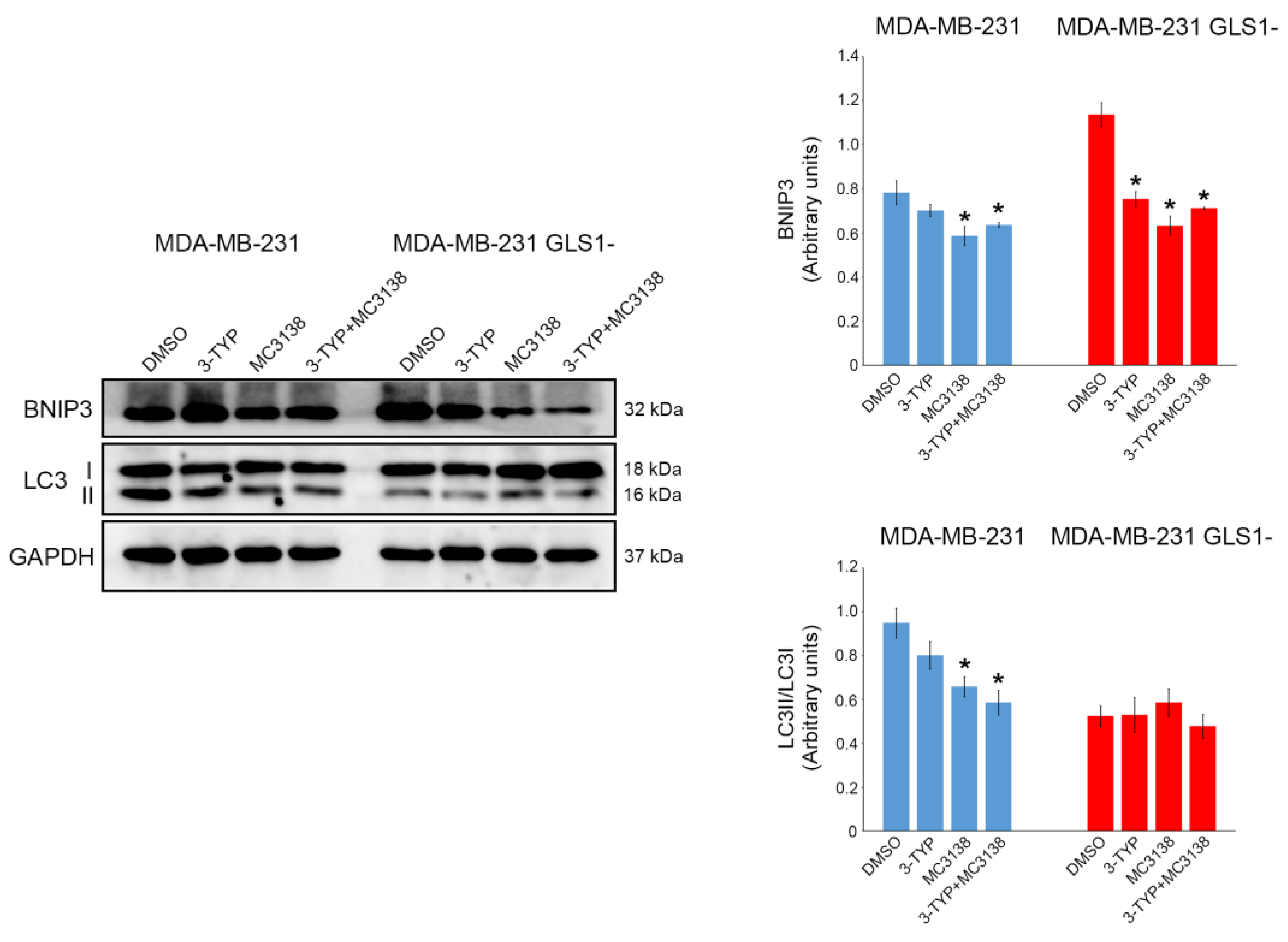
2.2. SIRT3 Inhibition and SIRT5 Activation Alter the Expression of Autophagy and Mitophagy Markers
2.3. SIRT3 Inhibition and SIRT5 Activation Alter the Morphology of MDA-MB-231 Spheroids
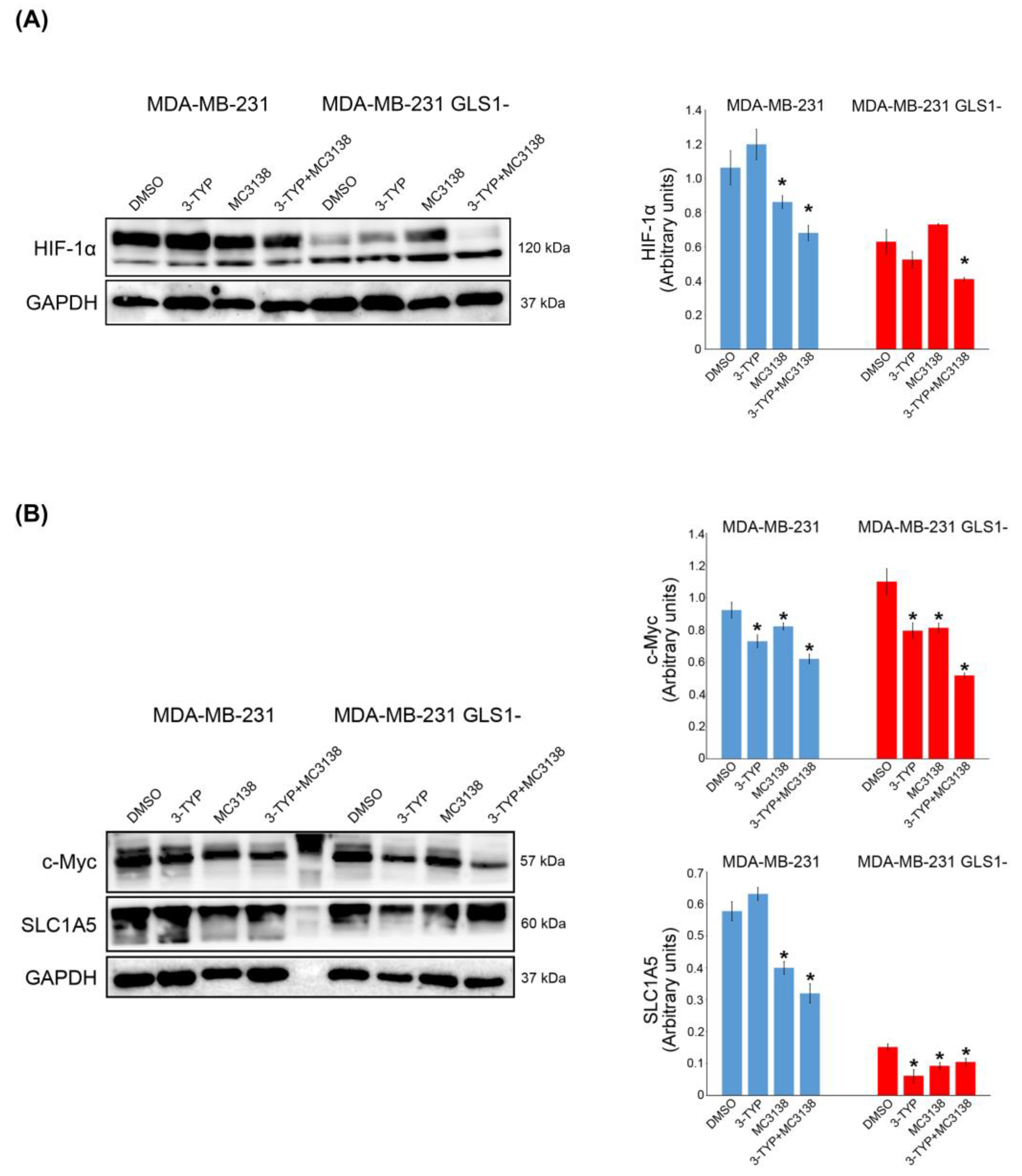
2.4. SIRT3 Inhibition and SIRT5 Activation Influence HIF-1α, c-Myc and SLC1A5 Expression
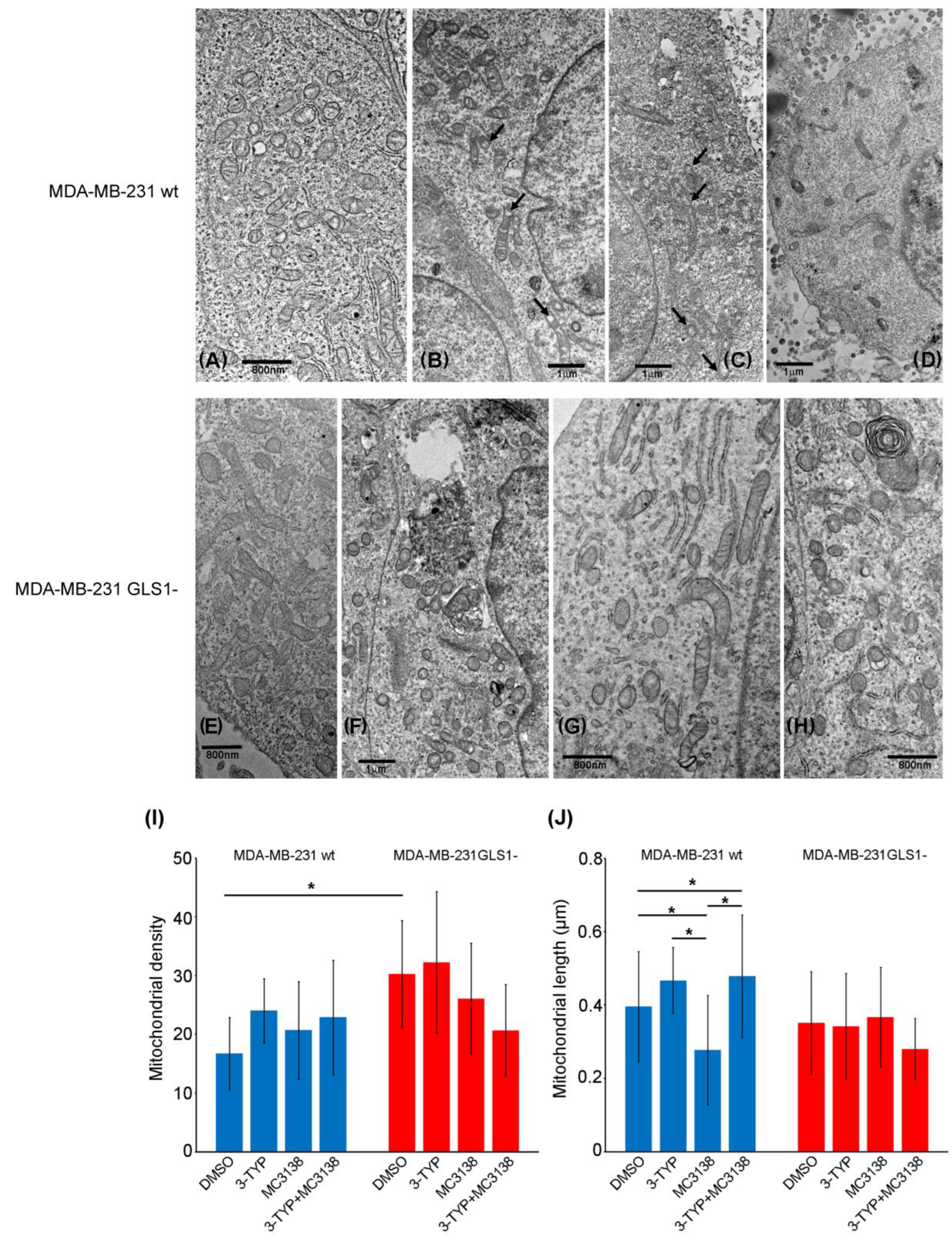
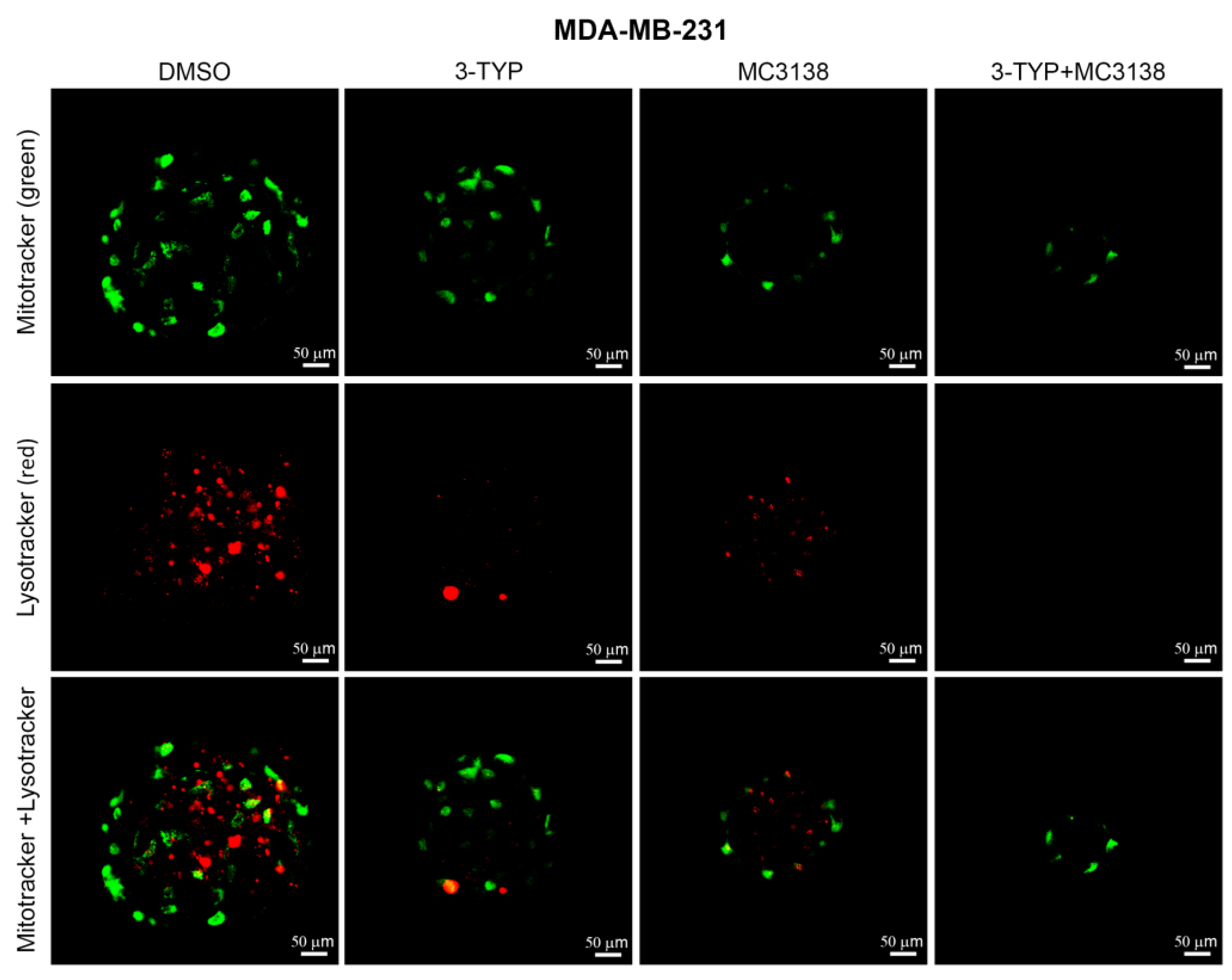
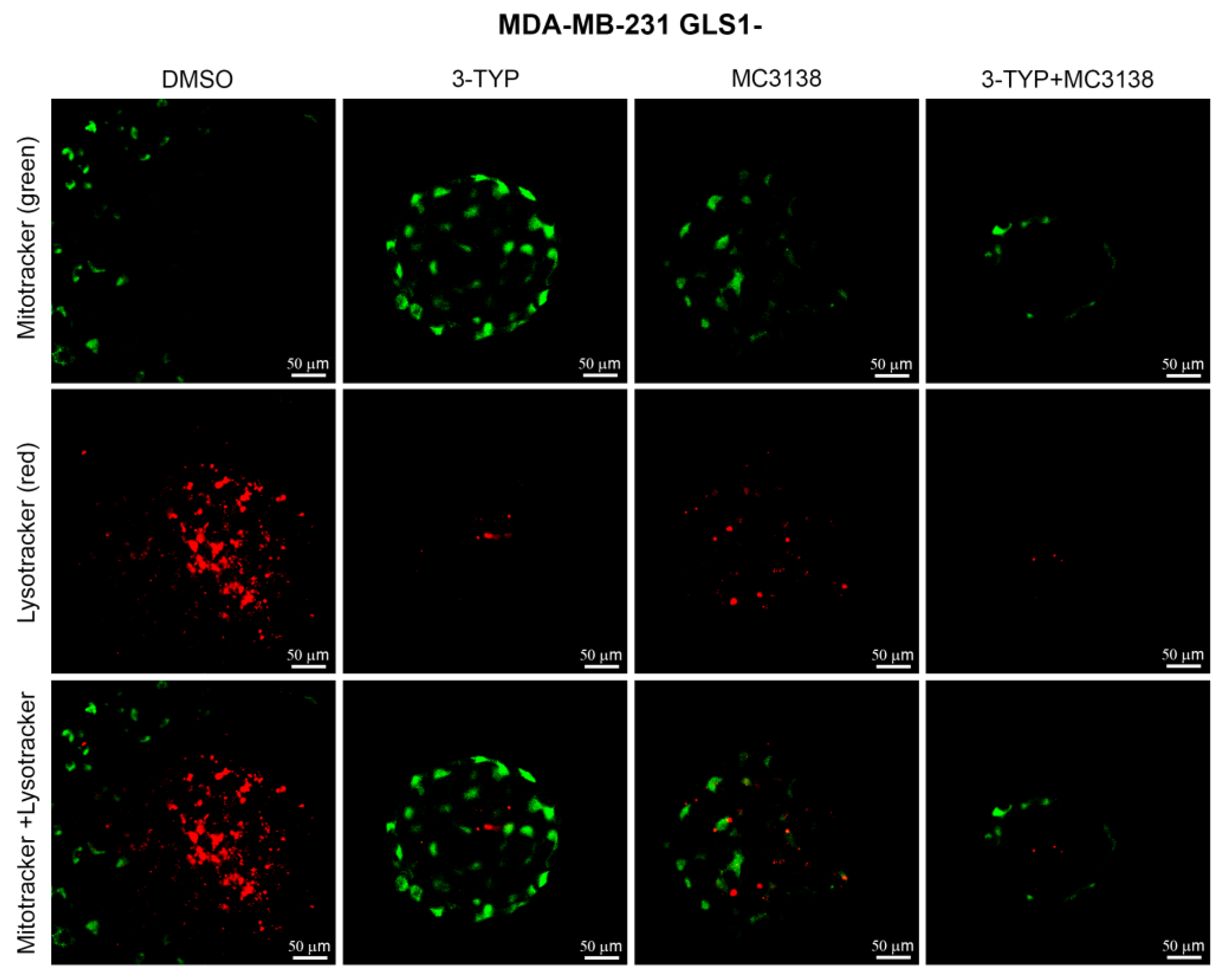
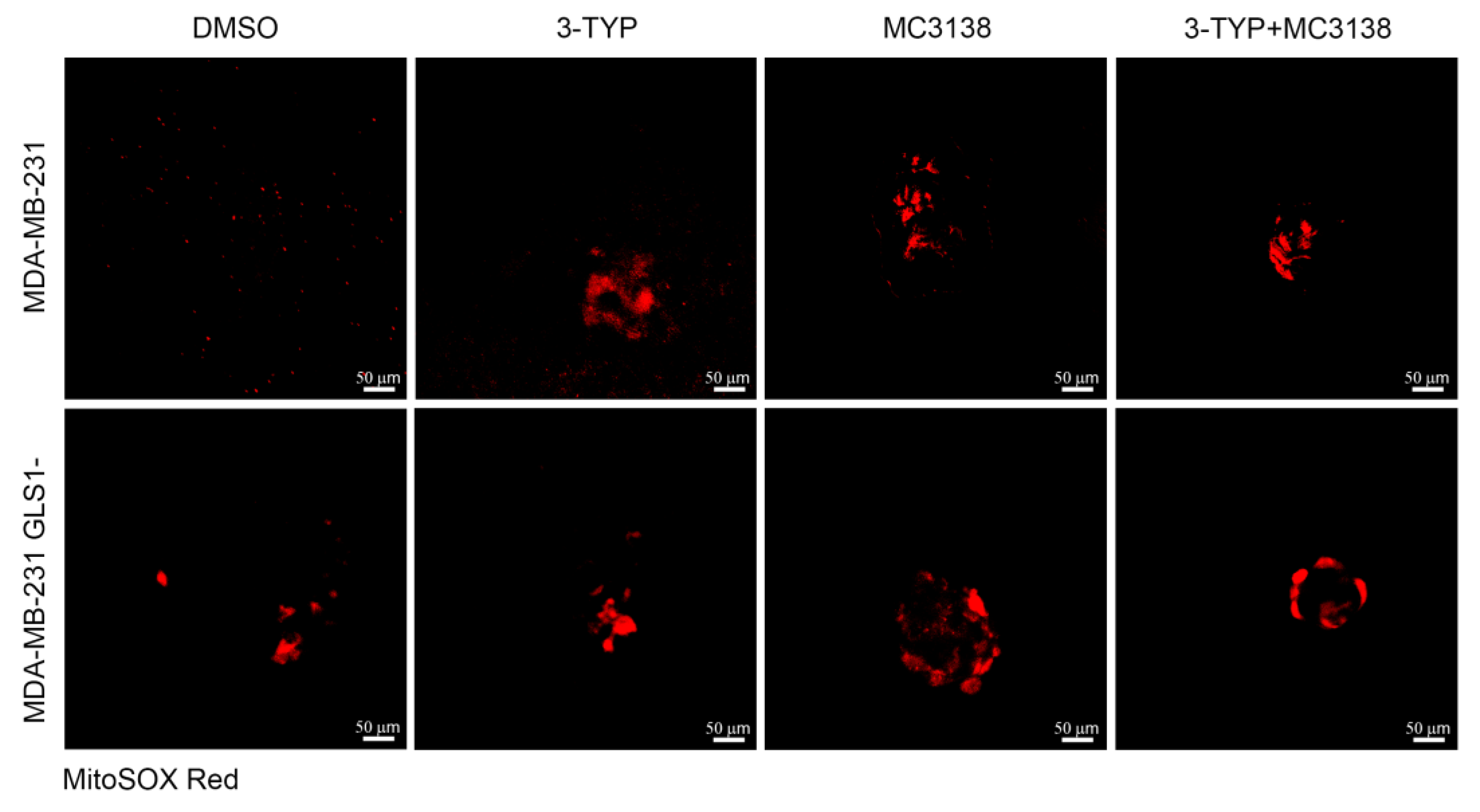
2.5. SIRT3 Inhibition and SIRT5 Activation Reduce Lysosomal Degradation of Mitochondria and Increase Mitochondrial ROS Production
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Trypan Blue Assay
4.3. Clonogenicity Assay
4.4. Spheroids Generation
4.5. Proteins Extraction and Immunoblotting
4.6. Immunofluorescence
4.7. Organelles Staining
4.8. Reactive Oxygen Species Staining
4.9. TEM
4.10. Morphometric Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, B.; Natarajan, E.; Balaji Raghavendran, H.R.; Markandan, U.D. Molecular Classification, Treatment, and Genetic Biomarkers in Triple-Negative Breast Cancer: A Review. Technol. Cancer Res. Treat. 2023, 22, 15330338221145246. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.; Krasnoselskyi, M.; Starikov, V.; Kartashov, S.; Zhulkevych, I.; Vlasenko, V.; Oleshko, K.; Bilodid, O.; Sadchikova, M.; Vinnyk, Y. Triple-negative breast cancer: Current treatment strategies and factors of negative prognosis. J. Med. Life 2022, 15, 153–161. [Google Scholar] [CrossRef]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Li, S.; Zeng, H.; Fan, J.; Wang, F.; Xu, C.; Li, Y.; Tu, J.; Nephew, K.P.; Long, X. Glutamine metabolism in breast cancer and possible therapeutic targets. Biochem. Pharmacol. 2023, 210, 115464. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Q.; Dong, C. Metabolic reprogramming in triple-negative breast cancer. Cancer Biol. Med. 2020, 17, 44–59. [Google Scholar] [CrossRef]
- El Ansari, R.; McIntyre, A.; Craze, M.L.; Ellis, I.O.; Rakha, E.A.; Green, A.R. Altered glutamine metabolism in breast cancer; subtype dependencies and alternative adaptations. Histopathology 2018, 72, 183–190. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Vassilopoulos, A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 2017, 16, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Aventaggiato, M.; Barreca, F.; Sansone, L.; Pellegrini, L.; Russo, M.A.; Cordani, M.; Tafani, M. Sirtuins and Hypoxia in EMT Control. Pharmaceuticals 2022, 15, 737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Dong, Z.; Ke, X.; Hou, J.; Zhao, E.; Zhang, K.; Wang, F.; Yang, L.; Xiang, Z.; Cui, H. The roles of sirtuins family in cell metabolism during tumor development. Semin. Cancer Biol. 2019, 57, 59–71. [Google Scholar] [CrossRef]
- Giblin, W.; Skinner, M.E.; Lombard, D.B. Sirtuins: Guardians of mammalian healthspan. Trends Genet. 2014, 30, 271–286. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, H. Mitochondrial sirtuins: Energy dynamics and cancer metabolism. Mol. Cells 2024, 47, 100029. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Guarente, L. The SirT3 divining rod points to oxidative stress. Mol. Cell 2011, 42, 561–568. [Google Scholar] [CrossRef]
- Li, R.; Quan, Y.; Xia, W. SIRT3 inhibits prostate cancer metastasis through regulation of FOXO3A by suppressing Wnt/β-catenin pathway. Exp. Cell Res. 2018, 364, 143–151. [Google Scholar] [CrossRef]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef]
- Barreca, F.; Aventaggiato, M.; Vitiello, L.; Sansone, L.; Russo, M.A.; Mai, A.; Valente, S.; Tafani, M. SIRT5 Activation and Inorganic Phosphate Binding Reduce Cancer Cell Vitality by Modulating Autophagy/Mitophagy and ROS. Antioxidants 2023, 12, 1635. [Google Scholar] [CrossRef]
- Aventaggiato, M.; Vernucci, E.; Barreca, F.; Russo, M.A.; Tafani, M. Sirtuins’ control of autophagy and mitophagy in cancer. Pharmacol. Ther. 2021, 221, 107748. [Google Scholar] [CrossRef]
- Carnevale, I.; Pellegrini, L.; D’Aquila, P.; Saladini, S.; Lococo, E.; Polletta, L.; Vernucci, E.; Foglio, E.; Coppola, S.; Sansone, L.; et al. SIRT1-SIRT3 Axis Regulates Cellular Response to Oxidative Stress and Etoposide. J. Cell. Physiol. 2017, 232, 1835–1844. [Google Scholar] [CrossRef]
- Garva, R.; Thepmalee, C.; Yasamut, U.; Sudsaward, S.; Guazzelli, A.; Rajendran, R.; Tongmuang, N.; Khunchai, S.; Meysami, P.; Limjindaporn, T.; et al. Sirtuin Family Members Selectively Regulate Autophagy in Osteosarcoma and Mesothelioma Cells in Response to Cellular Stress. Front. Oncol. 2019, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, key regulators of aging. Life Med. 2025, 4, lnaf019. [Google Scholar] [CrossRef]
- Bittman-Soto, X.S.; Thomas, E.S.; Ganshert, M.E.; Mendez-Santacruz, L.L.; Harrell, J.C. The Transformative Role of 3D Culture Models in Triple-Negative Breast Cancer Research. Cancers 2024, 16, 1859. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. 2020, 13, 5395–5405. [Google Scholar] [CrossRef]
- Riffle, S.; Pandey, R.N.; Albert, M.; Hegde, R.S. Linking hypoxia, DNA damage and proliferation in multicellular tumor spheroids. BMC Cancer 2017, 17, 338. [Google Scholar] [CrossRef]
- Jin, J.; Byun, J.K.; Choi, Y.K.; Park, K.G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Koh, E.; Lee, Y.S.; Lee, H.W.; Kang, H.G.; Yoon, Y.E.; Han, W.K.; Choi, K.H.; Kim, K.S. Mitochondrial Sirt3 supports cell proliferation by regulating glutamine-dependent oxidation in renal cell carcinoma. Biochem. Biophys. Res. Commun. 2016, 474, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Galli, U.; Mesenzani, O.; Coppo, C.; Sorba, G.; Canonico, P.L.; Tron, G.C.; Genazzani, A.A. Identification of a sirtuin 3 inhibitor that displays selectivity over sirtuin 1 and 2. Eur. J. Med. Chem. 2012, 55, 58–66. [Google Scholar] [CrossRef]
- Suenkel, B.; Valente, S.; Zwergel, C.; Weiss, S.; Di Bello, E.; Fioravanti, R.; Aventaggiato, M.; Amorim, J.A.; Garg, N.; Kumar, S.; et al. Potent and Specific Activators for Mitochondrial Sirtuins Sirt3 and Sirt5. J. Med. Chem. 2022, 65, 14015–14031. [Google Scholar] [CrossRef] [PubMed]
- Hackenbrock, C.R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria. J. Cell Biol. 1966, 30, 269–297. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef]
- Gao, P.; Tchernyshyov, I.; Chang, T.C.; Lee, Y.S.; Kita, K.; Ochi, T.; Zeller, K.I.; De Marzo, A.M.; Van Eyk, J.E.; Mendell, J.T.; et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, T.; Li, Z.; Wang, L.; Yuan, S.; Sun, L. The role of ASCT2 in cancer: A review. Eur. J. Pharmacol. 2018, 837, 81–87. [Google Scholar] [CrossRef]
- Du, D.; Liu, C.; Qin, M.; Zhang, X.; Xi, T.; Yuan, S.; Hao, H.; Xiong, J. Metabolic dysregulation and emerging therapeutical targets for hepatocellular carcinoma. Acta Pharm. Sin. B 2022, 12, 558–580. [Google Scholar] [CrossRef]
- Sun, J.; Ding, J.; Yue, H.; Xu, B.; Sodhi, A.; Xue, K.; Ren, H.; Qian, J. Hypoxia-induced BNIP3 facilitates the progression and metastasis of uveal melanoma by driving metabolic reprogramming. Autophagy 2025, 21, 191–209. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Li, T.; Copeland, C.; Le, A. Glutamine Metabolism in Cancer. Adv. Exp. Med. Biol. 2021, 1311, 17–38. [Google Scholar] [CrossRef]
- Ning, L.; Xie, N. SIRT3 Expression Predicts Overall Survival and Neoadjuvant Chemosensitivity in Triple-Negative Breast Cancer. Cancer Manag. Res. 2024, 16, 137–150. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Abril, Y.L.N.; Fernandez, I.R.; Hong, J.Y.; Chiang, Y.L.; Kutateladze, D.A.; Zhao, Q.; Yang, M.; Hu, J.; Sadhukhan, S.; Li, B.; et al. Pharmacological and genetic perturbation establish SIRT5 as a promising target in breast cancer. Oncogene 2021, 40, 1644–1658. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Yang, Y.; Dai, X.; Xu, S.; Li, T.; Zhang, Q.; Zhao, K.S.; Chen, Z. Polydatin ameliorates injury to the small intestine induced by hemorrhagic shock via SIRT3 activation-mediated mitochondrial protection. Expert Opin. Ther. Targets 2016, 20, 645–652. [Google Scholar] [CrossRef]
- Weinert, B.T.; Schölz, C.; Wagner, S.A.; Iesmantavicius, V.; Su, D.; Daniel, J.A.; Choudhary, C. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013, 4, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, H.; Guo, Z.; Zou, S.; Long, F.; Wu, J.; Li, P.; Zhao, G.P.; Zhao, W. Global Insights into Lysine Acylomes Reveal Crosstalk Between Lysine Acetylation and Succinylation in Streptomyces coelicolor Metabolic Pathways. Mol. Cell. Proteom. 2021, 20, 100148. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Mujahid, H.; Zhang, Y.; Peng, X.; Redoña, E.D.; Wang, C.; Peng, Z. Comprehensive Analysis of the Lysine Succinylome and Protein Co-modifications in Developing Rice Seeds. Mol. Cell. Proteom. 2019, 18, 2359–2372. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ganguly, D. Structural impairment of p53 C-terminal due to the effect of phosphorylation and acetylation: A study on the interdependence of PTM. J. Biomol. Struct. Dyn. 2024, 42, 13854–13863. [Google Scholar] [CrossRef]
- Kim, M.S.; Mun, Y.S.; Lee, S.E.; Cho, W.Y.; Han, S.H.; Kim, D.H.; Yoon, S.Y. Tau acetylation at K280 regulates tau phosphorylation. Int. J. Neurosci. 2023, 133, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.H.; Yu, K.; Lucas, J.; White, E.; Abraham, R.T. Ammonia derived from glutaminolysis is a diffusible regulator of autophagy. Sci. Signal. 2010, 3, ra31. [Google Scholar] [CrossRef]
- Mariño, G.; Kroemer, G. Ammonia: A diffusible factor released by proliferating cells that induces autophagy. Sci. Signal. 2010, 3, pe19. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Geng, H.; Liu, Q.; Xue, C.; David, L.L.; Beer, T.M.; Thomas, G.V.; Dai, M.S.; Qian, D.Z. HIF1α protein stability is increased by acetylation at lysine 709. J. Biol. Chem. 2012, 287, 35496–35505. [Google Scholar] [CrossRef]
- Huang, B.; Ding, J.; Guo, H.; Wang, H.; Xu, J.; Zheng, Q.; Zhou, L. SIRT3 Regulates the ROS-FPR1/HIF-1α Axis under Hypoxic Conditions to Influence Lung Cancer Progression. Cell Biochem. Biophys. 2023, 81, 813–821. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Li, Y.; Wei, S.; He, G.; Liu, J.; Li, X.; Yang, S.; Li, D.; Lin, W.; et al. Glutamine addiction in tumor cell: Oncogene regulation and clinical treatment. Cell Commun. Signal. CCS 2024, 22, 12. [Google Scholar] [CrossRef]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and cancer metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef]
- Amaya, M.L.; Inguva, A.; Pei, S.; Jones, C.; Krug, A.; Ye, H.; Minhajuddin, M.; Winters, A.; Furtek, S.L.; Gamboni, F.; et al. The STAT3-MYC axis promotes survival of leukemia stem cells by regulating SLC1A5 and oxidative phosphorylation. Blood 2022, 139, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Nam, H.S.; Cho, M.K.; Lee, S.H. Arctigenin induces necroptosis through mitochondrial dysfunction with CCN1 upregulation in prostate cancer cells under lactic acidosis. Mol. Cell. Biochem. 2020, 467, 45–56. [Google Scholar] [CrossRef]
- Regmi, S.; Raut, P.K.; Pathak, S.; Shrestha, P.; Park, P.H.; Jeong, J.H. Enhanced viability and function of mesenchymal stromal cell spheroids is mediated via autophagy induction. Autophagy 2021, 17, 2991–3010. [Google Scholar] [CrossRef]
- Hu, T.; Shukla, S.K.; Vernucci, E.; He, C.; Wang, D.; King, R.J.; Jha, K.; Siddhanta, K.; Mullen, N.J.; Attri, K.S.; et al. Metabolic Rewiring by Loss of Sirt5 Promotes Kras-Induced Pancreatic Cancer Progression. Gastroenterology 2021, 161, 1584–1600. [Google Scholar] [CrossRef]
- Kawaf, R.R.; Ramadan, W.S.; El-Awady, R. Deciphering the interplay of histone post-translational modifications in cancer: Co-targeting histone modulators for precision therapy. Life Sci. 2024, 346, 122639. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Zhang, X.; Wang, Z.; Cui, Z.; Li, C.; Han, Y.; Deng, W.; Zhou, X.; Wu, H.; Sun, J.; et al. Succinylation—Encoded metabolic codes: Cracking the molecular logic of cellular adaptation. Int. J. Surg. 2025, 111, 9560–9582. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Y.; Cui, Z.; Mao, J.; Xu, J.; Yao, R. The Selective SIRT3 Inhibitor 3-TYP Represses Primary Myeloma Growth by Reducing c-Myc Stability. Chem. Res. Toxicol. 2024, 37, 1062–1069. [Google Scholar] [CrossRef]
- Belli, M.; Cristina, M.; Calabrese, V.; Russo, M.; Granato, M.; Russo, M.A.; Sansone, L. Ultrastructural Changes of Neuroendocrine Pheochromocytoma Cell Line PC-12 Exposed In Vitro to Rotenone. Brain Sci. 2024, 14, 476. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Barreca, F.; Aventaggiato, M.; Cristina, M.; Sansone, L.; Belli, M.; Lista, M.B.; Francisci, G.; Valente, S.; Rotili, D.; Mai, A.; et al. A Combined SIRT5 Activation and SIRT3 Inhibition Prevents Breast Cancer Spheroids Growth by Reducing HIF-1α and Mitophagy. Pharmaceuticals 2026, 19, 23. https://doi.org/10.3390/ph19010023
Barreca F, Aventaggiato M, Cristina M, Sansone L, Belli M, Lista MB, Francisci G, Valente S, Rotili D, Mai A, et al. A Combined SIRT5 Activation and SIRT3 Inhibition Prevents Breast Cancer Spheroids Growth by Reducing HIF-1α and Mitophagy. Pharmaceuticals. 2026; 19(1):23. https://doi.org/10.3390/ph19010023
Chicago/Turabian StyleBarreca, Federica, Michele Aventaggiato, Mario Cristina, Luigi Sansone, Manuel Belli, Maria Beatrice Lista, Gaia Francisci, Sergio Valente, Dante Rotili, Antonello Mai, and et al. 2026. "A Combined SIRT5 Activation and SIRT3 Inhibition Prevents Breast Cancer Spheroids Growth by Reducing HIF-1α and Mitophagy" Pharmaceuticals 19, no. 1: 23. https://doi.org/10.3390/ph19010023
APA StyleBarreca, F., Aventaggiato, M., Cristina, M., Sansone, L., Belli, M., Lista, M. B., Francisci, G., Valente, S., Rotili, D., Mai, A., Russo, M. A., & Tafani, M. (2026). A Combined SIRT5 Activation and SIRT3 Inhibition Prevents Breast Cancer Spheroids Growth by Reducing HIF-1α and Mitophagy. Pharmaceuticals, 19(1), 23. https://doi.org/10.3390/ph19010023










