Dual-Targeting CSC Therapy: Acid-Responsive Cisplatin/CaCO3@siRNA Nanoplatform Overcomes HCC Chemoresistance
Abstract
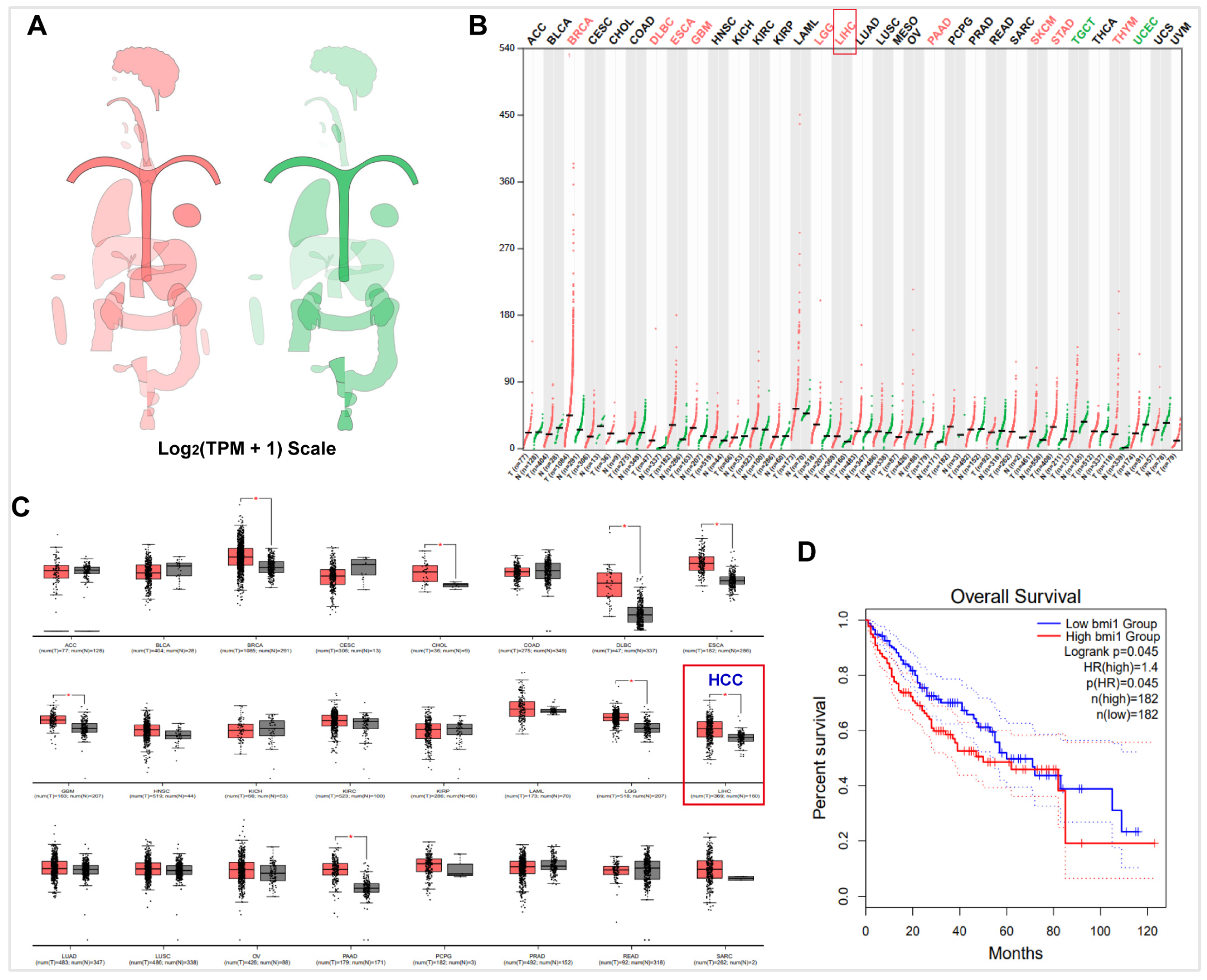
1. Introduction
2. Results and Discussion
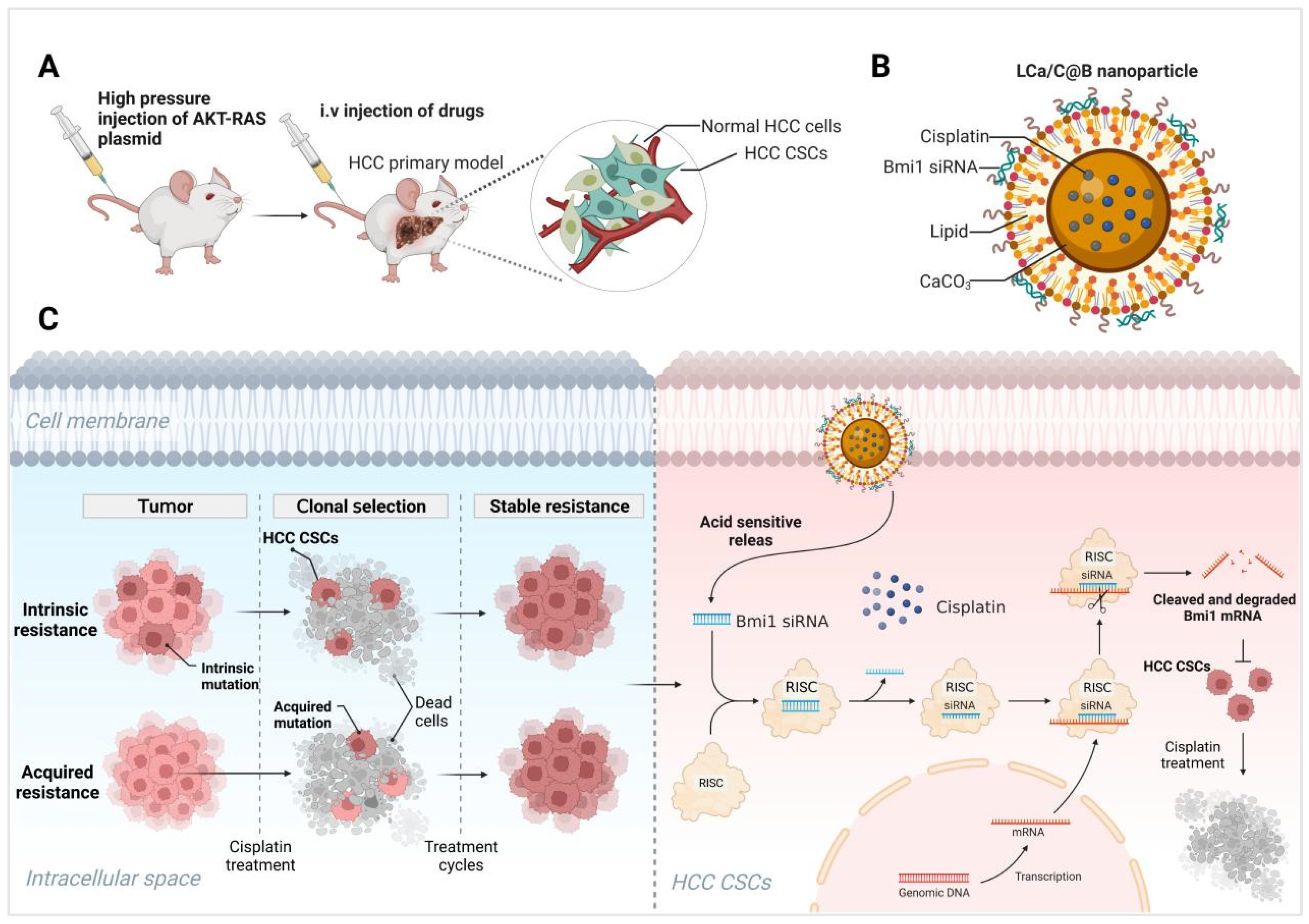
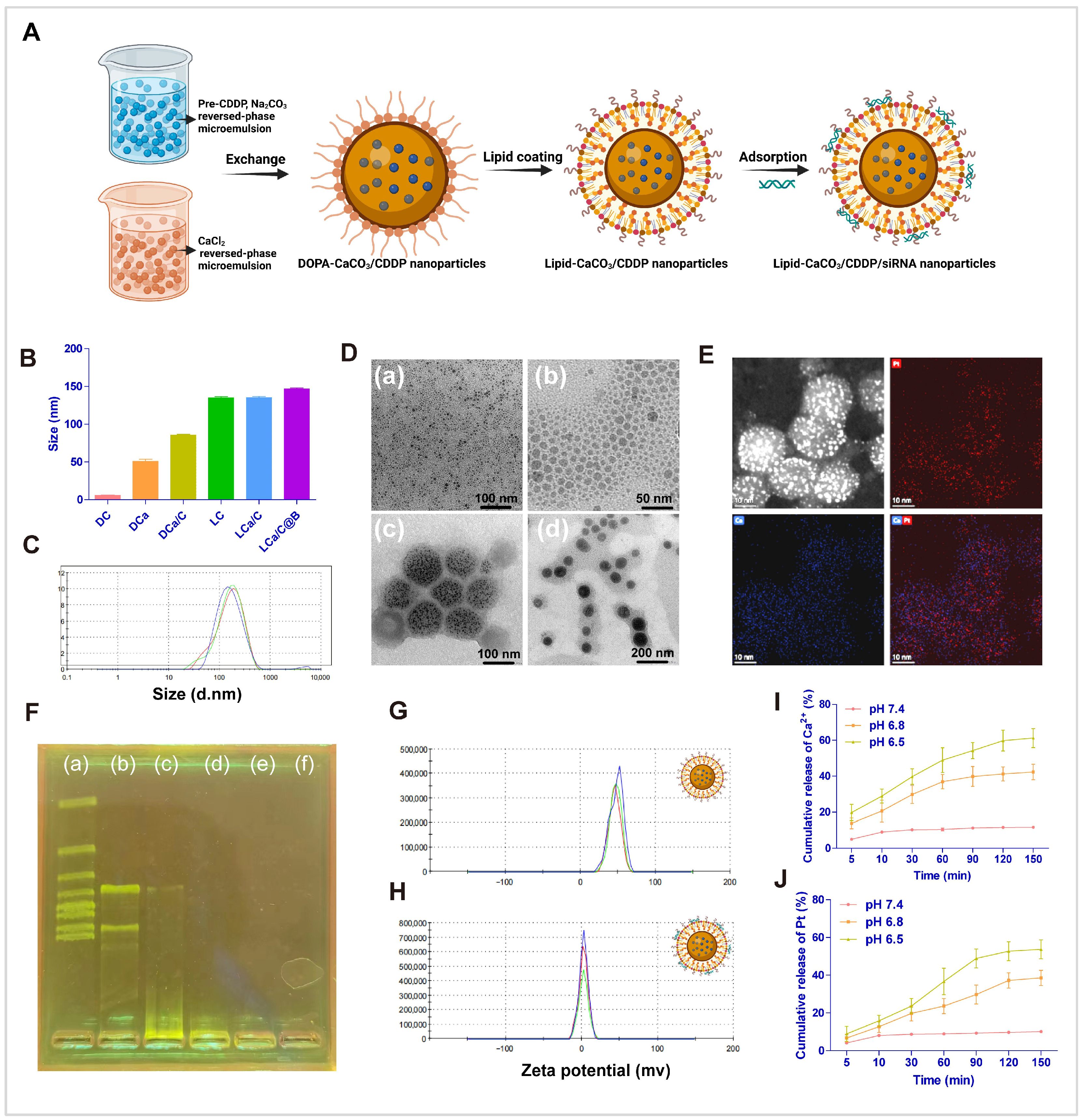
2.1. Subsection Preparation and Characterization of LCa/C@B
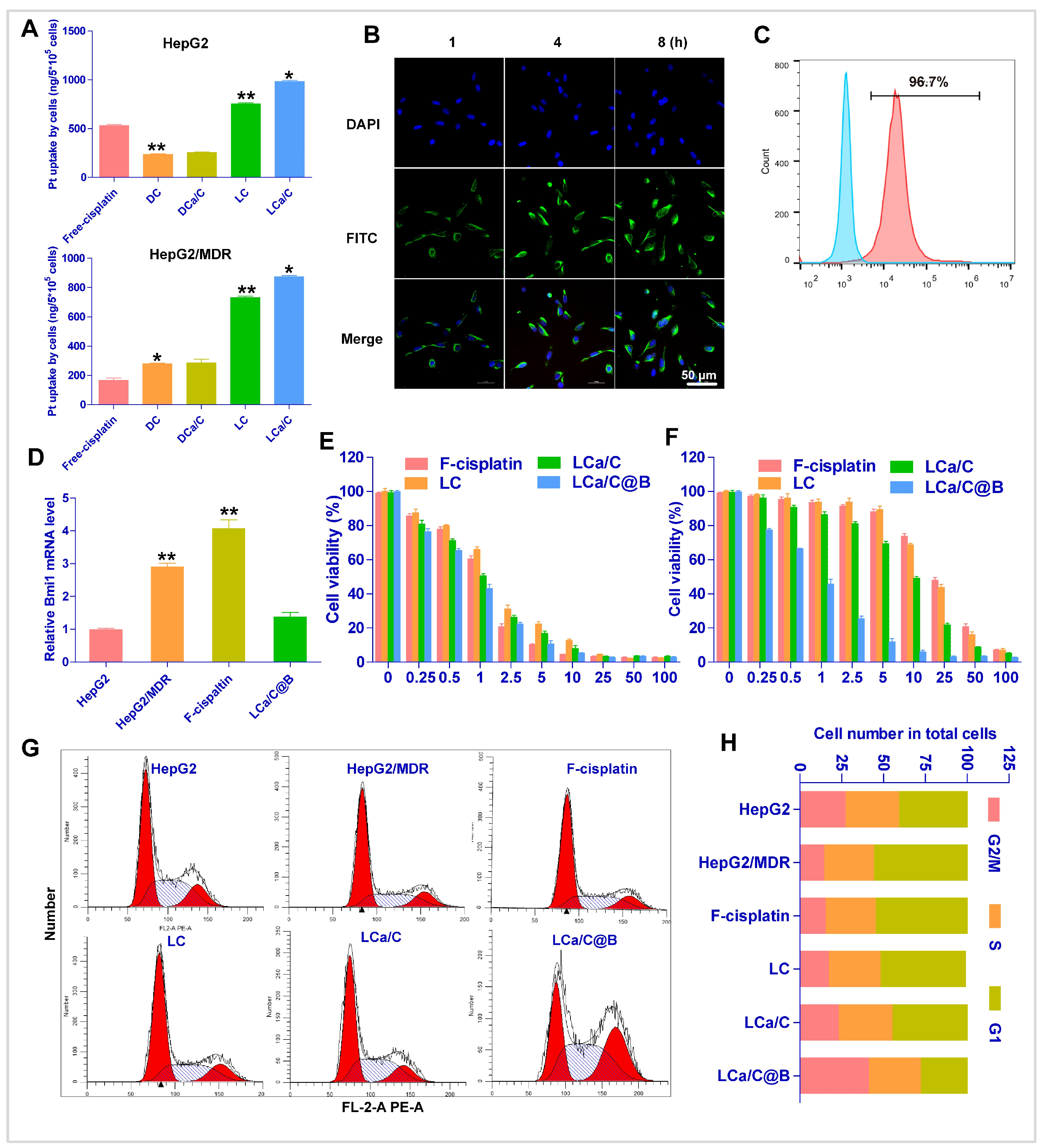
2.2. The Uptake and Anti-Tumor Effect of LCa/C@B in Resistant HCC Cells
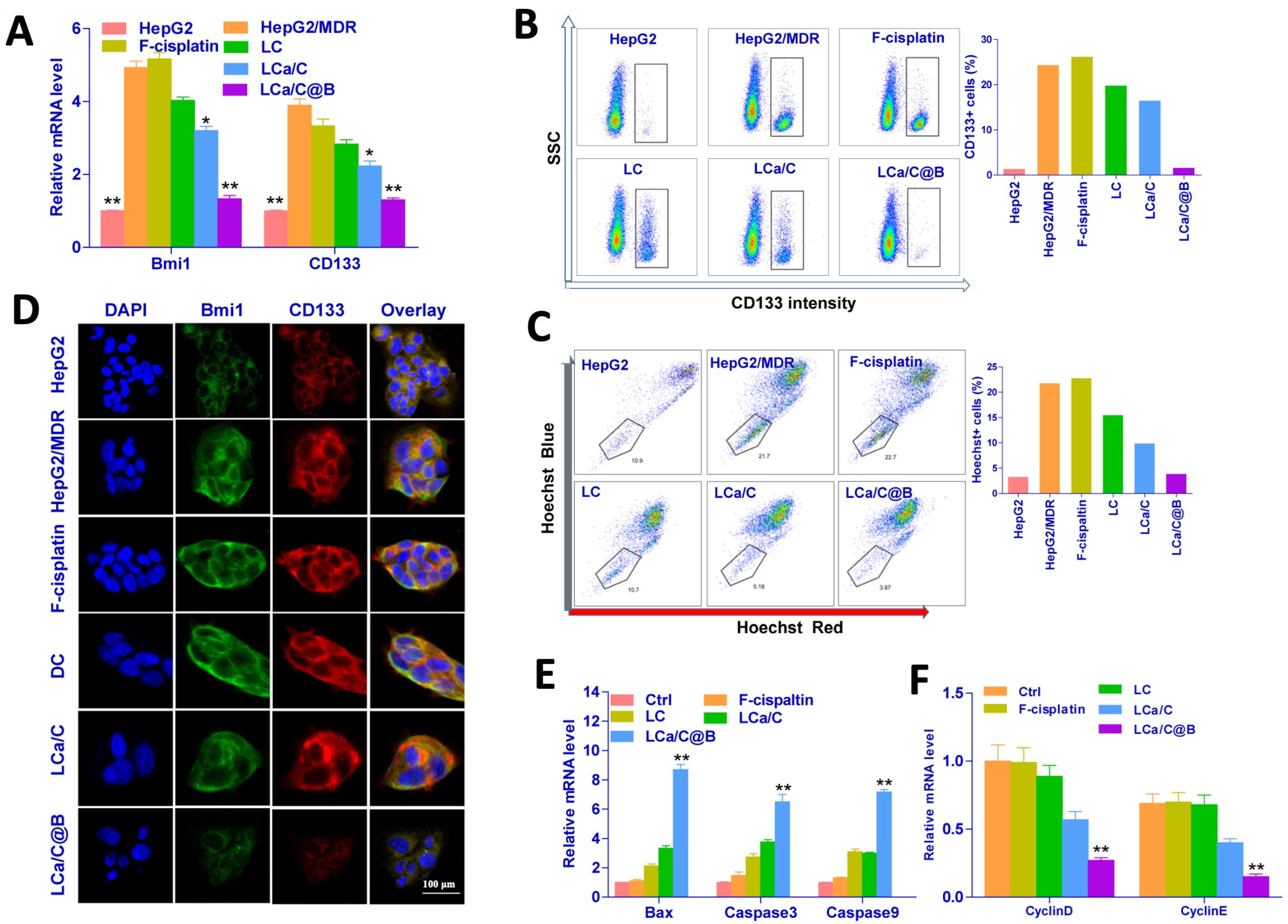
2.3. LCa/C@B Reversed Cisplatin-Resistant HCC Cells by Inhibiting CSC Expression
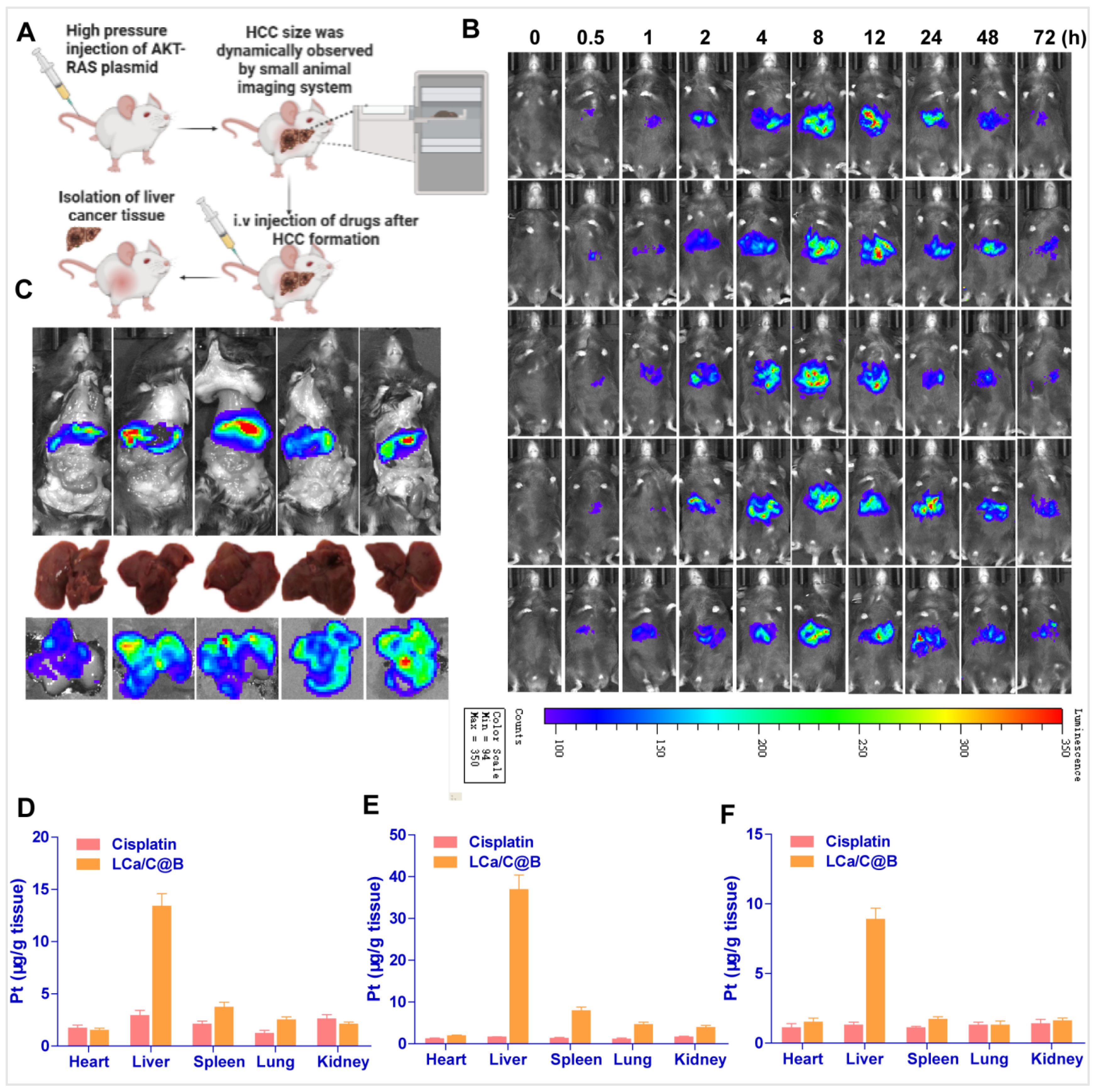
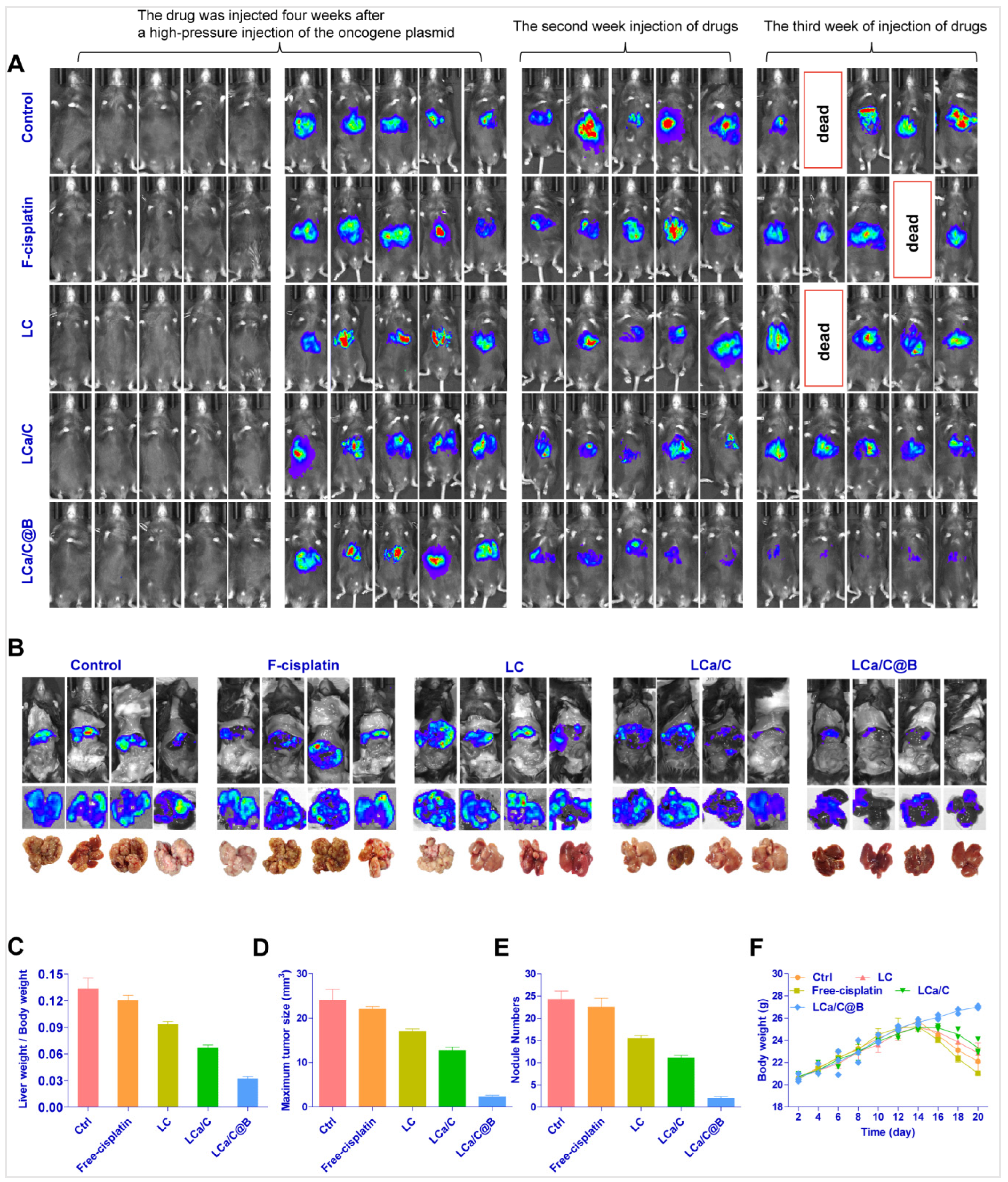
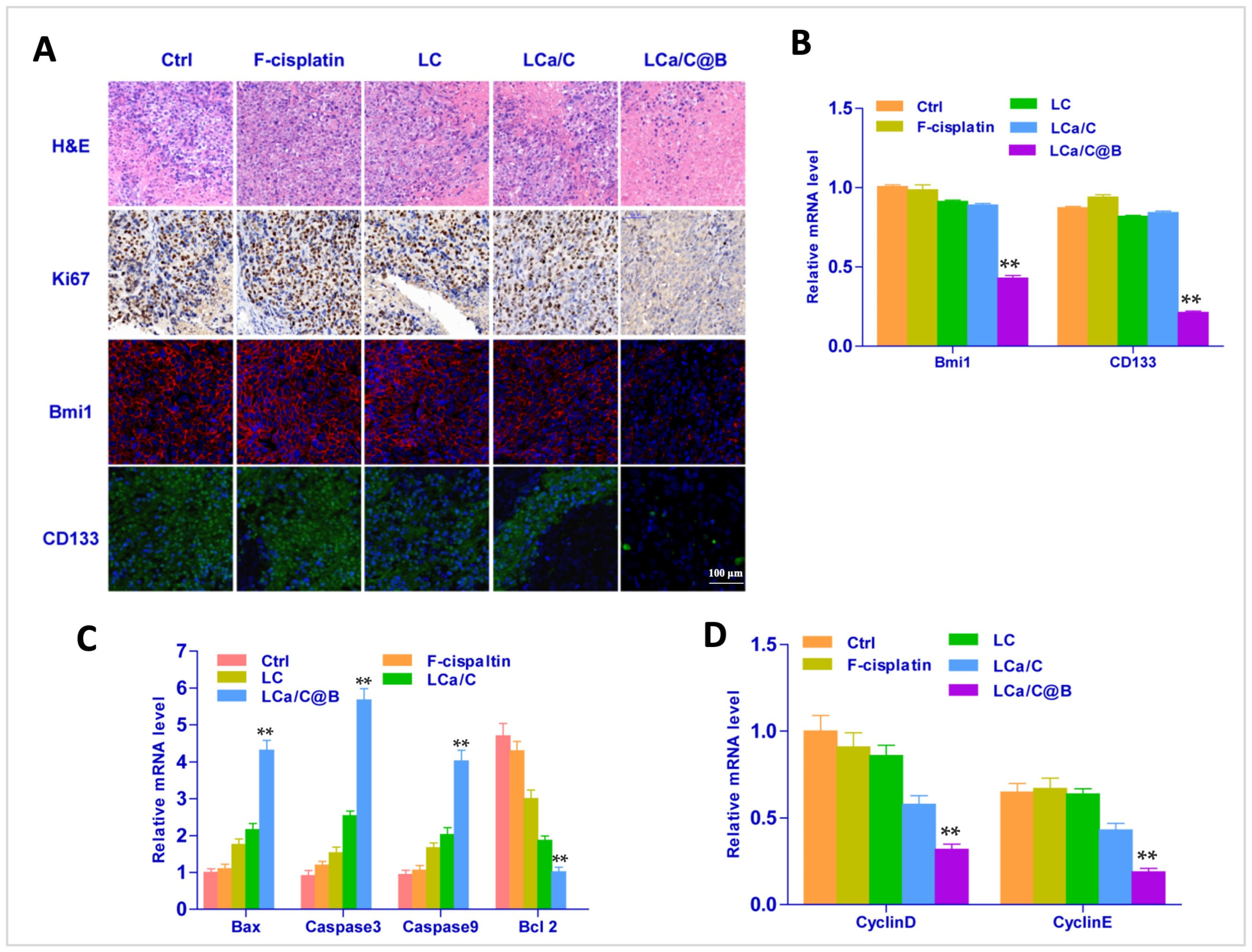
2.4. LCa/C@B Suppress Tumor Growth in Genedrives’ Primary HCC Model in Mice
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Cell Culture and Cisplatin-Resistant Cells
3.3. Synthesis the Cisplatin Precursor of Cis-[Pt(NH3)2(H2O)2](NO3)2
3.4. Preparation of Lipo-Coated CaCO3/Cisplatin Hybrid Watermelon-Shaped Nanoparticles (LCa/C@B)
3.5. Drug Loading Determination
3.6. Nanoparticle Characterization
3.7. In Vitro Acid-Responsiveness Release of Cisplatin Under Simulated Conditions
3.8. Intracellular Uptake of Nanoparticles
3.9. Cytotoxicity Determination
3.10. Cell Cycle Analyses
3.11. Flow Cytometry
3.12. Western Blotting Analysis
3.13. Mice
3.14. Primary HCC Mouse Model
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.D.; Roberts, L.R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, Y.; Han, J.; Liu, R.; Liu, C.; Zhao, Y.; Liu, Y. Ultrasound nanobubble-based combinational strategies of loaded miR-107-3p and CD133 Ab for anti-PD-L1 and anti-hepatocellular cancer stem cells. Int. J. Pharm. 2025, 670, 125140. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, L.; Guan, X.Y. The genetic and epigenetic alterations in human hepatocellular carcinoma: A recent update. Protein Cell 2014, 5, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Das, S.; Flanagan, R.; Oehler, M.; Menon, J.U. Targeting chronic liver diseases: Molecular markers, drug delivery strategies and future perspectives. Int. J. Pharm. 2024, 660, 124381. [Google Scholar] [CrossRef]
- Lin, X.; Ojo, D.; Wei, F.; Wong, N.; Gu, Y.; Tang, D. A Novel Aspect of Tumorigenesis-BMI1 Functions in Regulating DNA Damage Response. Biomolecules 2015, 5, 3396–3415. [Google Scholar] [CrossRef]
- Mekala, J.R.; Vaishnave, S. BMI1 and PTEN are key determinants of breast cancer therapy: A plausible therapeutic target in breast cancer. Gene 2018, 678, 302–311. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, W.; Wang, C.Y. BMI1 Inhibition Eliminates Residual Cancer Stem Cells after PD1 Blockade and Activates Antitumor Immunity to Prevent Metastasis and Relapse. Cell Stem Cell 2020, 27, 238–253.e6. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Z.; Tan, Y.; Lian, G.; Chen, S.; Chen, S.; Li, J.; Li, X.; Huang, K.; Chen, Y. Bmi-1-induced miR-27a and miR-155 promote tumor metastasis and chemoresistance by targeting RKIP in gastric cancer. Mol. Cancer 2020, 19, 109. [Google Scholar] [CrossRef]
- Zhang, L.; Qiang, J.; Yang, X.; Wang, D.; Rehman, A.U.; He, X.; Chen, W.; Sheng, D.; Zhou, L.; Jiang, Y.Z.; et al. IL1R2 Blockade Suppresses Breast Tumorigenesis and Progression by Impairing USP15-Dependent BMI1 Stability. Adv. Sci. 2020, 7, 1901728. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, L.; Zhu, K.Y.; Dong, M.; Xu, P.F.; Chen, Y.; Chen, S.J.; Chen, Z.; Deng, M.; Liu, T.X. Dominant-negative C/ebpalpha and polycomb group protein Bmi1 extend short-lived hematopoietic stem/progenitor cell life span and induce lethal dyserythropoiesis. Blood 2011, 118, 3842–3852. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Pardal, R.; Iwashita, T.; Park, I.K.; Clarke, M.F.; Morrison, S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003, 425, 962–967. [Google Scholar] [CrossRef]
- Tian, H.; Biehs, B.; Warming, S.; Leong, K.G.; Rangell, L.; Klein, O.D.; de Sauvage, F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011, 478, 255–259. [Google Scholar] [CrossRef]
- Dirks, P. Bmi1 and cell of origin determinants of brain tumor phenotype. Cancer Cell 2007, 12, 295–297. [Google Scholar] [CrossRef]
- Sherr, C.J. The INK4a/ARF network in tumour suppression. Nat. Rev. Mol. Cell Biol. 2001, 2, 731–737. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, D.; Yan, L.; Jiang, W.; Kim, J.S.; Gu, B.; Liu, Q.; Wang, R.; Xia, B.; Zhao, J.C.; et al. BMI1 regulates androgen receptor in prostate cancer independently of the polycomb repressive complex 1. Nat. Commun. 2018, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Mustafi, S.B.; Street, M.; Dey, A.; Dwivedi, S.K. Bmi-1: At the crossroads of physiological and pathological biology. Genes. Dis. 2015, 2, 225–239. [Google Scholar] [CrossRef]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rahimkhoei, V.; Akbari, A.; Jassim, A.Y.; Hussein, U.A.; Salavati-Niasari, M. Recent advances in targeting cancer stem cells by using nanomaterials. Int. J. Pharm. 2025, 673, 125381. [Google Scholar] [CrossRef]
- Zhou, J.N.; Zhang, B.; Wang, H.Y.; Wang, D.X.; Zhang, M.M.; Zhang, M.; Wang, X.K.; Fan, S.Y.; Xu, Y.C.; Zeng, Q.; et al. A Functional Screening Identifies a New Organic Selenium Compound Targeting Cancer Stem Cells: Role of c-Myc Transcription Activity Inhibition in Liver Cancer. Adv. Sci. 2022, 9, e2201166. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Jaffar Ali, D.; Qi, Y.; Li, Y.; Sun, B.; Liu, R.; Sun, B.; Xiao, Z. Engineered extracellular vesicles mediated CRISPR-induced deficiency of IQGAP1/FOXM1 reverses sorafenib resistance in HCC by suppressing cancer stem cells. J. Nanobiotechnol. 2023, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Sagar, J.; Chaib, B.; Sales, K.; Winslet, M.; Seifalian, A. Role of stem cells in cancer therapy and cancer stem cells: A review. Cancer Cell Int. 2007, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat. Commun. 2018, 9, 2997. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Begicevic, R.R.; Falasca, M. ABC Transporters in Cancer Stem Cells: Beyond Chemoresistance. Int. J. Mol. Sci. 2017, 18, 2362. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X.; Li, J.; Wang, H.; Xu, X.; Wu, Y.; Wang, J.; Wang, S.; Li, Y.; Zhang, Z. M2 macrophage microvesicle-inspired nanovehicles improve accessibility to cancer cells and cancer stem cells in tumors. J. Nanobiotechnol. 2021, 19, 397. [Google Scholar] [CrossRef]
- Lee, T.K.; Guan, X.Y.; Ma, S. Cancer stem cells in hepatocellular carcinoma—From origin to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 26–44. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef]
- Han, J.; Won, M.; Kim, J.H.; Jung, E.; Min, K.; Jangili, P.; Kim, J.S. Cancer stem cell-targeted bio-imaging and chemotherapeutic perspective. Chem. Soc. Rev. 2020, 49, 7856–7878. [Google Scholar] [CrossRef]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Romagosa, C.; Simonetti, S.; Lopez-Vicente, L.; Mazo, A.; Lleonart, M.E.; Castellvi, J.; Ramon y Cajal, S. p16(Ink4a) overexpression in cancer: A tumor suppressor gene associated with senescence and high-grade tumors. Oncogene 2011, 30, 2087–2097. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Liu, X.; Agarwal, P.; Zhao, S.; Conroy, D.W.; Ji, G.; Yu, J.; Jaroniec, C.P.; Liu, Z.; et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 562. [Google Scholar] [CrossRef]
- Ding, S.; Li, C.; Cheng, N.; Cui, X.; Xu, X.; Zhou, G. Redox Regulation in Cancer Stem Cells. Oxid. Med. Cell Longev. 2015, 2015, 750798. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Du, J.; Gu, Z.; Zhao, Y. Reactive Oxygen Species-Regulating Strategies Based on Nanomaterials for Disease Treatment. Adv. Sci. 2021, 8, 2002797. [Google Scholar] [CrossRef] [PubMed]
- Courtnay, R.; Ngo, D.C.; Malik, N.; Ververis, K.; Tortorella, S.M.; Karagiannis, T.C. Cancer metabolism and the Warburg effect: The role of HIF-1 and PI3K. Mol. Biol. Rep. 2015, 42, 841–851. [Google Scholar] [CrossRef]
- Kang, H.; Kim, B.; Park, J.; Youn, H.; Youn, B. The Warburg effect on radioresistance: Survival beyond growth. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188988. [Google Scholar] [CrossRef]
- Shamsi, M.; Saghafian, M.; Dejam, M.; Sanati-Nezhad, A. Mathematical Modeling of the Function of Warburg Effect in Tumor Microenvironment. Sci. Rep. 2018, 8, 8903. [Google Scholar] [CrossRef]
- Gu, J.; Zhao, G.; Yu, J.; Xu, P.; Yan, J.; Jin, Z.; Chen, S.; Wang, Y.; Zhang, L.W.; Wang, Y. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J. Nanobiotechnol. 2022, 20, 372. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Q.; Xiao, M.; Huang, X.; Wu, X. A versatile photothermal vaccine based on acid-responsive glyco-nanoplatform for synergistic therapy of cancer. Biomaterials 2021, 273, 120792. [Google Scholar] [CrossRef]
- Liu, Y.; Si, L.; Jiang, Y.; Jiang, S.; Zhang, X.; Li, S.; Chen, J.; Hu, J. Design of pH-Responsive Nanomaterials Based on the Tumor Microenvironment. Int. J. Nanomed. 2025, 20, 705–721. [Google Scholar] [CrossRef]
- Larroque-Lombard, A.L.; Chatelut, E.; Delord, J.P.; Imbs, D.C.; Rochaix, P.; Jean-Claude, B.; Allal, B. Design and Mechanism of Action of a New Prototype of Combi-Molecule “Programed” to Release Bioactive Species at a pH Range Akin to That of the Tumor Microenvironment. Pharmaceuticals 2021, 14, 160. [Google Scholar] [CrossRef]
- Ma, Y.; Lai, P.; Sha, Z.; Li, B.; Wu, J.; Zhou, X.; He, C.; Ma, X. TME-responsive nanocomposite hydrogel with targeted capacity for enhanced synergistic chemoimmunotherapy of MYC-amplified osteosarcoma. Bioact. Mater. 2025, 47, 83–99. [Google Scholar] [CrossRef]
- Li, J.; Dai, Y.; Wang, T.; Zhang, X.; Du, P.; Dong, Y.; Jiao, Z. Polyphenol-based pH-responsive nanoparticles enhance chemo-immunotherapy in pancreatic cancer. J. Control. Release 2025, 380, 615–629. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, P.; He, J.; Dong, S.; Li, P.; Zhang, C.Y.; Ma, T. TME-Responsive Polyprodrug Micelles for Multistage Delivery of Doxorubicin with Improved Cancer Therapeutic Efficacy in Rodents. Adv. Healthc. Mater. 2020, 9, e2000387. [Google Scholar] [CrossRef]
- Su, Q.; Wang, Z.; Li, P.; Wei, X.; Xiao, J.; Duan, X. pH and ROS Dual-Responsive Autocatalytic Release System Potentiates Immunotherapy of Colorectal Cancer. Adv. Healthc. Mater. 2024, 13, e2401126. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Wei, Z.J.; Long, K.; Sun, M.; Zhang, Z.; Wang, Y.; Wang, W.; Yuan, Z. pH-Responsive Persistent Luminescent Nanosystem with Sensitized NIR Imaging and Ratiometric Imaging Modes for Tumor Surgery Navigation. ACS Appl. Mater. Interfaces 2024, 16, 69071–69085. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, K.; Zhu, Y.Q.; Liu, L.; Kong, X.T.; Dai, Y.; Fu, X.J.; Li, Y.L.; Liu, M.H.; Zhang, D. Preparation of cancer cell membrane-coated Gambogic acid-loaded pH-sensitive liposomes to enhance targeted anti-hepatocellular carcinoma effect. Drug Deliv. Transl. Res. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, J.; Li, Z.; Feng, J.; Duan, X.; Yan, C.; Wen, G.; Qiu, X.; Shen, Z. Hollow mesoporous calcium peroxide nanoparticles for drug-free tumor calcicoptosis therapy. Acta Biomater. 2024, 185, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, H.; Wu, Z.; Chen, Q.; Zheng, W.; Shen, Q.; Wei, Q.; Shen, J.W.; Guo, Y. Tumor therapy utilizing dual-responsive nanoparticles: A multifaceted approach integrating calcium-overload and PTT/CDT/chemotherapy. J. Control. Release 2024, 376, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, H.; Liu, Y.; Li, B.; Wang, F.; Ye, P.; Xu, Y.; Lai, Y.; Yang, T. “Trinity” Comprehensively Regulates the Tumor Microenvironment of Lipid-Coated CaCO(3)@CuO(2) Nanoparticles Induces “Cuproptosis” in HCC. ACS Appl. Mater. Interfaces 2024, 16, 58203–58216. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Xing, R.; Zhao, M.; Li, Y.; Lu, H.; Liu, Z. Controllable Engineering and Functionalizing of Nanoparticles for Targeting Specific Proteins towards Biomedical Applications. Adv. Sci. 2021, 8, e2101713. [Google Scholar] [CrossRef]
- Guo, S.; Wang, Y.; Miao, L.; Xu, Z.; Lin, C.M.; Zhang, Y.; Huang, L. Lipid-coated Cisplatin nanoparticles induce neighboring effect and exhibit enhanced anticancer efficacy. ACS Nano 2013, 7, 9896–9904. [Google Scholar] [CrossRef]
- Chiba, T.; Seki, A.; Aoki, R.; Ichikawa, H.; Negishi, M.; Miyagi, S.; Oguro, H.; Saraya, A.; Kamiya, A.; Nakauchi, H.; et al. Bmi1 promotes hepatic stem cell expansion and tumorigenicity in both Ink4a/Arf-dependent and -independent manners in mice. Hepatology 2010, 52, 1111–1123. [Google Scholar] [CrossRef]
- Biehs, B.; Hu, J.K.; Strauli, N.B.; Sangiorgi, E.; Jung, H.; Heber, R.P.; Ho, S.; Goodwin, A.F.; Dasen, J.S.; Capecchi, M.R.; et al. BMI1 represses Ink4a/Arf and Hox genes to regulate stem cells in the rodent incisor. Nat. Cell Biol. 2013, 15, 846–852. [Google Scholar] [CrossRef]
- Liu, J.; Cao, L.; Chen, J.; Song, S.; Lee, I.H.; Quijano, C.; Liu, H.; Keyvanfar, K.; Chen, H.; Cao, L.Y.; et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 2009, 459, 387–392. [Google Scholar] [CrossRef]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef]
- Xia, H.; Li, F.; Hu, X.; Park, W.; Wang, S.; Jang, Y.; Du, Y.; Baik, S.; Cho, S.; Kang, T.; et al. pH-Sensitive Pt Nanocluster Assembly Overcomes Cisplatin Resistance and Heterogeneous Stemness of Hepatocellular Carcinoma. ACS Cent. Sci. 2016, 2, 802–811. [Google Scholar] [CrossRef]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yu, K.H.; Wong, T.L.; Zhang, Z.; Chan, C.H.; Loong, J.H.; Che, N.; Yu, H.J.; Tan, K.V.; Tong, M.; et al. Lineage tracing and single-cell analysis reveal proliferative Prom1+ tumour-propagating cells and their dynamic cellular transition during liver cancer progression. Gut 2022, 71, 1656–1668. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Solenne, T.; Wang, H.; Li, B.; Liu, Y.; Wang, F.; Yang, T. Core-shell cisplatin/SiO2 nanocapsules combined with PTC-209 overcome chemotherapy-Acquired and intrinsic resistance in hepatocellular carcinoma. Acta Biomater. 2023, 170, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.C.; Xue, H.; Yun, W.J. An overview of mouse models of hepatocellular carcinoma. Infect. Agent. Cancer 2023, 18, 49. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, F.; Lin, M.; Liu, Y.; Wang, H.; Li, B.; Yang, T.; Li, W. Dual-Targeting CSC Therapy: Acid-Responsive Cisplatin/CaCO3@siRNA Nanoplatform Overcomes HCC Chemoresistance. Pharmaceuticals 2026, 19, 22. https://doi.org/10.3390/ph19010022
Wang F, Lin M, Liu Y, Wang H, Li B, Yang T, Li W. Dual-Targeting CSC Therapy: Acid-Responsive Cisplatin/CaCO3@siRNA Nanoplatform Overcomes HCC Chemoresistance. Pharmaceuticals. 2026; 19(1):22. https://doi.org/10.3390/ph19010022
Chicago/Turabian StyleWang, Fei, Ming Lin, Yong Liu, Han Wang, Bin Li, Tan Yang, and Weijie Li. 2026. "Dual-Targeting CSC Therapy: Acid-Responsive Cisplatin/CaCO3@siRNA Nanoplatform Overcomes HCC Chemoresistance" Pharmaceuticals 19, no. 1: 22. https://doi.org/10.3390/ph19010022
APA StyleWang, F., Lin, M., Liu, Y., Wang, H., Li, B., Yang, T., & Li, W. (2026). Dual-Targeting CSC Therapy: Acid-Responsive Cisplatin/CaCO3@siRNA Nanoplatform Overcomes HCC Chemoresistance. Pharmaceuticals, 19(1), 22. https://doi.org/10.3390/ph19010022





