Obesity-Related Cancers in Relation to Use of Statins and Testosterone Replacement Therapy Among Older Women: SEER-Medicare 2007–2015
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Data
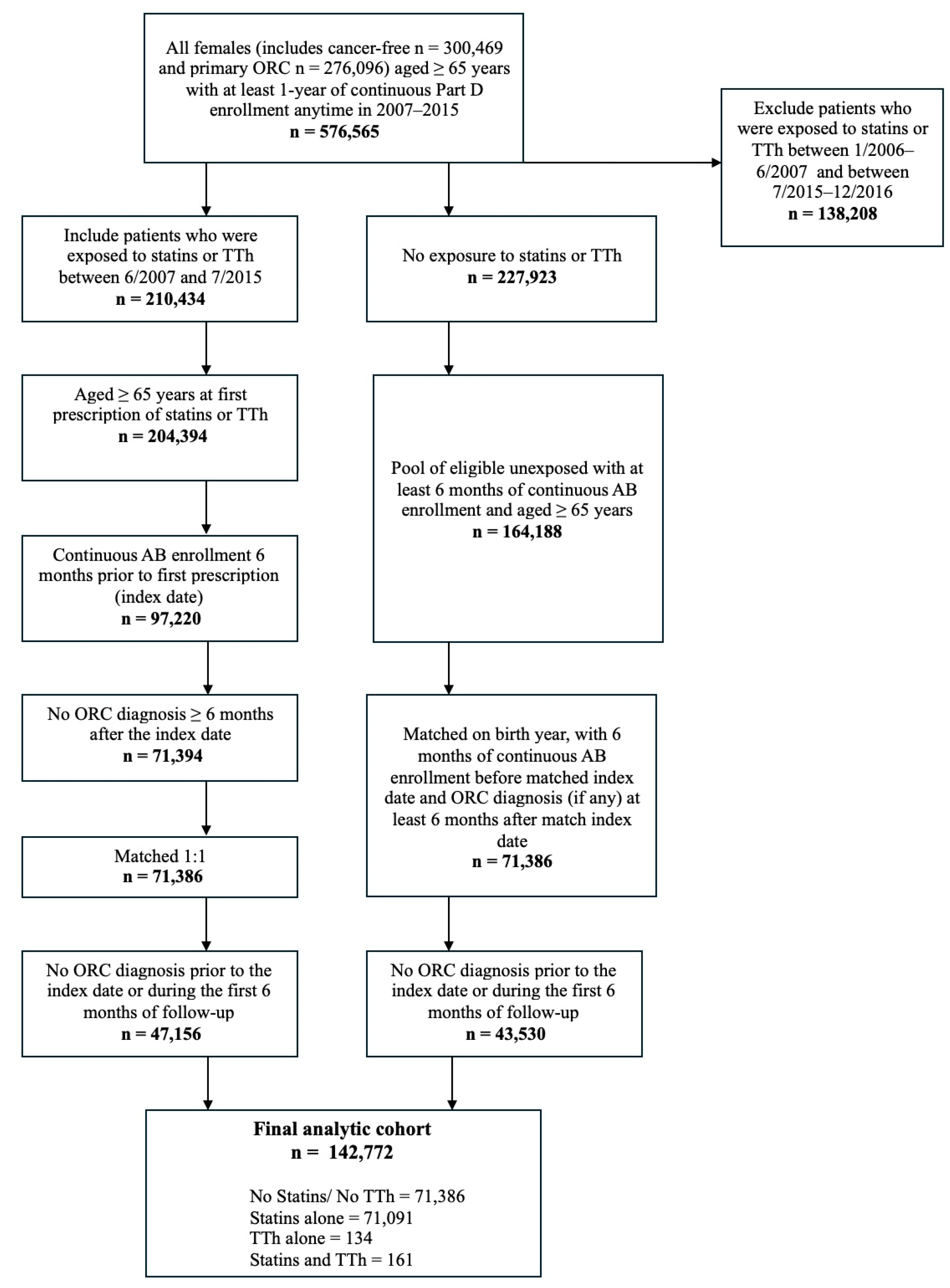
4.2. Study Cohort
4.3. Primary Exposure: Use of Statins and TTh
4.4. Primary Outcome: Incident ORC
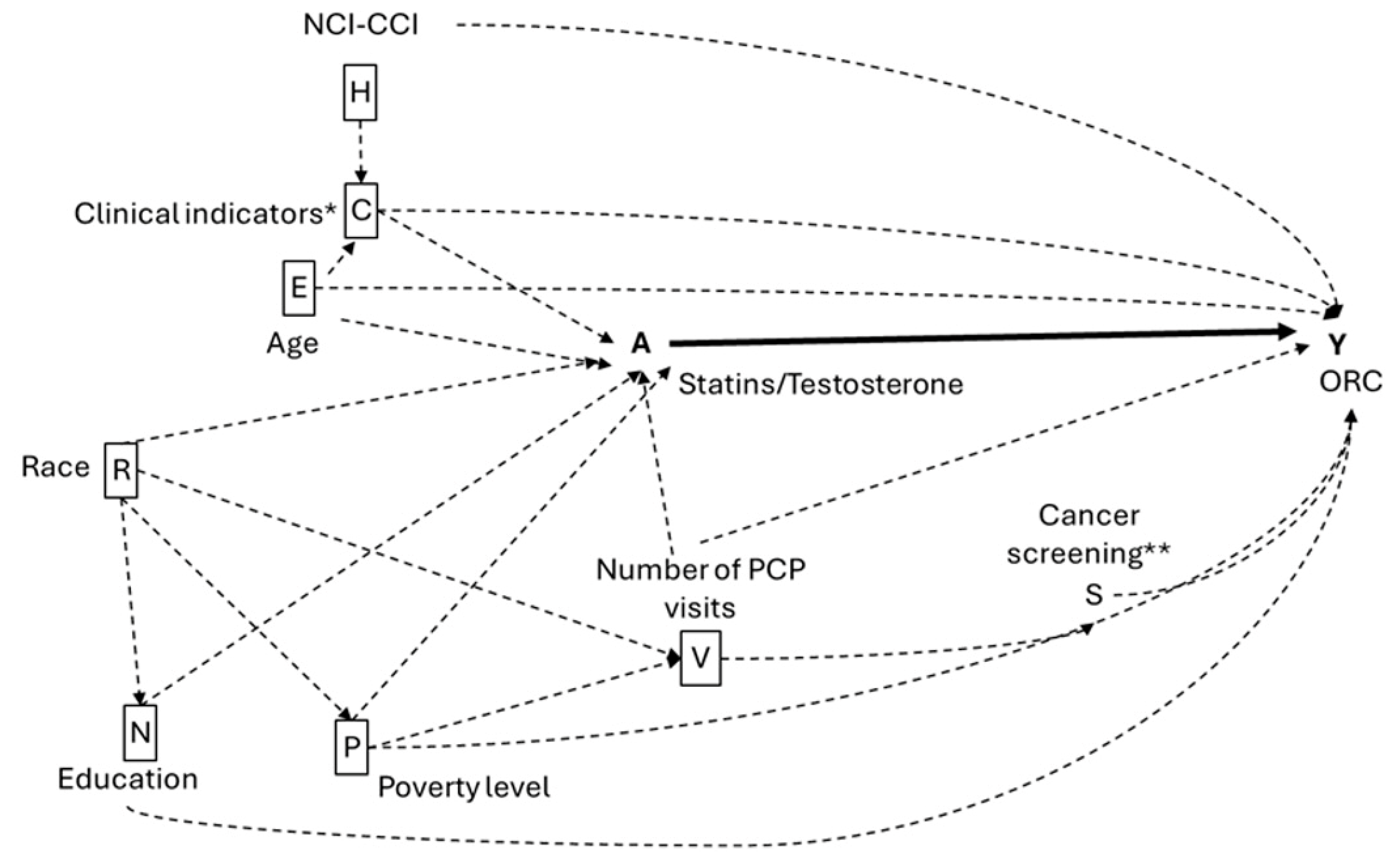
4.5. Study Covariates
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJCC | American joint committee on cancer |
| BrCa | Breast cancer |
| CCI | Charlson comorbidity index |
| CI | Confidence interval |
| CRC | Colorectal cancer |
| CVD | Cardiovascular disease |
| DHEA | Dehydroepiandrosterone |
| EPIC | European prospective investigation into cancer and nutrition |
| FDA | Food and drug Aaministration |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme |
| HR | Hazard ratio |
| hs-CRP | High-sensitivity C-reactive protein |
| IRB | Institutional review board |
| LDL | Low-density lipoprotein |
| NDC | National drug code |
| ORC | Obesity-related cancer |
| PCOS | Polycystic ovary syndrome |
| PCP | Primary care physician |
| SD | Standard deviation |
| SHBG | Sex hormone-binding globulin |
| SEER | Surveillance, epidemiology, and end results |
| SES | Socioeconomic status |
| TTh | Testosterone replacement therapy |
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. PharmacoEconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Ye, P.; Xi, Y.; Huang, Z.; Xu, P. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights. Cancers 2020, 12, 1408. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- Graaf, M.R.; Beiderbeck, A.B.; Egberts, A.C.; Richel, D.J.; Guchelaar, H.J. The risk of cancer in users of statins. J. Clin. Oncol. 2004, 22, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Cauley, J.A.; Zmuda, J.M.; Lui, L.Y.; Hillier, T.A.; Ness, R.B.; Stone, K.L.; Cummings, S.R.; Bauer, D.C. Lipid-lowering drug use and breast cancer in older women: A prospective study. J. Women’s Health 2003, 12, 749–756. [Google Scholar] [CrossRef]
- Irvin, S.; Clarke, M.A.; Trabert, B.; Wentzensen, N. Systematic review and meta-analysis of studies assessing the relationship between statins use and risk of ovarian cancer. Cancer Causes Control. CCC 2020, 31, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Poynter, J.N.; Gruber, S.B.; Higgins, P.D.; Almog, R.; Bonner, J.D.; Rennert, H.S.; Low, M.; Greenson, J.K.; Rennert, G. Statins and the risk of colorectal cancer. N. Engl. J. Med. 2005, 352, 2184–2192. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, J.W.; He, X.R.; Jin, W.L.; He, X.Y. Statins: A repurposed drug to fight cancer. J. Exp. Clin. Cancer Res. CR 2021, 40, 241. [Google Scholar] [CrossRef]
- Grabarek, B.O.; Boroń, D.; Morawiec, E.; Michalski, P.; Palazzo-Michalska, V.; Pach, Ł.; Dziuk, B.; Świder, M.; Zmarzły, N. Crosstalk between Statins and Cancer Prevention and Therapy: An Update. Pharmaceuticals 2021, 14, 1220. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, F.; Song, Z.; Chen, P.; Liu, S.; Ouyang, L. Statins use and the risk of ovarian and endometrial cancers: A meta-analysis. BMC Cancer 2019, 19, 730. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Flordellis, C.S.; Sitaras, N.M. Statins and the risk of colorectal cancer: A meta-analysis of 18 studies involving more than 1.5 million patients. J. Clin. Oncol. 2007, 25, 3462–3468. [Google Scholar] [CrossRef]
- Cheng, M.H.; Chiu, H.F.; Ho, S.C.; Tsai, S.S.; Wu, T.N.; Yang, C.Y. Statins use and the risk of colorectal cancer: A population-based case-control study. World J. Gastroenterol. 2011, 17, 5197–5202. [Google Scholar] [CrossRef]
- Donovitz, G.; Cotten, M. Breast Cancer Incidence Reduction in Women Treated with Subcutaneous Testosterone: Testosterone Therapy and Breast Cancer Incidence Study. Eur. J. Breast Health 2021, 17, 150–156. [Google Scholar] [CrossRef]
- Davis, S.R.; Baber, R.; Panay, N.; Bitzer, J.; Perez, S.C.; Islam, R.M.; Kaunitz, A.M.; Kingsberg, S.A.; Lambrinoudaki, I.; Liu, J.; et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. Climacteric 2019, 22, 429–434. [Google Scholar] [CrossRef]
- Donovitz, G.S. A Personal Prospective on Testosterone Therapy in Women-What We Know in 2022. J. Pers. Med. 2022, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Morgentaler, A. Androgen therapy in women with testosterone insufficiency: Looking back and looking ahead. Androg. Clin. Res. Ther. 2022, 3, 2–13. [Google Scholar] [CrossRef]
- Dimitrakakis, C.; Jones, R.A.; Liu, A.; Bondy, C.A. Breast cancer incidence in postmenopausal women using testosterone in addition to usual hormone therapy. Menopause 2004, 11, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, R.; Khurana, V.; Bejjanki, H.; Caldito, G.; Fort, C. Statins to reduce breast cancer risk: A case control study in U.S. female veterans. J. Clin. Oncol. 2005, 23 (Suppl. S16), 514. [Google Scholar] [CrossRef]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch. Intern. Med. 2005, 165, 2264–2271. [Google Scholar] [CrossRef]
- Cauley, J.A.; McTiernan, A.; Rodabough, R.J.; LaCroix, A.; Bauer, D.C.; Margolis, K.L.; Paskett, E.D.; Vitolins, M.Z.; Furberg, C.D.; Chlebowski, R.T. Women’s Health Initiative Research Group Statins use and breast cancer: Prospective results from the Women’s Health Initiative. J. Natl. Cancer Inst. 2006, 98, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, W.; Wang, J.; Xie, L.; Li, T.; He, Y.; Deng, Y.; Peng, Q.; Li, S.; Qin, X. Association between statins use and colorectal cancer risk: A meta-analysis of 42 studies. Cancer Causes Control. CCC 2014, 25, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.K.; Sehgal, V.S.; Kashfi, K. Molecular targets of statins and their potential side effects: Not all the glitter is gold. Eur. J. Pharmacol. 2022, 922, 174906. [Google Scholar] [CrossRef]
- Chalhoub, I.G.; Boulos, R.T.; Dagher, Y.G.; El Helou, S.; Haifa, K.G.; Atallah, B.; Nasr, F.; Kassab, I.; Chahine, M.N. Statins, commonly coprescribed drugs, and concomitant risk factors: A protective, neutral, or harmful association with common cancer types development: A 10-year multicentric retrospective lebanese study. Medicine 2023, 102, e34562. [Google Scholar] [CrossRef]
- Alvarez-Jimenez, L.; Morales-Palomo, F.; Moreno-Cabañas, A.; Ortega, J.F.; Mora-Rodríguez, R. Effects of statin therapy on glycemic control and insulin resistance: A systematic review and meta-analysis. Eur. J. Pharmacol. 2023, 947, 175672. [Google Scholar] [CrossRef]
- Rodríguez-Miguel, A.; Fernández-Antón, E.; Barreira-Hernández, D.; García-Rodríguez, L.A.; Gil, M.; García-Lledó, A.; De Abajo, F.J. Statins and Colorectal Cancer Risk: A Population-Based Case-Control Study and Synthesis of the Epidemiological Evidence. J. Clin. Med. 2022, 11, 1528. [Google Scholar] [CrossRef]
- Han, K.T.; Kim, S. Do cholesterol levels and continuity of statin use affect colorectal cancer incidence in older adults under 75 years of age? PLoS ONE 2021, 16, e0250716. [Google Scholar] [CrossRef]
- Cheung, K.S.; Chen, L.; Chan, E.W.; Seto, W.K.; Wong, I.C.K.; Leung, W.K. Statins reduce the progression of non-advanced adenomas to colorectal cancer: A postcolonoscopy study in 187 897 patients. Gut 2019, 68, 1979–1985. [Google Scholar] [CrossRef]
- Lavie, O.; Pinchev, M.; Rennert, H.S.; Segev, Y.; Rennert, G. The effect of statins on risk and survival of gynecological malignancies. Gynecol. Oncol. 2013, 130, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.; van Leeuwen, J.E.; Elbaz, M.; Branchard, E.; Penn, L.Z. Statins as Anticancer Agents in the Era of Precision Medicine. Clin. Cancer Res. 2020, 26, 5791–5800. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhu, Q.; Liu, Q.; Wang, Y.; Xie, W.; Hu, L. Statins use and endometrial cancer risk: A meta-analysis. Oncotarget 2017, 8, 62425–62434. [Google Scholar] [CrossRef]
- Tamimi, R.M.; Hankinson, S.E.; Chen, W.Y.; Rosner, B.; Colditz, G.A. Combined estrogen and testosterone use and risk of breast cancer in postmenopausal women. Arch. Intern. Med. 2006, 166, 1483–1489. [Google Scholar] [CrossRef]
- Dorgan, J.F.; Longcope, C.; Stephenson, H.E.; Falk, R.T., Jr.; Miller, R.; Franz, C.; Kahle, L.; Campbell, W.S.; Tangrea, J.A.; Schatzkin, A. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 533–539. [Google Scholar] [PubMed]
- Bouras, E.; Papandreou, C.; Tzoulaki, I.; Tsilidis, K.K. Endogenous sex steroid hormones and colorectal cancer risk: A systematic review and meta-analysis. Discov. Oncol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Lukanova, A.; Lundin, E.; Akhmedkhanov, A.; Micheli, A.; Rinaldi, S.; Zeleniuch-Jacquotte, A.; Lenner, P.; Muti, P.; Biessy, C.; Krogh, V.; et al. Circulating levels of sex steroid hormones and risk of ovarian cancer. Int. J. Cancer 2003, 104, 636–642. [Google Scholar] [CrossRef]
- Allen, N.E.; Key, T.J.; Dossus, L.; Rinaldi, S.; Cust, A.; Lukanova, A.; Peeters, P.H.; Onland-Moret, N.C.; Lahmann, P.H.; Berrino, F.; et al. Endogenous sex hormones and endometrial cancer risk in women in the European Prospective Investigation into Cancer and Nutrition (EPIC). Endocr. Relat. Cancer 2008, 15, 485–497. [Google Scholar] [CrossRef]
- Lopez, D.S.; Huang, D.; Tsilidis, K.K.; Canfield, S.; Khera, M.; Baillargeon, J.G.; Kuo, Y.F.; Peek, M.K.; Platz, E.A.; Markides, K. The role of testosterone replacement therapy and statins use, and their combination, in prostate cancer. Cancer Causes Control. CCC 2021, 32, 965–976. [Google Scholar] [CrossRef]
- Newman, C.B.; Preiss, D.; Tobert, J.A.; Jacobson, T.A.; Page, R.L., II; Goldstein, L.B.; Chin, C.; Tannock, L.R.; Miller, M.; Raghuveer, G.; et al. Statin Safety and Associated Adverse Events: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e38–e81. [Google Scholar] [CrossRef]
- Krysiak, R.; Gilowski, W.; Okopień, B. The effect of testosterone on cardiometabolic risk factors in atorvastatin-treated men with late-onset hypogonadism. Pharmacol. Rep. PR 2016, 68, 196–200. [Google Scholar] [CrossRef]
- Bachmann, G.; Oza, D. Female androgen insufficiency. Obstet. Gynecol. Clin. N. Am. 2006, 33, 589–598. [Google Scholar] [CrossRef]
- Somboonporn, W.; Davis, S.R.; National Health and Medical Research Council. Testosterone effects on the breast: Implications for testosterone therapy for women. Endocr. Rev. 2004, 25, 374–388. [Google Scholar] [CrossRef]
- Mokarram, P.; Alizadeh, J.; Razban, V.; Barazeh, M.; Solomon, C.; Kavousipour, S. Interconnection of Estrogen/Testosterone Metabolism and Mevalonate Pathway in Breast and Prostate Cancers. Curr. Mol. Pharmacol. 2017, 10, 86–114. [Google Scholar] [CrossRef]
- Newman, C.B.; Blaha, M.J.; Boord, J.B.; Cariou, B.; Chait, A.; Fein, H.G.; Ginsberg, H.N.; Goldberg, I.J.; Murad, M.H.; Subramanian, S.; et al. Lipid Management in Patients with Endocrine Disorders: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2020, 105, dgaa674. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kostantinou, A.; Kougias, M.; Kazazis, C. Statins and cancer. Anti-Cancer Agents Med. Chem. 2014, 14, 706–712. [Google Scholar] [CrossRef]
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals 2020, 13, 422. [Google Scholar] [CrossRef]
- Zeleznik, O.A.; Irvin, S.R.; Samimi, G.; Trabert, B. The Role of Statins in the Prevention of Ovarian and Endometrial Cancers. Cancer Prev. Res. 2023, 16, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Braunstein, G.D. Safety of testosterone use in women. Maturitas 2009, 63, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in women--the clinical significance. Lancet Diabetes Endocrinol. 2015, 3, 980–992. [Google Scholar] [CrossRef]
- Wierman, M.E.; Arlt, W.; Basson, R.; Davis, S.R.; Miller, K.K.; Murad, M.H.; Rosner, W.; Santoro, N. Androgen therapy in women: A reappraisal: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2014, 99, 3489–3510. [Google Scholar] [CrossRef]
- Lévesque, L.E.; Hanley, J.A.; Kezouh, A.; Suissa, S. Problem of immortal time bias in cohort studies: Example using statins for preventing progression of diabetes. BMJ 2010, 340, b5087. [Google Scholar] [CrossRef]
- Reiner, Ž. Statins in the primary prevention of cardiovascular disease. Nature reviews. Cardiology 2013, 10, 453–464. [Google Scholar] [CrossRef] [PubMed]
- SEER-Medicare Linked Data Resource. Analytic Support for Researchers. Measures that Are Limited or Not Available in the Data. Available online: https://healthcaredelivery.cancer.gov/seermedicare/considerations/measures.html (accessed on 2 July 2025).
- Bhasin, S.; Lincoff, A.M.; Nissen, S.E.; Wannemuehler, K.; McDonnell, M.E.; Peters, A.L.; Khan, N.; Snabes, M.C.; Li, X.; Li, G.; et al. Effect of Testosterone on Progression From Prediabetes to Diabetes in Men With Hypogonadism: A Substudy of the TRAVERSE Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Schooling, C.M.; Yeung, S.L.A.; Freeman, G.; Cowling, B.J. The effect of statins on testosterone in men and women, a systematic review and meta-analysis of randomized controlled trials. BMC Med. 2013, 11, 57. [Google Scholar] [CrossRef]
- Oluleye, O.W.; Kronmal, R.A.; Folsom, A.R.; Vaidya, D.M.; Ouyang, P.; Duprez, D.A.; Dobs, A.S.; Yarmohammadi, H.; Konety, S.H. Association Between Statin Use and Sex Hormone in the Multi-Ethnic Study of Atherosclerosis Cohort. J. Clin. Endocrinol. Metab. 2019, 104, 4600–4606. [Google Scholar] [CrossRef]
- Warren, J.L.; Klabunde, C.N.; Schrag, D.; Bach, P.B.; Riley, G.F. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med. Care 2002, 40 (Suppl. S8), IV-3–IV-18. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Jeong, G.H.; Lee, K.H.; Kim, J.Y.; Eisenhut, M.; Kronbichler, A.; van der Vliet, H.J.; Shin, J.I.; Gamerith, G. Statinsand Cancer Mortality and Survival: An Umbrella Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 326. [Google Scholar] [CrossRef]
- Baillargeon, J.; Urban, R.J.; Raji, M.A.; Westra, J.R.; Williams, S.B.; Lopez, D.S.; Kuo, Y.-F. Testosterone prescribing among women in the USA, 2002–2017. J. Gen. Intern. Med. 2020, 35, 1891–1893. [Google Scholar] [CrossRef]
- Hernán, M.A.; Hernández-Díaz, S.; Werler, M.M.; Mitchell, A.A. Causal knowledge as a prerequisite for confounding evaluation: An application to birth defects epidemiology. Am. J. Epidemiol. 2002, 155, 176–184. [Google Scholar] [CrossRef] [PubMed]
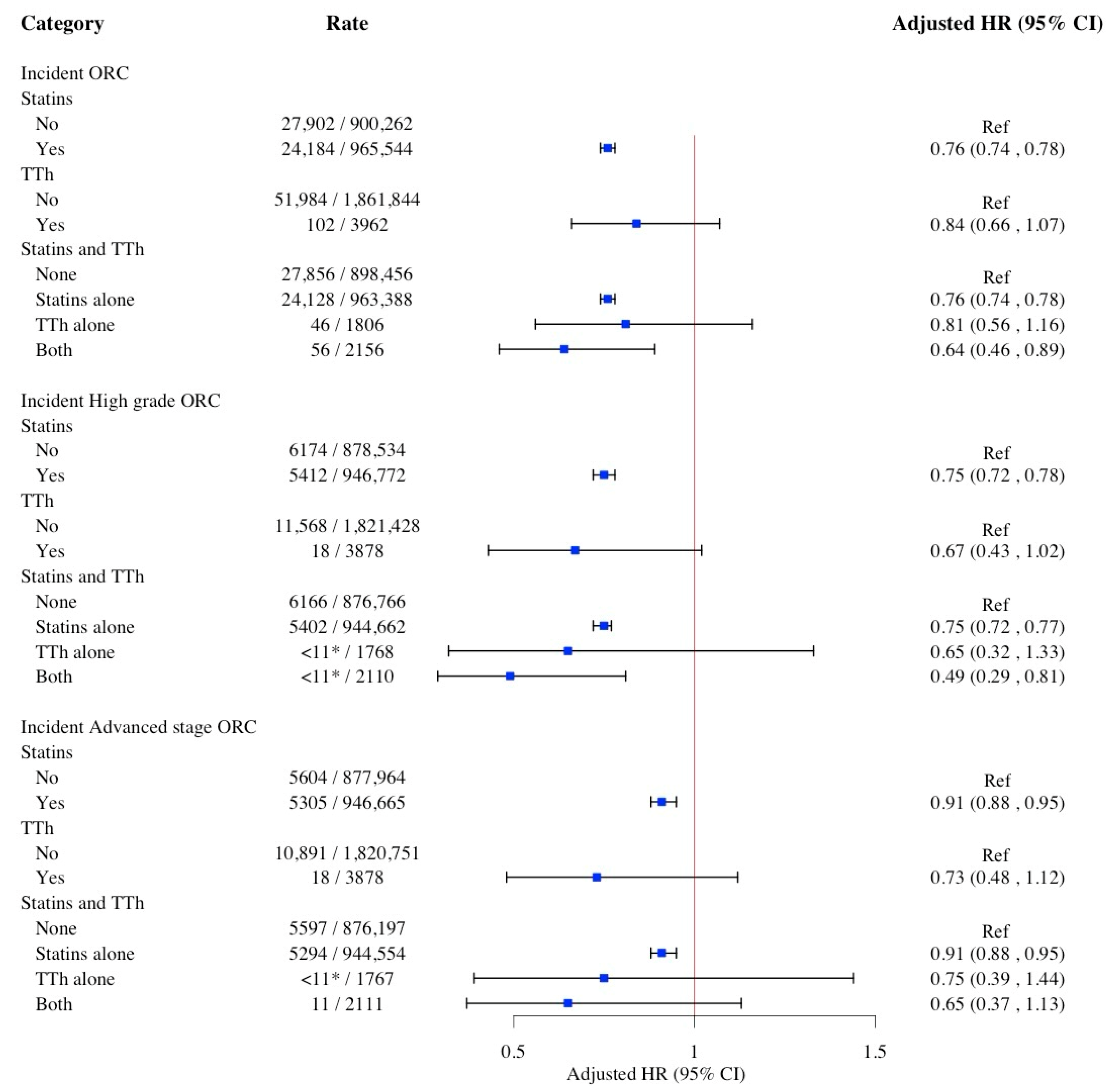
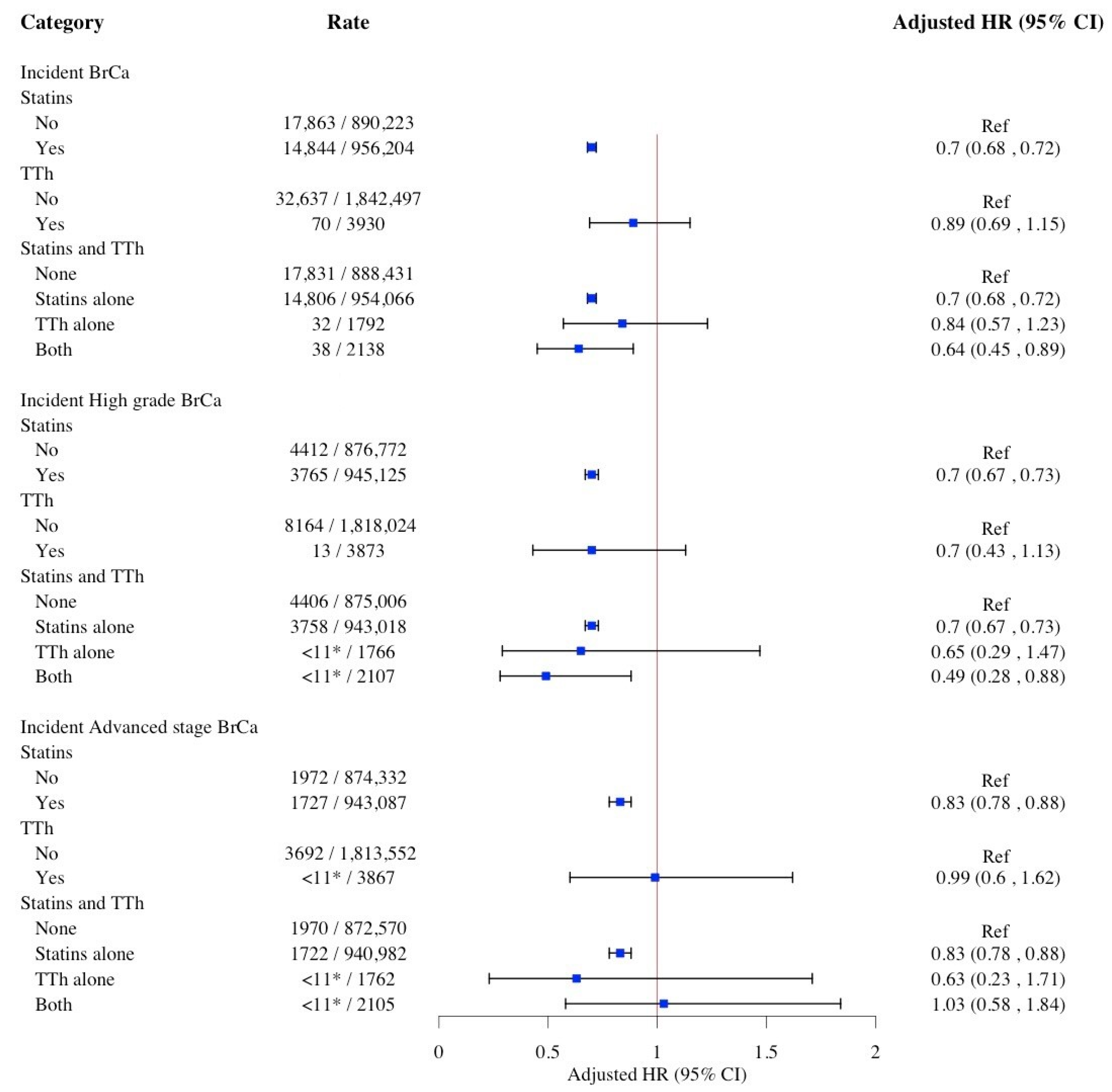

| Characteristics | No. (%) | p-Value | |||
|---|---|---|---|---|---|
| No Statins/No TTh (n = 71,386) | Statins Alone (n = 71,091) | TTh Alone (n = 134) | Statins and TTh (n = 161) | ||
| ORC | |||||
| 17,831 (24.98) | 14,806 (20.83) | >13 (>9.70) ** | >23 (>14.29) ** | 0.0001 * |
| 5705 (7.99) | 5351(7.53) | <11 (<8.21) ** | <11 (<6.83) ** | 0.0019 * |
| 1276 (1.79) | 1318 (1.85) | <11 (<8.21) ** | <11 (<6.83) ** | 0.2707 |
| 3044 (4.26) | 2653 (3.73) | <11 (<8.21) ** | <11 (<6.83) ** | 0.0001 * |
| ORC Stage a | |||||
| 22,259 (31.18) | 18,834 (26.49) | 39 (29.1) | 45 (27.95) | 0.0001 * |
| 5597 (7.84) | 5294 (7.45) | <11 (<8.21) ** | 11 (6.83) | |
| 43,530 (60.98) | 46,963 (66.06) | >84 (>62.69) ** | 105 (65.22) | |
| ORC Grade b | |||||
| 21,690 (30.38) | 18,726 (26.34) | 38 (28.36) | 46 (28.57) | 0.0001 * |
| 6166 (8.64) | 5402 (7.6) | <11 (<8.21) ** | <11 (<6.83) ** | |
| 43,530 (60.98) | 46,963 (66.06) | >85 (>63.43) ** | >104 (>64.60) ** | |
| Age | |||||
| 6401 (8.97) | 22,977 (32.32) | 50 (37.31) | 71 (44.1) | 0.0001 * |
| 18,268 (25.59) | 17,183 (24.17) | 29 (21.64) | 42 (26.09) | |
| 16,453 (23.05) | 12,881 (18.12) | 24 (17.91) | 23 (14.29) | |
| 30,264 (42.39) | 18,050 (25.39) | 31 (23.13) | 25 (15.53) | |
| Race/ethnicity | |||||
| 58,929 (82.55) | 56,069 (78.87) | >101 (>75.37) ** | >122 (>75.78) ** | <0.0001 * |
| 5748 (8.05) | 7493 (10.54) | <11 (<8.21) ** | 13 (8.07) | |
| 1708 (2.39) | 2296 (3.23) | <11 (<8.21) ** | <11 (<6.83) ** | |
| 5001 (7.01) | 5233 (7.36) | <11 (<8.21) ** | 15 (9.32) | |
| Hyperlipidemia | 14,889 (20.86) | 44,860 (63.1) | 41 (30.6) | 99 (61.49) | 0.0001 * |
| Hypertension | 32,339 (45.3) | 53,304 (74.98) | 90 (67.16) | 128 (79.5) | 0.0001 * |
| Muscular wasting and atrophy | 580 (0.81) | 799 (1.12) | <11 (<8.21) ** | <11 (<6.83) ** | 0.0001 * |
| Malaise and fatigue | 9799 (13.73) | 14,752 (20.75) | 50 (37.31) | 59 (36.65) | 0.0001 * |
| Osteoporosis | 9155 (12.82) | 10,635 (14.96) | 35 (26.12) | 29 (18.01) | 0.0001 * |
| Anterior pituitary disorder | 50 (0.07) | 55 (0.08) | <11 (<8.21) ** | <11 (<6.83) ** | 0.9238 |
| Depression disorder | 3603 (5.05) | 5624 (7.91) | 12 (8.96) | 12 (7.45) | 0.0001 * |
| Diabetes | 8607 (12.06) | 23,294 (32.77) | 29 (21.64) | 38 (23.6) | 0.0001 * |
| Cardiovascular disease | 28,784 (40.32) | 41,621 (58.55) | 78 (58.21) | 105 (65.22) | 0.0001 * |
| Use of insulin | 1038 (1.45) | 3998 (5.62) | <11 (<8.21) ** | <11 (<6.83) ** | 0.0001 * |
| Charlson comorbidity | |||||
| 50,321 (70.49) | 36,752 (51.7) | 58 (43.28) | 83 (51.55) | 0.0001 * |
| 14081 (19.73) | 17,837 (25.09) | 39 (29.1) | 49 (30.43) | |
| 4500 (6.3) | 8758 (12.32) | 19 (14.18) | 16 (9.94) | |
| 2484 (3.48) | 7744 (10.89) | 18 (13.43) | 13 (8.07) | |
| Number of BrCa screening c, mean (SD) | 0.18 (0.43) | 0.21 (0.44) | 0.25 (0.47) | 0.21 (0.42) | <0.0001 * |
| Number of CRC screening d, mean (SD) | 0.04 (0.21) | 0.05 (0.23) | 0.06 (0.24) | 0.05 (0.22) | <0.0001 * |
| Number of ovarian cancer screening e, mean (SD) | 0.02 (0.15) | 0.03 (0.17) | 0.06 (0.27) | 0.06 (0.40) | <0.0001 * |
| Number of PCP visits, mean (SD) | 8.34 (8.99) | 11.96 (10.82) | 17.25 (12.65) | 16.28 (12.09) | <0.0001 * |
| Percent of adults with <12 years education, mean (SD) | 18.37 (11.81) | 20.16 (12.44) | 18.50 (11.13) | 22.36 (13.56) | <0.0001 * |
| Percent of adults below poverty, mean (SD) | 11.26 (8.16) | 11.96 (8.68) | 12.93 (8.65) | 14.87 (9.67) | <0.0001 * |
| Incident CRC | High-Grade CRC | Advanced-Stage CRC | ||||
|---|---|---|---|---|---|---|
| Rate | HR (95% CI) | Rate | HR (95% CI) | Rate | HR (95% CI) | |
| Statins | ||||||
| No | 5710/878,070 | Ref | 864/873,224 | Ref | 2142/874,502 | Ref |
| Yes | 5360/946,720 | 0.98 (0.94, 1.02) | 813/942,173 | 1.0 (0.92, 1.09) | 2086/943,446 | 1.03 (0.97, 1.09) |
| TTh | ||||||
| No | 11,056/1,820,916 | Ref | 676/1,811,536 | Ref | 4225/18,144,085 | Ref |
| Yes | 14/3874 | 0.67 (0.43, 1.03) | <11/3861 * | 0.33 (0.04, 2.37) | <11/3863 * | 0.38 (0.12, 1.18) |
| Statins and TTh | ||||||
| None | 5705/876,305 | Ref | 864/871,464 | Ref | 2141/872,741 | Ref |
| Statins Alone | 5351/944,611 | 0.98 (0.94, 1.02) | 812/940,072 | Not Calculated | 2084/941,344 | 1.03 (0.97, 1.09) |
| TTh Alone | <11/1765 * | 0.57 (0.28, 1.14) | <11/1760 * | Not Calculated | <11/1761 * | 0.32 (0.04, 2.26) |
| Both | <11/2109 * | 0.72 (0.42, 1.26) | <11/2101 * | Not Calculated | <11/2102 * | 0.43 (0.11, 1.75) |
| Incident Ovarian Cancer | High-Grade Ovarian Cancer | Advanced-Stage Ovarian Cancer | ||||
|---|---|---|---|---|---|---|
| Rate | HR (95% CI) | Rate | HR (95% CI) | Rate | HR (95% CI) | |
| Statins | ||||||
| No | 1281/873,641 | Ref | 308/872,668 | Ref | 855/873,215 | Ref |
| Yes | 1320/942,680 | 0.96 (0.89, 1.04) | 295/941,655 | 0.80 (0.69, 0.93) | 891/942,251 | 0.94 (0.85, 1.03) |
| TTh | ||||||
| No | 2594/1,8124,54 | Ref | 600/1,810,460 | Ref | 1740/1,811,600 | Ref |
| Yes | <11/3867 * | 0.84 (0.38, 1.87) | <11/3863 * | 1.29 (0.31, 5.27) | <11/3866 * | 1.0 (0.42, 2.37) |
| Statins and TTh | ||||||
| None | 1276/871,876 | Ref | 306/870,906 | Ref | 851/87,1451 | Ref |
| Statins Alone | 1318/940,578 | Not Calculated | 294/939,554 | Not Calculated | 889/940,149 | Not Calculated |
| TTh Alone | <11/1765 * | Not Calculated | <11/1762 * | Not Calculated | <11/1764 * | Not Calculated |
| Both | <11/2102 * | Not Calculated | <11/2101 * | Not Calculated | <11/2102 * | Not Calculated |
| Incident Endometrial Cancer | High-Grade Endometrial Cancer | Advanced-Stage Endometrial Cancer | ||||
|---|---|---|---|---|---|---|
| Rate | HR (95% CI) | Rate | HR (95% CI) | Rate | HR (95% CI) | |
| Statins | ||||||
| No | 3048/875,408 | Ref | 590/872,950 | Ref | 635/872,995 | Ref |
| Yes | 2660/944,020 | 0.67 (0.64, 0.70) | 539/941,899 | 0.70 (0.62, 0.78) | 601/941,961 | 0.76 (0.68, 0.84) |
| TTh | ||||||
| No | 5697/1,815,557 | Ref | 1128/1,810,988 | Ref | 1234/1,811,094 | Ref |
| Yes | 11/3871 | 0.79 (0.47, 1.35) | <11/3861 * | 0.42 (0.37, 0.48) | <11/3861 * | 0.74 (0.27, 1.98) |
| Statins and TTh | ||||||
| None | 3044/873,644 | Ref | 590/871,190 | Ref | 635/871,235 | Ref |
| Statins Alone | 2653/941,913 | 0.67 (0.63, 0.70) | 538/939,798 | Not Calculated | 599/939,859 | Not Calculated |
| TTh Alone | <11/1764 * | 0.48 (0.187, 1.22) | <11/1760 * | Not Calculated | <11/1760 * | Not Calculated |
| Both | <11/2107 * | 0.70 (0.37, 1.33) | <11/2101 * | Not Calculated | <11/2102 * | Not Calculated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.R.; Wu, S.; Saab, D.; Digbeu, B.; Abdelgadir, O.; Hernandez-Perez, J.G.; Torres-Sanchez, L.E.; Leonard, T.; Cano, M.; Kuo, Y.-F.; et al. Obesity-Related Cancers in Relation to Use of Statins and Testosterone Replacement Therapy Among Older Women: SEER-Medicare 2007–2015. Pharmaceuticals 2025, 18, 1413. https://doi.org/10.3390/ph18091413
Hussain MR, Wu S, Saab D, Digbeu B, Abdelgadir O, Hernandez-Perez JG, Torres-Sanchez LE, Leonard T, Cano M, Kuo Y-F, et al. Obesity-Related Cancers in Relation to Use of Statins and Testosterone Replacement Therapy Among Older Women: SEER-Medicare 2007–2015. Pharmaceuticals. 2025; 18(9):1413. https://doi.org/10.3390/ph18091413
Chicago/Turabian StyleHussain, Maryam R., Shannon Wu, Diane Saab, Biai Digbeu, Omer Abdelgadir, Jesus Gibran Hernandez-Perez, Luisa E. Torres-Sanchez, Tammy Leonard, Miguel Cano, Yong-Fang Kuo, and et al. 2025. "Obesity-Related Cancers in Relation to Use of Statins and Testosterone Replacement Therapy Among Older Women: SEER-Medicare 2007–2015" Pharmaceuticals 18, no. 9: 1413. https://doi.org/10.3390/ph18091413
APA StyleHussain, M. R., Wu, S., Saab, D., Digbeu, B., Abdelgadir, O., Hernandez-Perez, J. G., Torres-Sanchez, L. E., Leonard, T., Cano, M., Kuo, Y.-F., Villasante-Tezanos, A., & Lopez, D. S. (2025). Obesity-Related Cancers in Relation to Use of Statins and Testosterone Replacement Therapy Among Older Women: SEER-Medicare 2007–2015. Pharmaceuticals, 18(9), 1413. https://doi.org/10.3390/ph18091413





