Therapeutic Potential of Food-Derived Rutin Phytosome Nanoparticles: Anti-Tumor, Antioxidant, and Anti-Inflammatory Activity in Ehrlich Ascites Carcinoma
Abstract
1. Introduction
2. Results
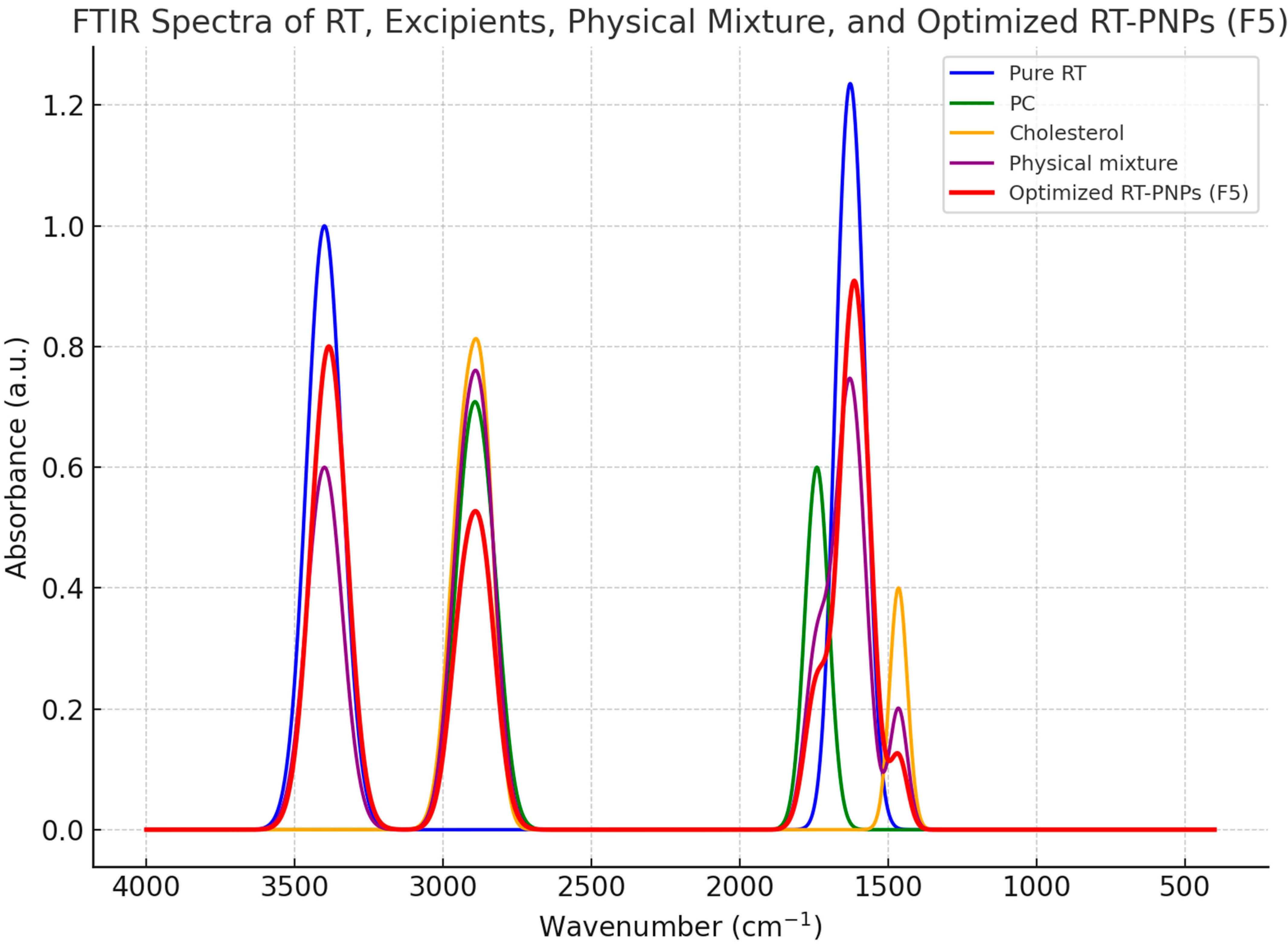
2.1. Characterization of Rutin-Loaded Nano-Phytosomes (RT-PNPs)
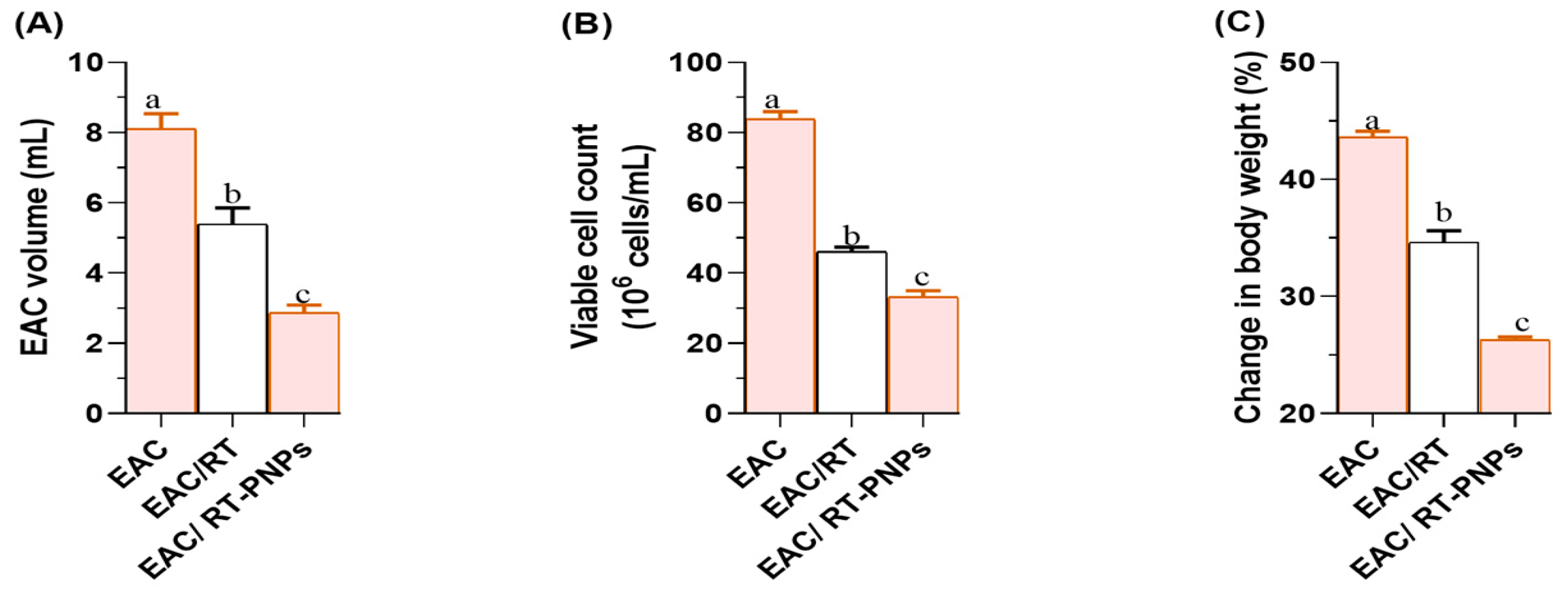
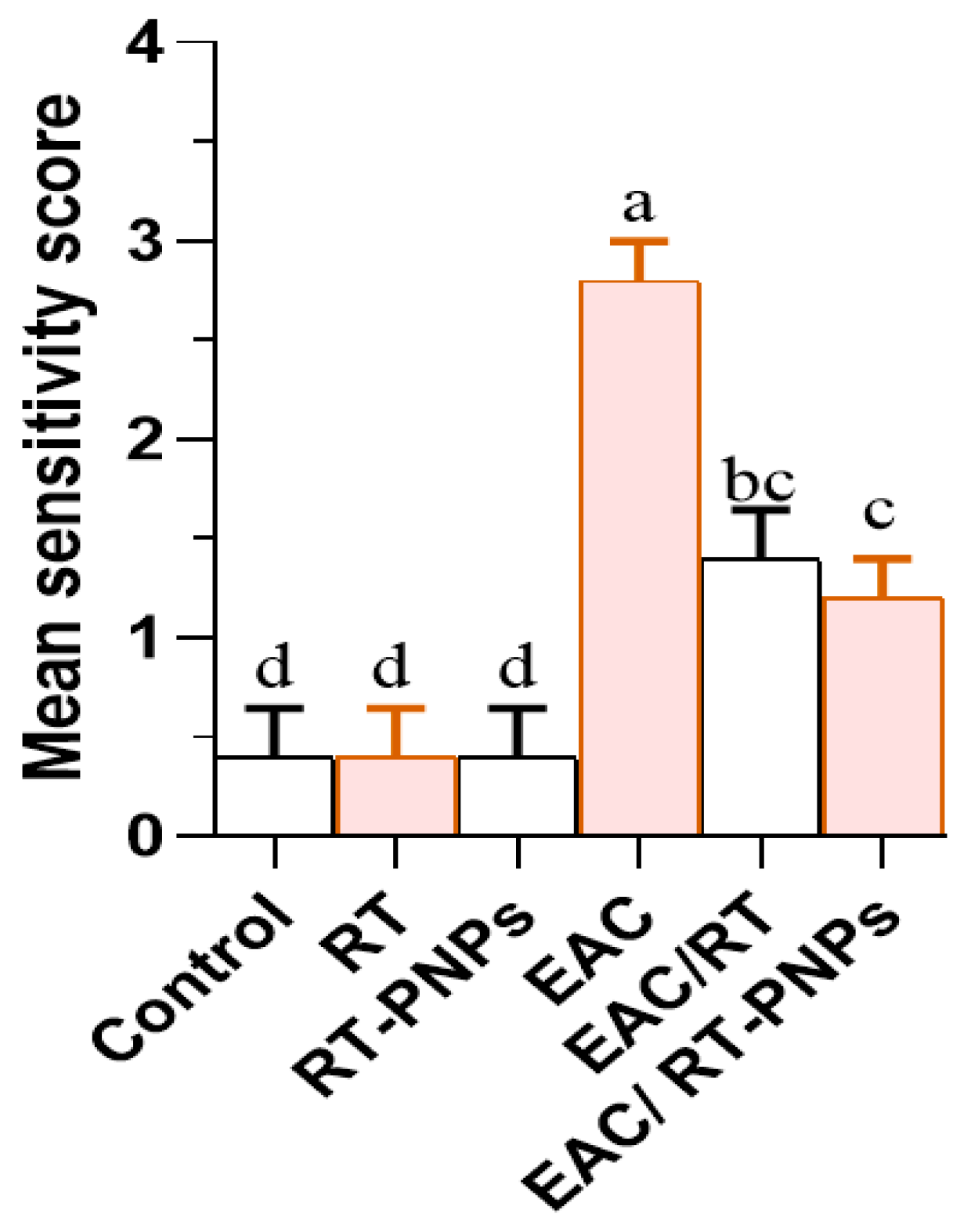
2.2. Changes in Cell Volume, Viability, Body Weight, and the Mean Survival Time
2.3. Hematology Parameters
2.4. Blood Chemistry
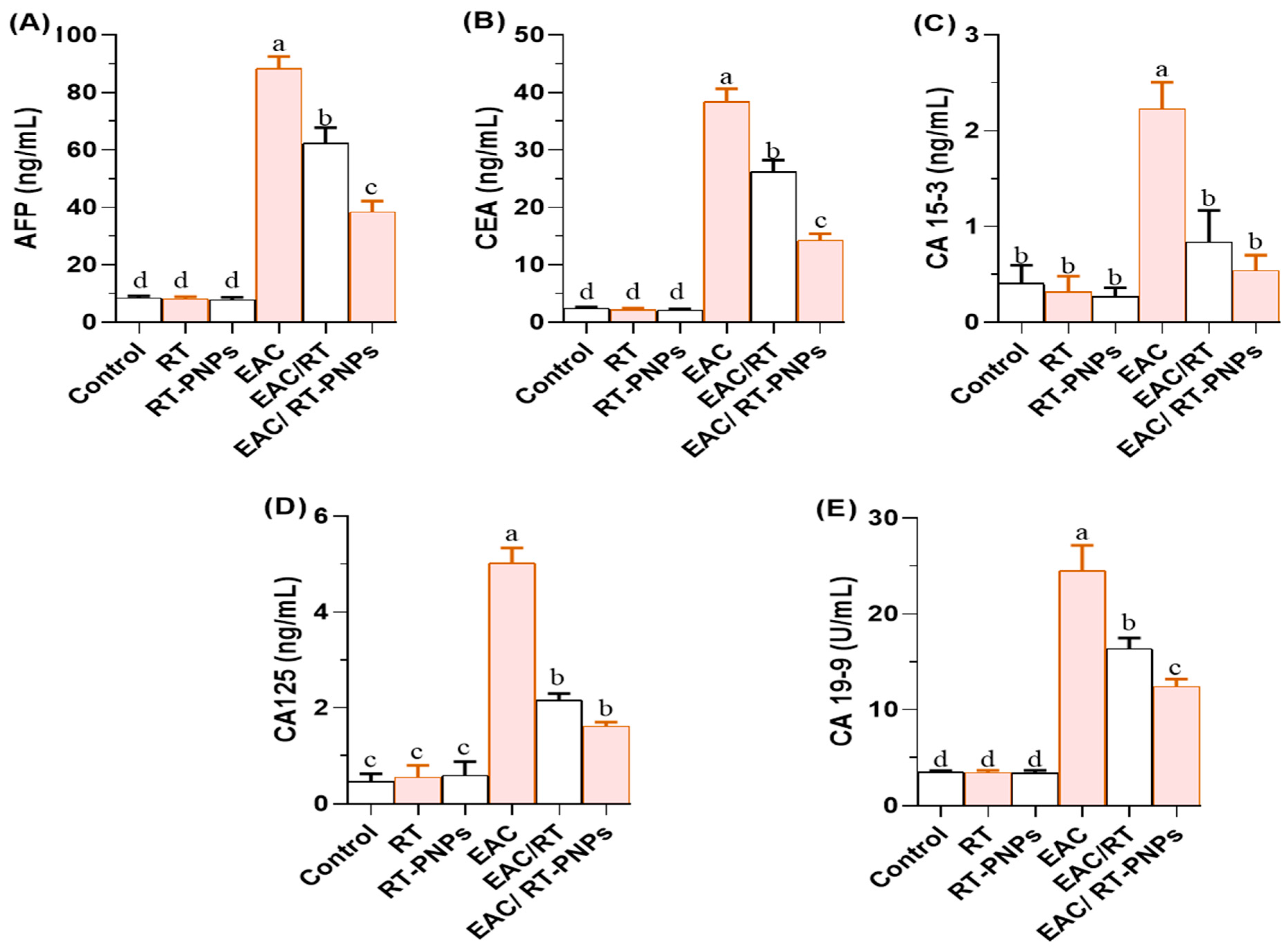
2.5. Tumor-Associated Biomarkers
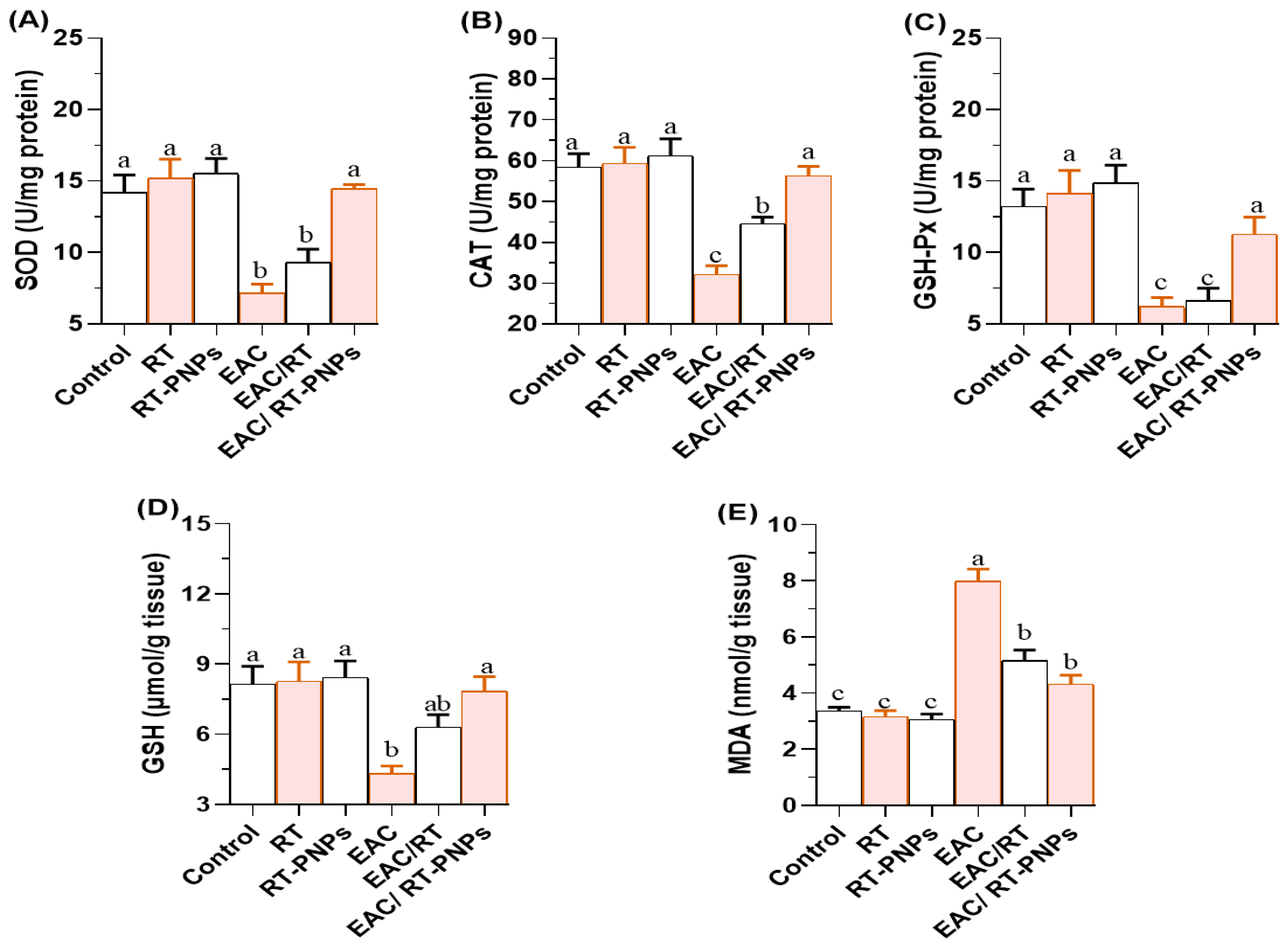
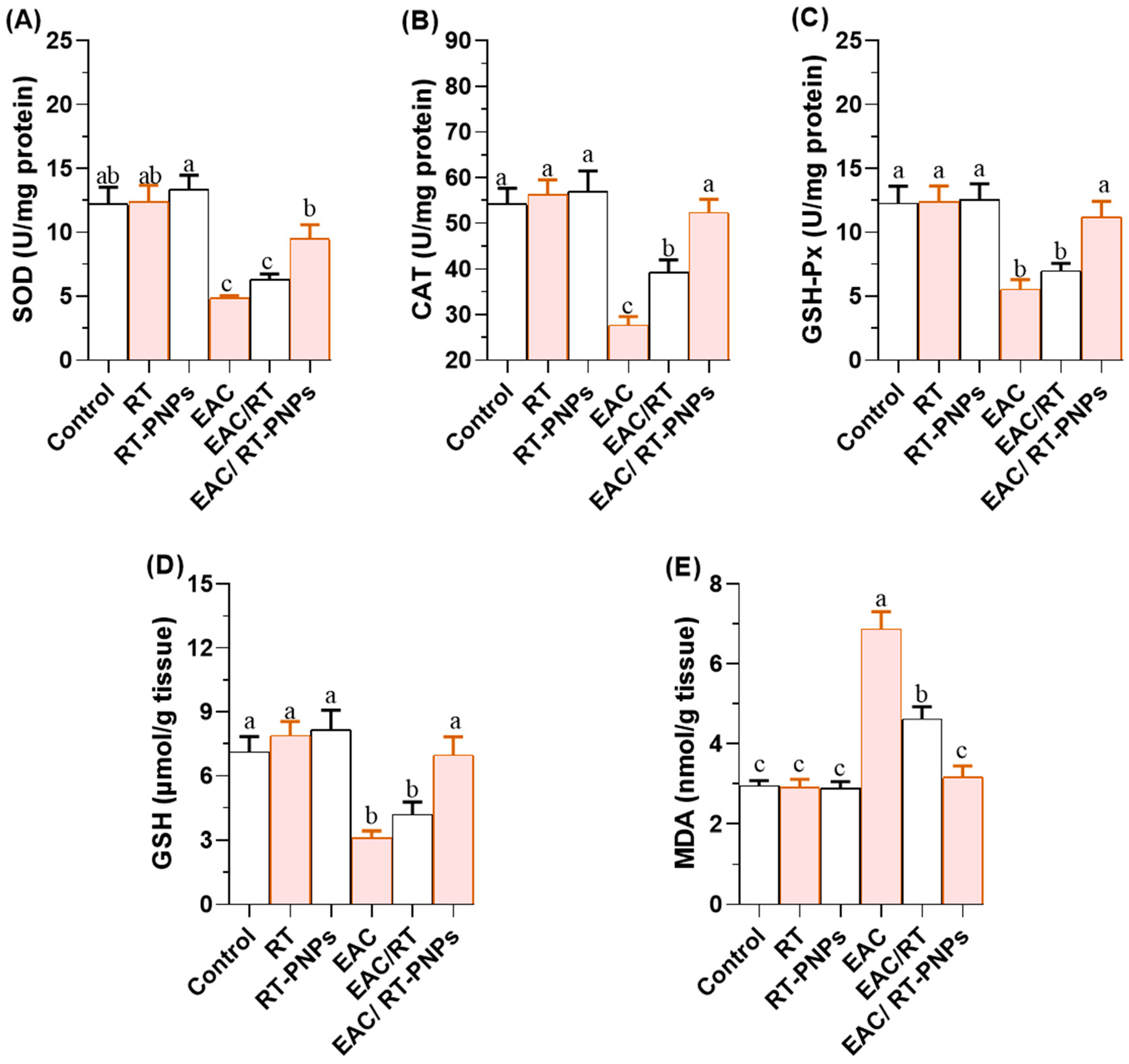
2.6. Redox Status
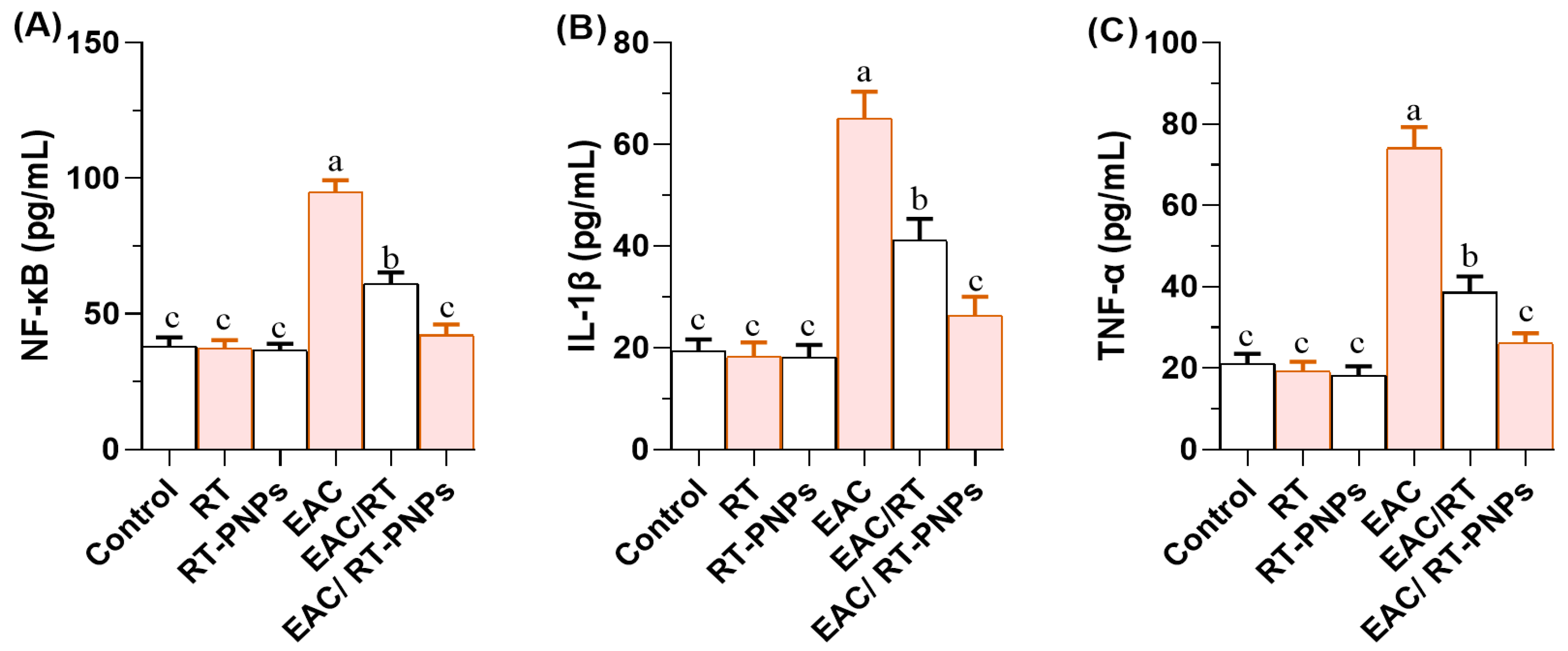
2.7. Inflammatory Reaction
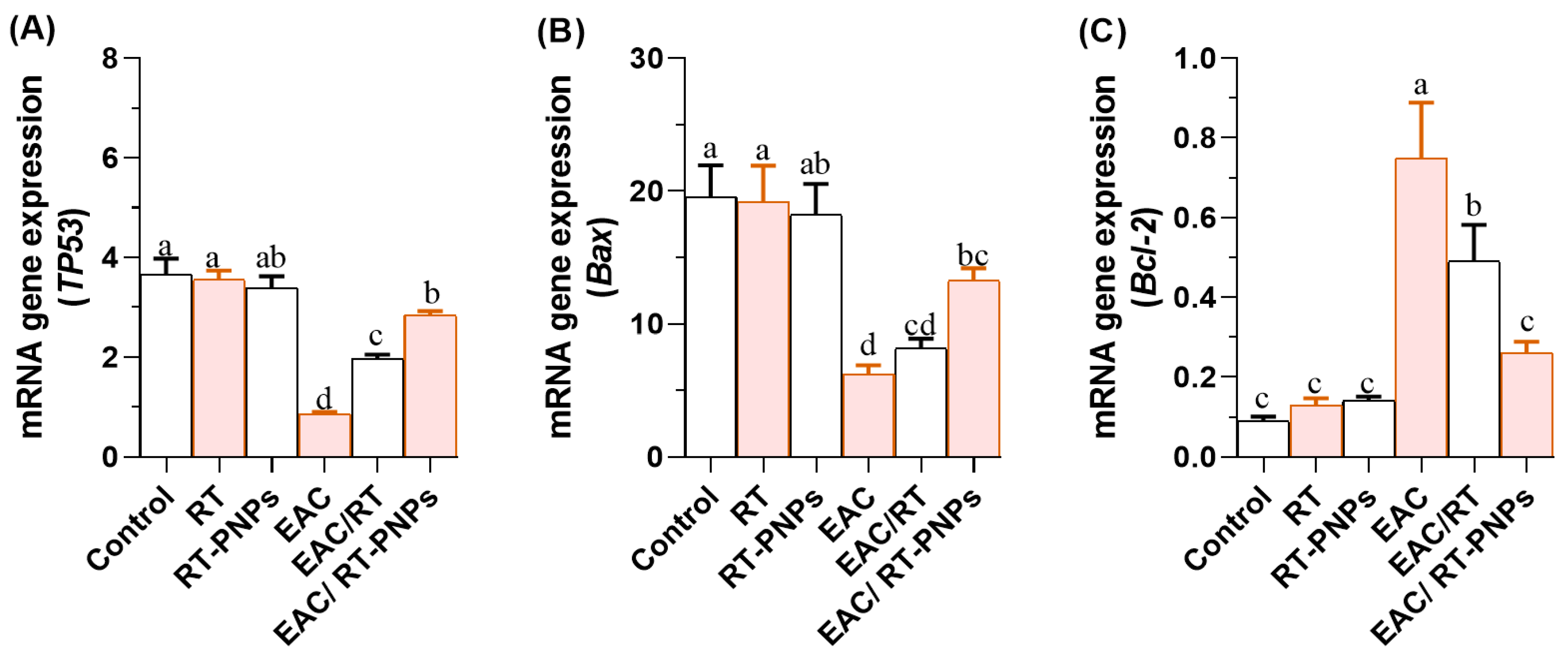
2.8. Expression Profiles of TP53, Bax, and Bcl-2 Genes
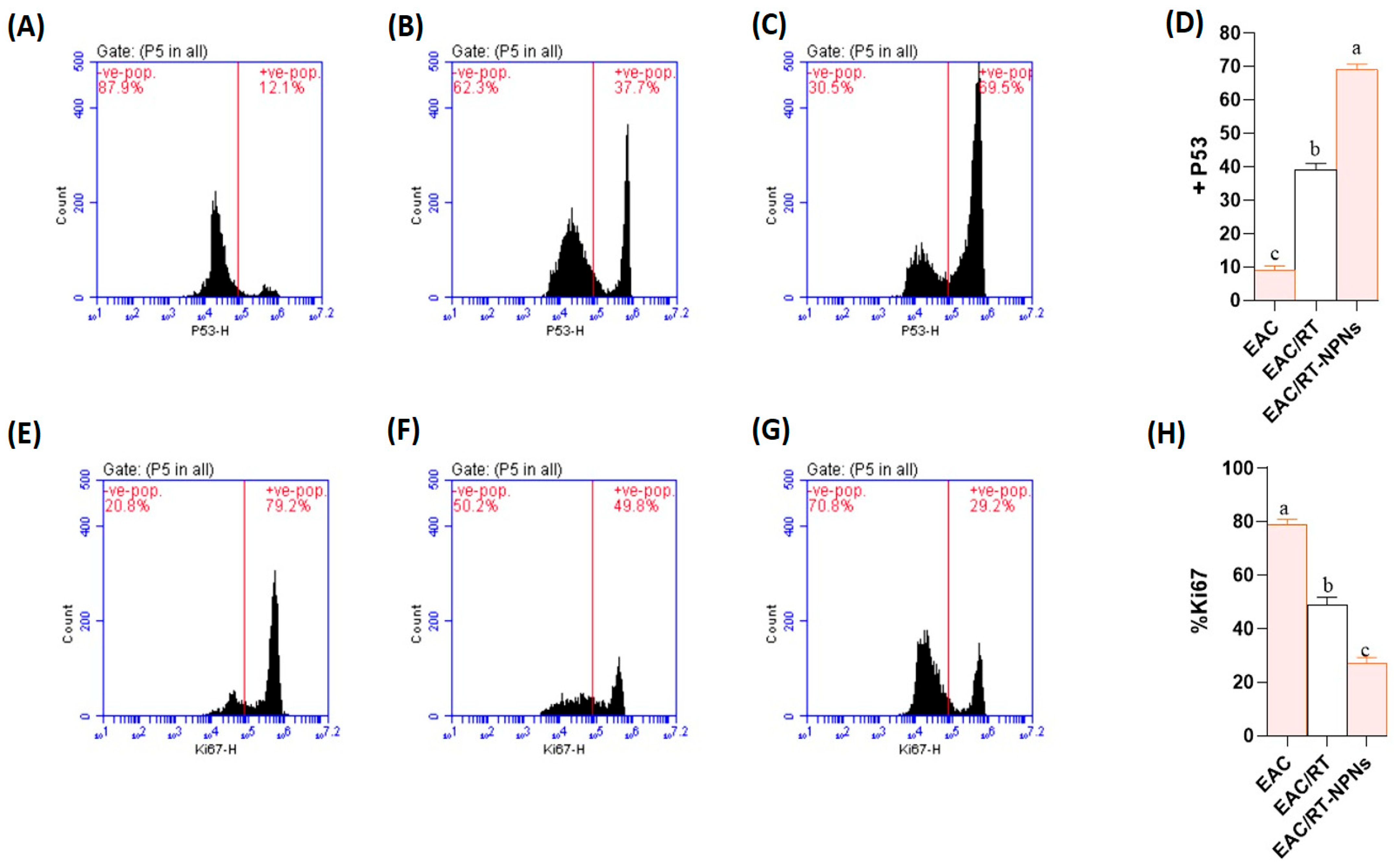
2.9. Flow Cytometric Analysis of p53 and Ki-67 Expression Results
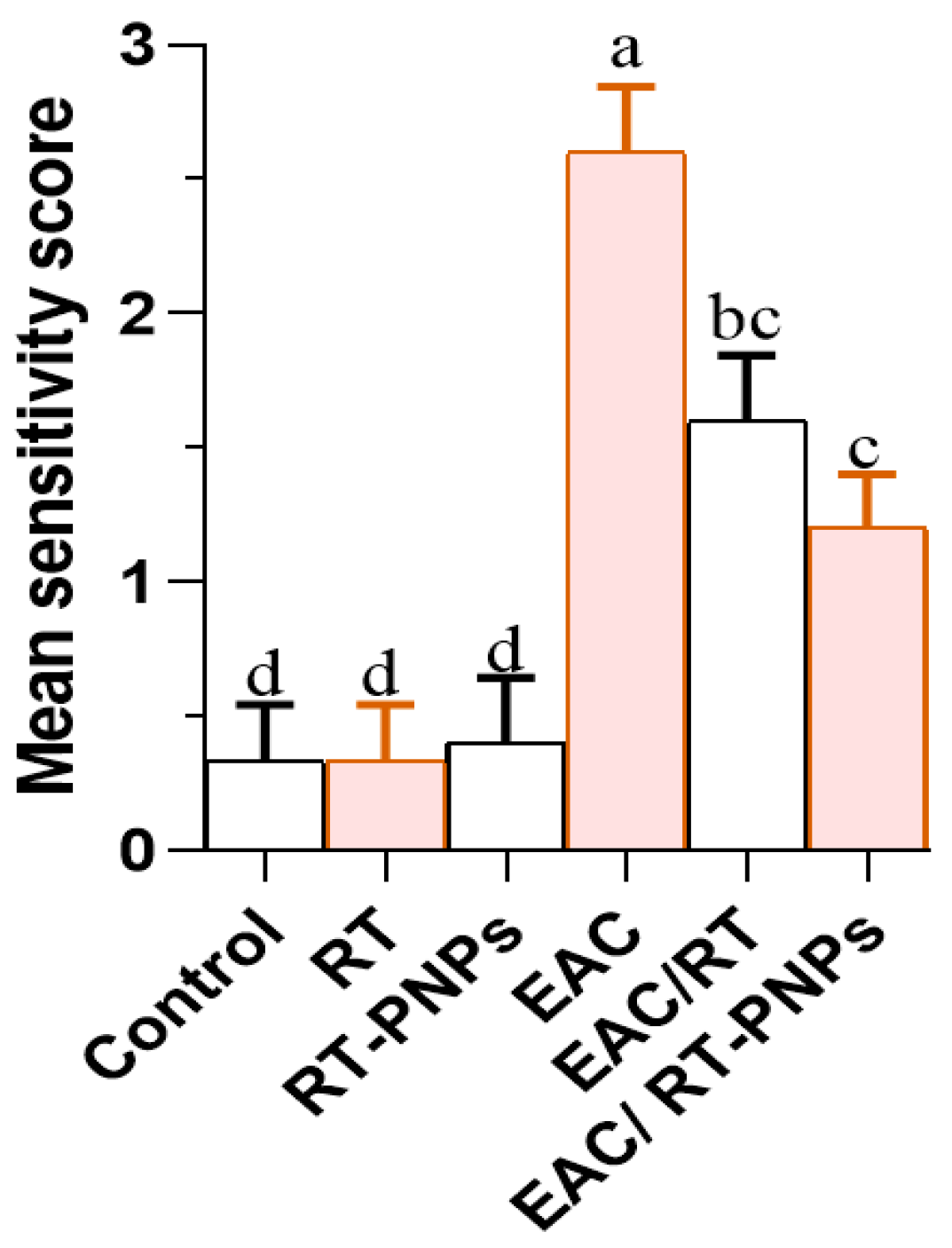
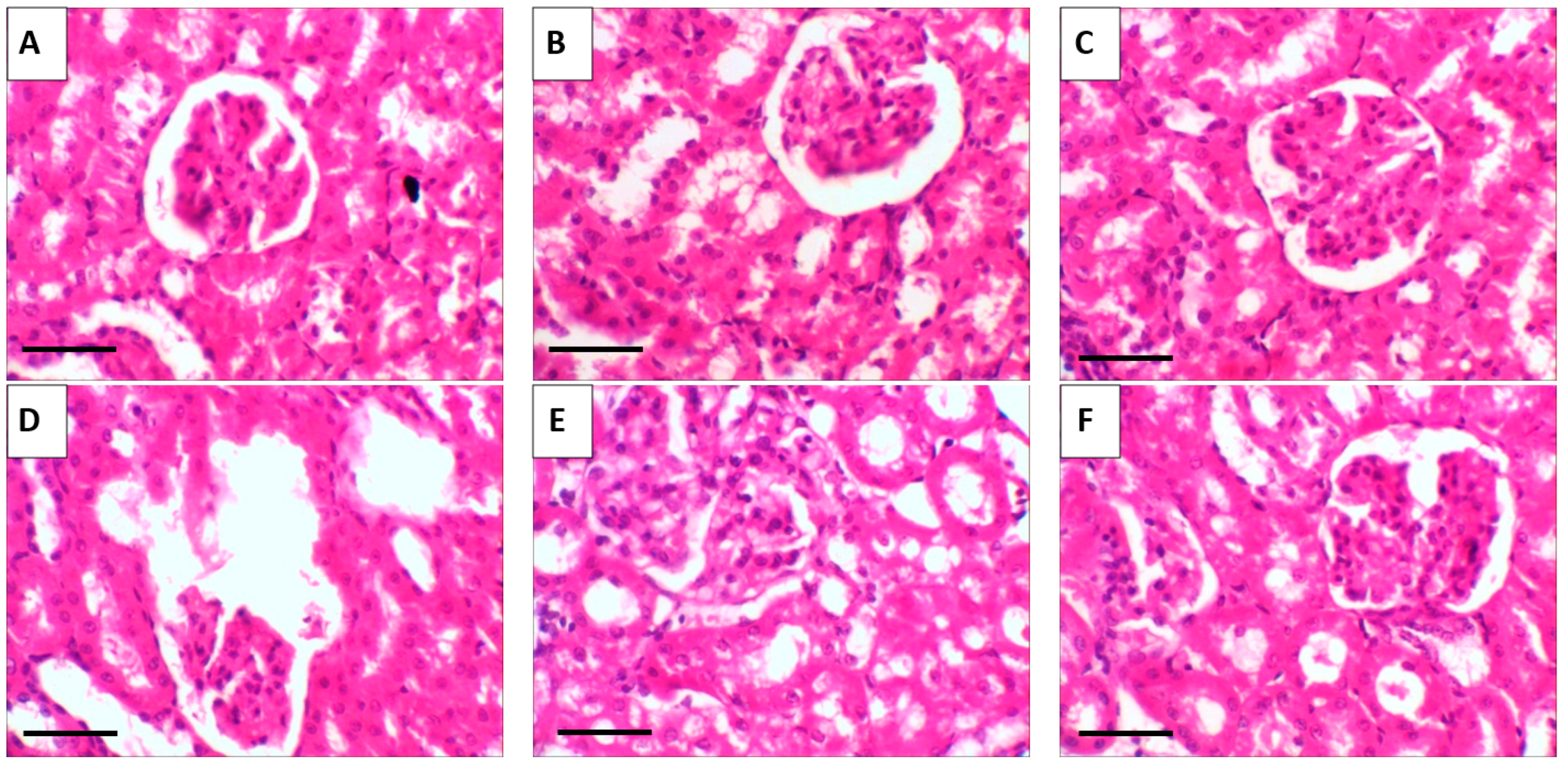
2.10. Histopathological Results
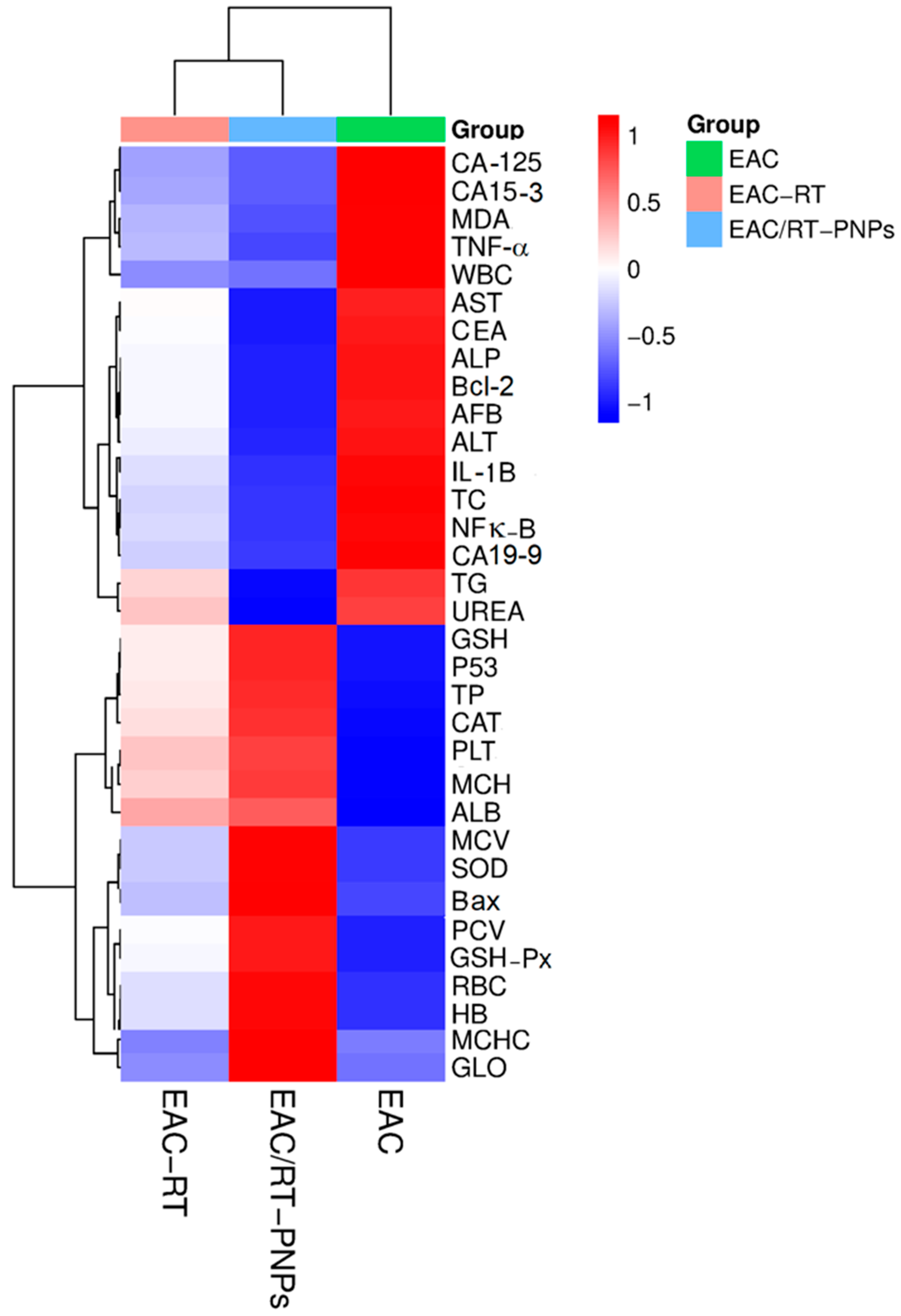
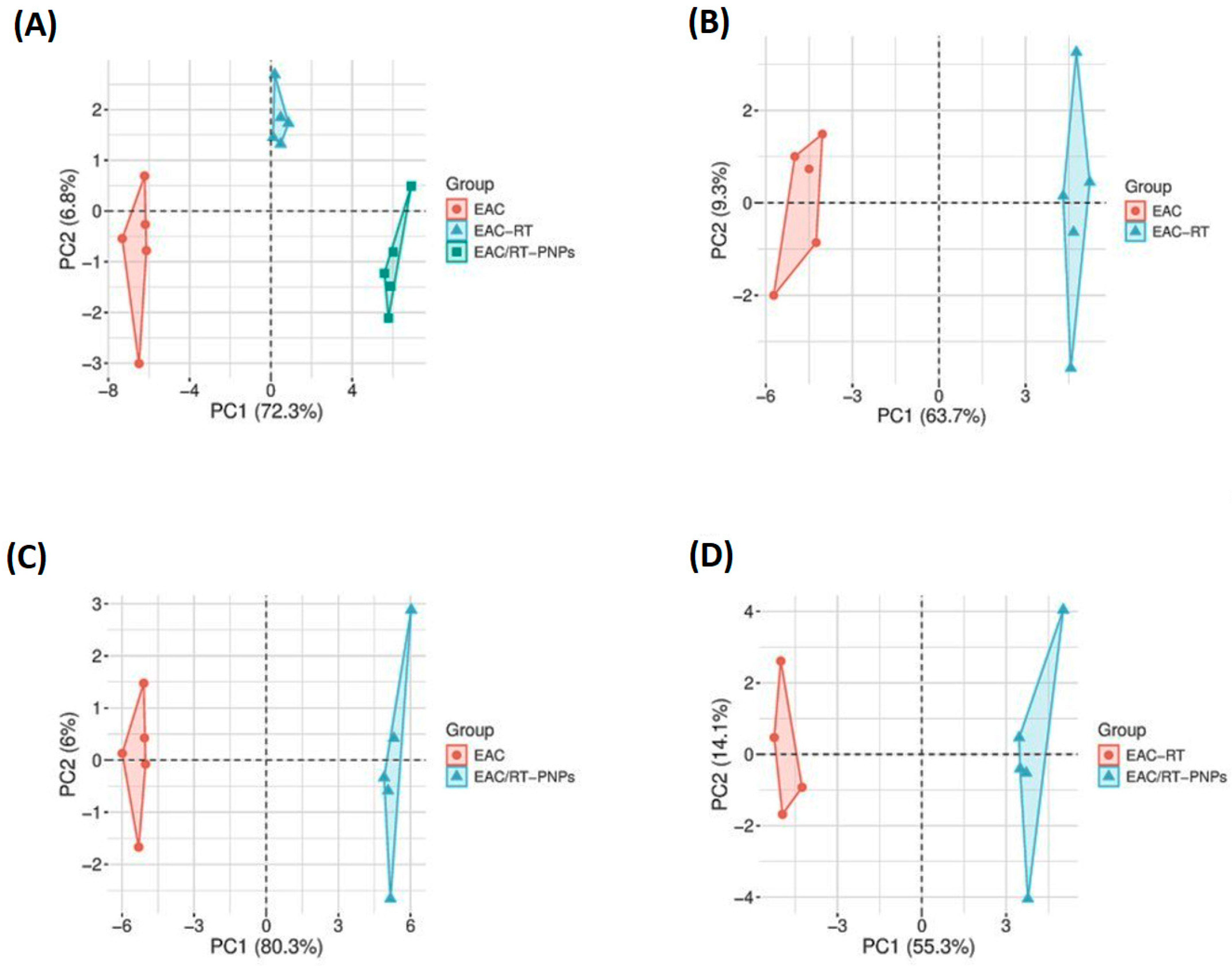
2.11. Multivariable Data Analyses
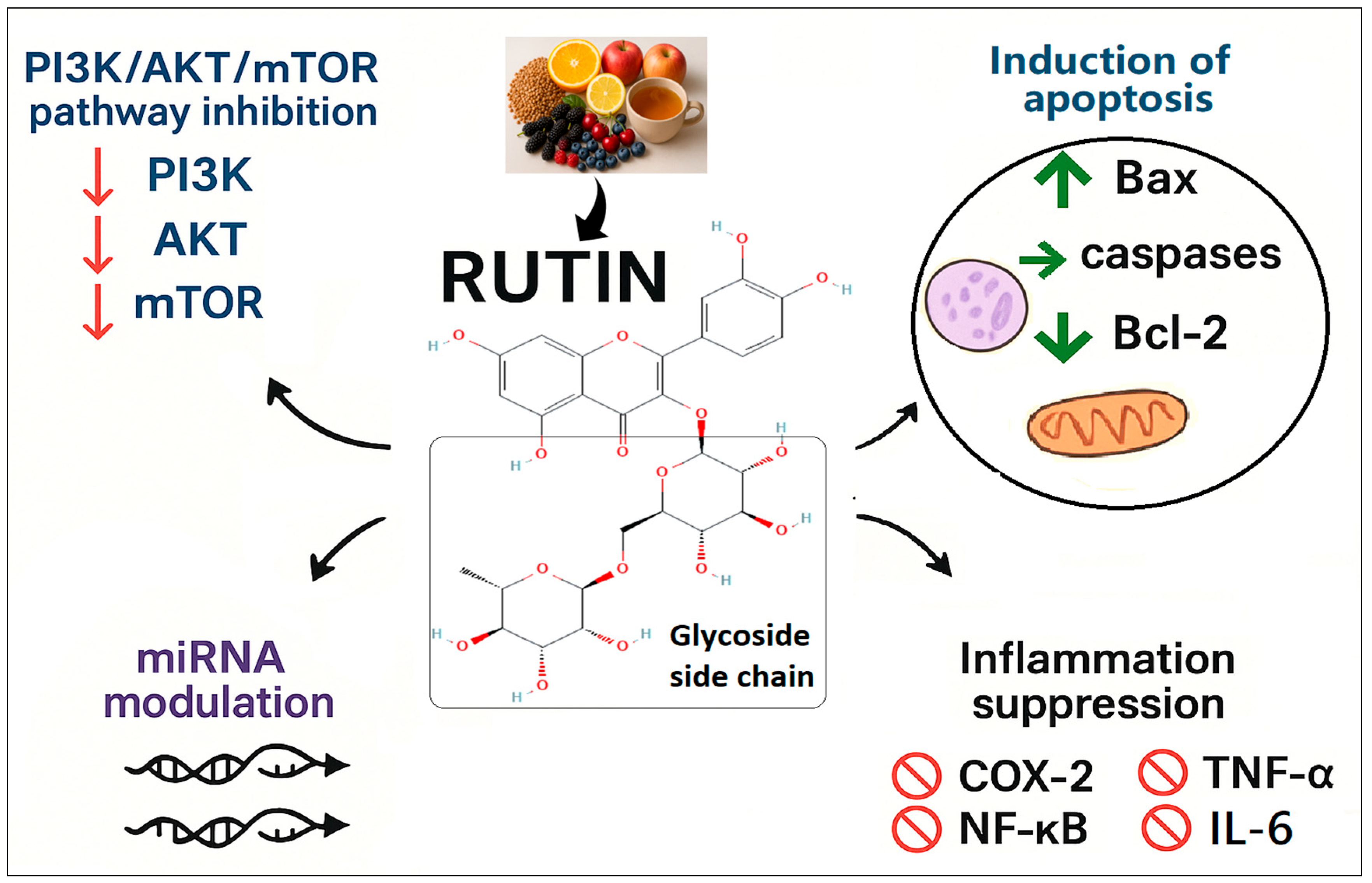
3. Discussion
4. Materials and Methods
4.1. Preparation of Rutin-Loaded Phytosome Nanoparticles (RT-PNPs)
4.2. Induction of EAC in Mice
4.3. Animal Handling and Experimental Protocol
4.4. Sample Collection
4.5. Hematological Profile Analysis
4.6. Serum Biochemical Analysis
4.7. Quantification of Tumor Volume and Viable Cell Count in Ascitic Fluid
4.8. Antioxidant Status
4.9. Expression Levels of P53, Bax, and Bcl2 Genes
4.10. Flow Cytometric Analysis of p53 and Ki-67 Expression
4.11. Histopathological Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ALP | Alkaline phosphatase |
| AFP | Alpha fetoprotein |
| AST | Aspartate aminotransferase |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| CA-125 | Cancer antigen 125 |
| CA 15-3 | Cancer antigen 15-3 |
| CA 19-9 | Cancer antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CAT | Catalase |
| cDNA | Complementary DNA |
| COX-2 | Cyclooxygenase-2 |
| DLS | Dynamic light scattering |
| EAC | Ehrlich ascites carcinoma |
| ELFA | Enzyme-linked fluorescent assay |
| FMO | Fluorescence minus one |
| GSH | Glutathione (reduced) |
| GSH-Px | Glutathione peroxidase |
| GSK-3β | Glycogen synthase kinase-3 beta |
| H&E | Hematoxylin and eosin |
| Hb | Hemoglobin |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| MDA | Malondialdehyde |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MCV | Mean corpuscular volume |
| MFI | Mean fluorescence intensity |
| MST | Mean survival time |
| miRNAs | MicroRNAs |
| NCI | National Cancer Institute |
| NF-κB | Nuclear factor kappa B |
| PCV | Packed cell volume |
| PLT | Platelet count |
| PDI | Polydispersity index |
| KCl | Potassium chloride |
| PCA | Principal component analysis |
| RBC | Red blood cell |
| RT | Rutin |
| RT-PNPs | Rutin-loaded phytosome nanoparticles |
| SOD | Superoxide dismutase |
| TBARS | Thiobarbituric acid reactive substances |
| TC | Total cholesterol |
| TP | Total protein |
| TEM | Transmission electron microscopy |
| TGs | Triglycerides |
| TNF-α | Tumor necrosis factor-alpha |
| WBC | White blood cell |
References
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Manzari-Tavakoli, A.; Babajani, A.; Tavakoli, M.M.; Safaeinejad, F.; Jafari, A. Integrating natural compounds and nanoparticle-based drug delivery systems: A novel strategy for enhanced efficacy and selectivity in cancer therapy. Cancer Med. 2024, 13, e7010. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Sayed, H.M.; Said, M.M.; Morcos, N.Y.S.; El Gawish, M.A.; Ismail, A.F.M. Antitumor and Radiosensitizing Effects of Zinc Oxide-Caffeic Acid Nanoparticles against Solid Ehrlich Carcinoma in Female Mice. Integr. Cancer Ther. 2021, 20, 15347354211021920. [Google Scholar] [CrossRef]
- Yılmaz, S.; Doğanyiğit, Z.; Oflamaz, A.O.; Ateş, Ş.; Söylemez, E.S.A.; Nisari, M.; Farooqı, A.A. Determination of Rutin’s antitumoral effect on EAC solid tumor by AgNOR count and PI3K/AKT/mTOR signaling pathway. Med. Oncol. 2023, 40, 131. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Nafees, S.; Mehdi, S.H.; Zafaryab, M.; Zeya, B.; Sarwar, T.; Rizvi, M.A. Synergistic Interaction of Rutin and Silibinin on Human Colon Cancer Cell Line. Arch. Med. Res. 2018, 49, 226–234. [Google Scholar] [CrossRef]
- Huang, F.M.; Chang, Y.C.; Lee, M.W.; Su, N.Y.; Yang, L.C.; Kuan, Y.H. Rutin alleviates bisphenol A-glycidyl methacrylate-induced generation of proinflammatory mediators through the MAPK and NF-κB pathways in macrophages. Environ. Toxicol. 2023, 38, 628–634. [Google Scholar] [CrossRef]
- Dos Santos, C.A.L.; de Araújo Monteiro, A.A.; da Silva, P.A.G.; Kamdem, J.P.; Duarte, A.E.; Almutairi, M.M.; Ali, A.; Anwar, S.; Ibrahim, M. Protective capacity of Rutin against oxidative damage induced by saline stress in the roots of the model organism Allium cepa. Sci. Rep. 2025, 15, 24447. [Google Scholar] [CrossRef]
- Przybylski, T.; Czerniel, J.; Dobrosielski, J.; Stawny, M. Flavonol Technology: From the Compounds’ Chemistry to Clinical Research. Molecules 2025, 30, 3113. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, S.; Amjadi-Moheb, F.; Tabaripour, R.; Ashrafi, G.H.; Akhavan-Niaki, H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020, 262, 118513. [Google Scholar] [CrossRef] [PubMed]
- Talebi, H.; Farahpour, M.R.; Hamishehkar, H. The effectiveness of Rutin for prevention of surgical induced endometriosis development in a rat model. Sci. Rep. 2021, 11, 7180. [Google Scholar] [CrossRef]
- Zargar, S.; Altwaijry, N.; Wani, T.A.; Alkahtani, H.M. Evaluation of the Possible Pathways Involved in the Protective Effects of Quercetin, Naringenin, and Rutin at the Gene, Protein and miRNA Levels Using In-Silico Multidimensional Data Analysis. Molecules 2023, 28, 4904. [Google Scholar] [CrossRef]
- Muvhulawa, N.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.H.; Mthiyane, F.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. Rutin ameliorates inflammation and improves metabolic function: A comprehensive analysis of scientific literature. Pharmacol. Res. 2022, 178, 106163. [Google Scholar] [CrossRef]
- Gęgotek, A.; Ambrożewicz, E.; Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch. Dermatol. Res. 2019, 311, 203–219. [Google Scholar] [CrossRef]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Barani, M.; Sangiovanni, E.; Angarano, M.; Rajizadeh, M.A.; Mehrabani, M.; Piazza, S.; Gangadharappa, H.V.; Pardakhty, A.; Mehrbani, M.; Dell’Agli, M.; et al. Phytosomes as Innovative Delivery Systems for Phytochemicals: A Comprehensive Review of Literature. Int. J. Nanomed. 2021, 16, 6983–7022. [Google Scholar] [CrossRef]
- Radulski, D.; Stipp, M.; Galindo, C.; Acco, A. Features and applications of Ehrlich tumor model in cancer studies: A literature review. Transl. Breast Cancer Res. 2023, 4, 22. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhao, Y.-G.; Zhang, Y. Enhancing Drug Solubility, Bioavailability, and Targeted Therapeutic Applications through Magnetic Nanoparticles. Molecules 2024, 29, 4854. [Google Scholar] [CrossRef]
- Oladipo, A.O.; Lebelo, S.L.; Msagati, T.A.M. Nanocarrier design–function relationship: The prodigious role of properties in regulating biocompatibility for drug delivery applications. Chem. Biol. Interact. 2023, 377, 110466. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Almughem, F.A.; Almehmady, A.M.; Jarallah, S.J.; Alsharif, W.K.; Alzahrani, N.M.; Alshehri, A.A. Phytosomes as an Emerging Nanotechnology Platform for the Topical Delivery of Bioactive Phytochemicals. Pharmaceutics 2021, 13, 1475. [Google Scholar] [CrossRef]
- Mohamed, W. The antitumor activity of Arthrospira platensis and/or cisplatin in a murine model of Ehrlich ascites carcinoma with hematinic and hepato-renal protective action. J. Funct. Foods 2020, 66, 103831. [Google Scholar] [CrossRef]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Rahmani, S.; Naraki, K.; Roohbakhsh, A.; Hayes, A.W.; Karimi, G. The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Sci. Nutr. 2023, 11, 39–56. [Google Scholar] [CrossRef]
- Savas, I.N.; Coskun, A. The Future of Tumor Markers: Advancing Early Malignancy Detection Through Omics Technologies, Continuous Monitoring, and Personalized Reference Intervals. Biomolecules 2025, 15, 1011. [Google Scholar] [CrossRef]
- Turlej, E.; Domaradzka, A.; Radzka, J.; Drulis-Fajdasz, D.; Kulbacka, J.; Gizak, A. Cross-Talk Between Cancer and Its Cellular Environment—A Role in Cancer Progression. Cells 2025, 14, 403. [Google Scholar] [CrossRef]
- Elsadek, M.F.; Essam El-Din, M.M.; Ahmed, B.M. Evaluation of anticarcinogenic and antioxidant properties of Eruca sativa extracts versus Ehrlich ascites carcinoma in mice. J. King Saud Univ. Sci. 2021, 33, 101435. [Google Scholar] [CrossRef]
- Frost, Z.; Bakhit, S.; Amaefuna, C.; Powers, R.; Ramana, K. Recent Advances on the Role of B Vitamins in Cancer Prevention and Progression. Int. J. Mol. Sci. 2025, 26, 1967. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Allam, T.; Korany, R.M.S.; Abdelfattah, A.M.; Omran, A.M.; Abd Eldaim, M.A.; Hassan, A.M.; El-Borai, N.B. Protective and Therapeutic Efficacy of Hesperidin versus Cisplatin against Ehrlich Ascites Carcinoma-Induced Renal Damage in Mice. Pharmaceuticals 2022, 15, 294. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, S.; Yang, L.; Song, P.; Liu, Z.; Liu, X.; Yan, X.; Dong, Q. Roles of reactive oxygen species in inflammation and cancer. MedComm 2024, 5, e519. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.A.; El Raey, M.A.; Ibrahim, A.Y.; Abdelfattah, M.A.O.; Abdelmageed, A.M.; Sobeh, M. A sulfated polyphenols-rich extract from Sabal yapa exhibits antitumor activities in Ehrlich ascites carcinoma. Saudi J. Biol. Sci. 2021, 28, 3117–3125. [Google Scholar] [CrossRef]
- Han, X.; Xue, X.; Zhao, Y.; Li, Y.; Liu, W.; Zhang, J.; Fan, S. Rutin-Enriched Extract from Coriandrum sativum L. Ameliorates Ionizing Radiation-Induced Hematopoietic Injury. Int. J. Mol. Sci. 2017, 18, 942. [Google Scholar] [CrossRef]
- Hussein, J.; El-khayat, Z.; Farouk, H. Antitumor Activity of Selenium in Ehrlich Ascites Carcinoma Bearing Mice. Biomed. Pharmacol. J. 2023, 16, 1353–1364. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Zhou, X.; Prochownik, E.V.; Wang, F.; Li, Y. Posttranslational control of lipogenesis in the tumor microenvironment. J. Hematol. Oncol. 2022, 15, 120. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Gad El Hak, H.N.; Nafie, M.S.; Saad, R.; Abdelrazek, H.M.A.; Kilany, O.E. Evaluation of antioxidant, anti-inflammatory, anticancer activities and molecular docking of Moringa oleifera seed oil extract against experimental model of Ehrlich ascites carcinoma in Swiss female albino mice. BMC Complement. Med. Ther. 2023, 23, 457. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Anwar, S.; Sarwar, T.; Khan, A.A.; Rahmani, A.H. Therapeutic Applications and Mechanisms of Superoxide Dismutase (SOD) in Different Pathogenesis. Biomolecules 2025, 15, 1130. [Google Scholar] [CrossRef]
- Grujicic, J.; Allen, A.R. Manganese Superoxide Dismutase: Structure, Function, and Implications in Human Disease. Antioxidants 2025, 14, 848. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Rupasinghe, H.P.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Emeka, P.M.; Badger-Emeka, L.I.; Thirugnanasambantham, K.; Alatawi, A.S. Rutin-Activated Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Attenuates Corneal and Heart Damage in Mice. Pharmaceuticals 2024, 17, 1523. [Google Scholar] [CrossRef]
- Donia, T.I.K.; Gerges, M.N.; Mohamed, T.M. Amelioration effect of Egyptian sweet orange hesperidin on Ehrlich ascites carcinoma (EAC) bearing mice. Chem. Biol. Interact. 2018, 285, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Abd Eldaim, M.A.; Tousson, E.; El Sayed, I.E.T.; Abd Elmaksoud, A.Z.; Ahmed, A.A.S. Ameliorative effects of 9-diaminoacridine derivative against Ehrlich ascites carcinoma-induced hepatorenal injury in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 21835–21850. [Google Scholar] [CrossRef] [PubMed]
- Abosharaf, H.A.; Gebreel, D.T.; Allam, S.; El-Atrash, A.; Tousson, E. Ehrlich ascites carcinoma provokes renal toxicity and DNA injury in mice: Therapeutic impact of chitosan and maitake nanoparticles. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Jelic, M.; Mandic, A.; Maricic, S.; Srdjenovic, B. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2019, 17, 22–28. [Google Scholar] [CrossRef]
- Dragoș, D.; Enache, I.I.; Manea, M.M. Oxidative Stress and Nutritional Antioxidants in Renal Diseases: A Narrative Review. Antioxidants 2025, 14, 757. [Google Scholar] [CrossRef]
- Medhat, D.; Hussein, J.; El-Naggar, M.E.; Attia, M.F.; Anwar, M.; Latif, Y.A.; Booles, H.F.; Morsy, S.; Farrag, A.R.; Khalil, W.K.B.; et al. Effect of Au-dextran NPs as anti-tumor agent against EAC and solid tumor in mice by biochemical evaluations and histopathological investigations. Biomed. Pharmacother. 2017, 91, 1006–1016. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Roberti, A.; Chaffey, L.E.; Greaves, D.R. NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology 2022, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; Tousson, E.; El-Masry, T.A.; Altwaijry, N.; Saleh, A. Ehrlich ascites carcinoma as model for studying the cardiac protective effects of curcumin nanoparticles against cardiac damage in female mice. Environ. Toxicol. 2021, 36, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Sannappa Gowda, N.G.; Shiragannavar, V.D.; Prabhuswamimath, S.C.; Tuladhar, S.; Chidambaram, S.B.; Santhekadur, P.K. Ehrlich Ascites carcinoma mice model for studying liver inflammation and fibrosis. Adv. Cancer Biol. Metastasis 2022, 4, 100029. [Google Scholar] [CrossRef]
- Eltahir, Z.; Ibrahim, M.; Mohieldeen, M.Y.; Bayoumi, A.; Ahmed, S.M. Thymoquinone Nanoparticles (TQ-NPs) in Kidney Toxicity Induced by Ehrlich Ascites Carcinoma (EAC): An In Vivo Study. Can. J. Kidney Health Dis. 2024, 11, 20543581241258812. [Google Scholar] [CrossRef]
- Silva-Santana, G.; Bax, J.C.; Fernandes, D.C.S.; Bacellar, D.T.L.; Hooper, C.; Dias, A.; Silva, C.B.; de Souza, A.M.; Ramos, S.; Santos, R.A.; et al. Clinical hematological and biochemical parameters in Swiss, BALB/c, C57BL/6 and B6D2F1 Mus musculus. Anim. Model. Exp. Med. 2020, 3, 304–315. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; Tulbah, F.S.A.; El-ghor, A.A.; Eissa, S.M. Suppression of tumor growth and apoptosis induction by pomegranate seed nano-emulsion in mice bearing solid Ehrlich carcinoma cells. Sci. Rep. 2023, 13, 5525. [Google Scholar] [CrossRef]
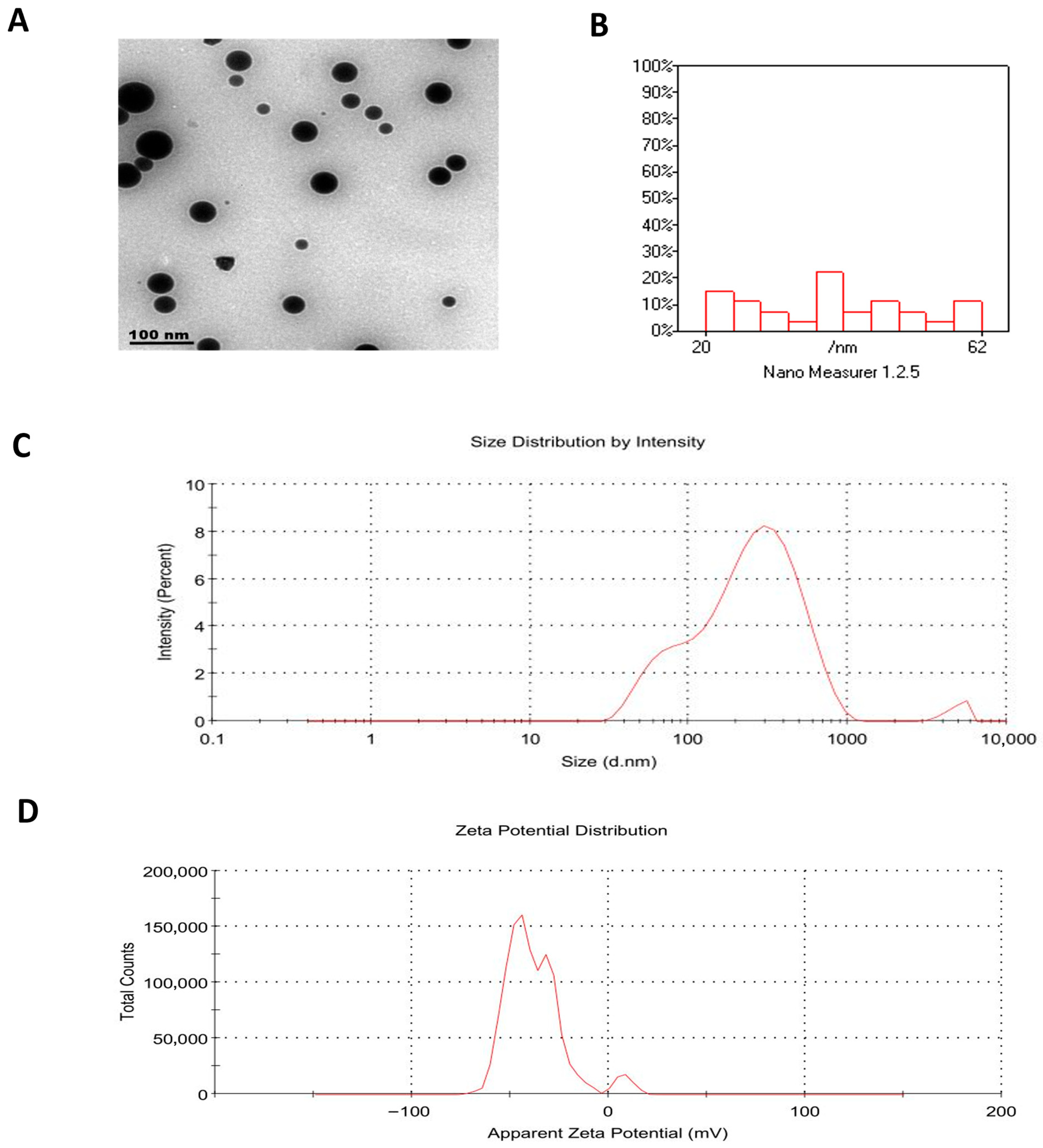

| Formulations | PC: RT Molar Ratio | Cholesterol (% w/w of Total Lipid) | PDI | Particle Size (nm, DLS) | Zeta Potential (mV) | Encapsulation Efficiency (EE%) |
|---|---|---|---|---|---|---|
| F1 | 1:1 | 0% | 0.471 | 262 | −22.3 | 66.31 |
| F2 | 1:1 | 5% | 0.447 | 231 | −27.9 | 71.52 |
| F3 | 1:1 | 10% | 0.423 | 224 | −29.5 | 74.03 |
| F4 | 1:2 | 0% | 0.401 | 201 | −32.4 | 79.24 |
| F5 | 1:2 | 5% | 0.392 | 185 | −37.0 | 84.62 |
| F6 | 1:2 | 10% | 0.410 | 193 | −35.2 | 83.14 |
| F7 | 1:3 | 5% | 0.452 | 214 | −31.1 | 78.47 |
| Parameters | Control | RT | RT-PNPs | EAC | EAC/RT | EAC/RT-PNPs |
|---|---|---|---|---|---|---|
| RBCs (106/μL) | 7.14 ± 0.29 a | 7.22 ± 0.34 a | 7.26 ± 0.39 a | 4.15 ± 0.22 c | 5.21 ± 0.28 b | 6.85 ± 0.30 a |
| Hb (g/dL) | 13.8 ± 0.62 a | 14.02 ± 0.59 a | 14.21 ± 0.44 a | 8.84 ± 0.48 b | 10.23 ± 0.53 b | 12.36 ± 0.76 a |
| PLT (×103/µL) | 534.12 ± 6.23 a | 539.37 ± 7.14 a | 540.23 ± 7.25 a | 336.21 ± 6.36 c | 465.21 ± 8.96 b | 520.39 ± 10.15 a |
| PCV (%) | 46.25 ± 2.33 ab | 47.21 ± 3.15 ab | 48.53 ± 4.24 a | 30.21 ± 2.20 c | 36.26 ± 3.02 bc | 42.58 ± 5.15 ab |
| WBC (103/μL) | 8.62 ± 0.32 b | 8.56 ± 0.29 b | 8.44 ± 0.35 b | 14.21 ± 0.69 a | 9.32 ± 0.78 b | 9.03 ± 0.68 b |
| MCH (pg) | 19.32 ± 0.08 b | 19.42 ± 0.09 b | 19.57 ± 0.44 b | 21.30 ± 0.03 a | 19.63 ± 0.04 b | 18.03 ± 0.32 c |
| MCHC (%) | 34.69 ± 2.03 a | 35.21 ± 3.45 a | 36.23 ± 3.92 a | 31.15 ± 4.63 a | 31.16 ± 2.88 a | 34.22 ± 3.42 a |
| MCV (fl) | 55.82 ± 3.03 a | 55.53 ± 5.74 a | 54.58 ± 7.18 a | 69.40 ± 10.34 a | 63.38 ± 6.01 a | 52.99 ± 4.38 a |
| Parameters | Control | RT | RT-PNPs | EAC | EAC/RT | EAC/RT-PNPs |
|---|---|---|---|---|---|---|
| TP (g/dL) | 6.74 ± 0.31 a | 6.82 ± 0.29 a | 6.91 ± 0.32 a | 4.37 ± 0.19 c | 5.44 ± 0.21 b | 6.21 ± 0.28 ab |
| Glo (g/dL) | 2.77 ± 0.17 a | 2.81 ± 0.18 a | 2.72 ± 0.22 a | 2.09 ± 0.13 b | 2.13 ± 0.03 b | 2.67 ± 0.12 a |
| Alb (g/dL) | 3.97 ± 0.14 a | 4.01 ± 0.11 a | 4.19 ± 0.10 a | 2.28 ± 0.06 c | 3.31 ± 0.18 b | 3.54 ± 0.16 b |
| TG (mg/dL) | 86.44 ± 2.29 c | 83.32 ± 3.55 c | 84.57 ± 4.14 c | 153.32 ± 5.25 a | 133.62 ± 4.93 b | 97.41 ± 5.37 c |
| TC (mg/dL) | 118.41 ± 3.23 c | 121.12 ± 4.12 c | 123.27 ± 4.97 c | 183.33 ± 5.33 a | 144.54 ± 4.36 b | 124.03 ± 3.97 c |
| Cr (mg/dL) | 0.63 ± 0.06 b | 0.62 ± 0.04 b | 0.59 ± 0.07 b | 1.29 ± 0.10 a | 0.84 ± 0.09 b | 0.76 ± 0.08 b |
| Urea (mg/dL) | 27.71 ± 1.63 d | 26.32 ± 2.55 d | 25.41 ± 1.83 d | 59.32 ± 2.11 a | 52.13 ± 3.03 b | 35.27 ± 2.37 c |
| ALP (U/L) | 84.74 ± 3.78 cd | 82.12 ± 2.65 cd | 79.49 ± 3.44 d | 157.41 ± 5.12 a | 123.26 ± 4.16 b | 93.34 ± 5.02 c |
| ALT (U/L) | 39.64 ± 2.30 c | 36.63 ± 2.76 c | 35.24 ± 3.63 c | 89.94 ± 4.16 a | 63.37 ± 3.19 b | 42.32 ± 3.90 c |
| AST (U/L) | 67.41 ± 2.53 c | 65.27 ± 2.37 c | 64.46 ± 1.93 c | 117.12 ± 3.68 a | 96.32 ± 5.32 b | 74.52 ± 4.12 c |
| Gene | Sequences (5′-3′) | Length (bp) |
|---|---|---|
| TP53 | F: CCCCTGTCATCTTTTGTCCCT R: AGCTGGCAGAATAGCTTATTGAG | 137 |
| Bcl-2 | F: CATCGCCCTGTGGATGACTG R: GGCCATATAGTTCCACAAAGGC | 95 |
| Bax | F: GTCTCCGGCGAATTGGAGAT R: ACCCGGAAGAAGACCTCTCG | 100 |
| GAPDH | F: GTATCGGACGCCTGGTTAC R: CTTGCCGTGGGTAGAGTCAT | 128 |
| Score | Integrated Scoring System for Semi-Quantitative Assessment of Hepatic Lesions |
|---|---|
| 0 (none) | Normal histological architecture was observed. |
| 1 (mild) | Occasional hepatocellular degeneration and necrosis were observed, with minimal to absent inflammatory infiltration and rare vascular congestion. |
| 2 (moderate) | Hepatic tissue displayed moderate vacuolar degeneration, multifocal hepatocyte necrosis, scattered inflammatory cell infiltration, and mild congestion of blood vessels. |
| 3 (severe) | Hepatic tissue exhibited diffuse and severe degenerative changes, extensive hepatocellular necrosis, widespread leukocytic infiltration, and moderate to severe congestion of hepatic vasculature. |
| Score | Degenerative and Necrotic Changes in Renal Tubules | Structural Changes in Glomeruli | Inflammation | Hemorrhage |
|---|---|---|---|---|
| 0 | None | None | None | None |
| 1 | Histopathological examination revealed degenerative changes in 1 to 2 out of 12 fields analyzed. | Mild glomerular atrophy was confined to 1–2 out of 12 assessed fields. | Histological evaluation revealed mild inflammation in 1 to 2 of the 12 assessed areas. | Histological analysis revealed mild glomerular and interstitial congestion with infrequent hemorrhage in 1 to 2 of the 12 evaluated sections. |
| 2 | Histological analysis revealed degeneration with multifocal epithelial sloughing in 3 to 6 out of 12 fields. | Mild to moderate glomerular shrinkage appeared in 3 to 4 of the 12 inspected sections. | Histological examination revealed moderate inflammation in 3 to 4 of the 12 assessed areas. | Histopathological evaluation revealed low-grade interstitial congestion and focal hemorrhagic areas in 3 to 4 out of 12 fields. |
| 3 | Histopathological assessment revealed widespread tubular necrosis in 7 to 9 of the 12 examined areas. | Evidence of lamellar fusion was detected in 5 to 6 of the 12 inspected fields. | Histopathological analysis revealed inflammation in 5–7 of the 12 inspected fields. | Histopathological assessment revealed widespread interstitial congestion and hemorrhagic foci in 5 to 6 out of 12 tissue sections. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfawaz, M.; Elmorsy, E.M.; Samy, A.; Shams, A.S.; Salem, M.A.; Shaalan, A.A.M.; Fawzy, M.S.; Hosny, N. Therapeutic Potential of Food-Derived Rutin Phytosome Nanoparticles: Anti-Tumor, Antioxidant, and Anti-Inflammatory Activity in Ehrlich Ascites Carcinoma. Pharmaceuticals 2025, 18, 1410. https://doi.org/10.3390/ph18091410
Alfawaz M, Elmorsy EM, Samy A, Shams AS, Salem MA, Shaalan AAM, Fawzy MS, Hosny N. Therapeutic Potential of Food-Derived Rutin Phytosome Nanoparticles: Anti-Tumor, Antioxidant, and Anti-Inflammatory Activity in Ehrlich Ascites Carcinoma. Pharmaceuticals. 2025; 18(9):1410. https://doi.org/10.3390/ph18091410
Chicago/Turabian StyleAlfawaz, M., Ekramy M. Elmorsy, Alaa Samy, Ahmed S. Shams, Mai A. Salem, Aly A. M. Shaalan, Manal S. Fawzy, and Nora Hosny. 2025. "Therapeutic Potential of Food-Derived Rutin Phytosome Nanoparticles: Anti-Tumor, Antioxidant, and Anti-Inflammatory Activity in Ehrlich Ascites Carcinoma" Pharmaceuticals 18, no. 9: 1410. https://doi.org/10.3390/ph18091410
APA StyleAlfawaz, M., Elmorsy, E. M., Samy, A., Shams, A. S., Salem, M. A., Shaalan, A. A. M., Fawzy, M. S., & Hosny, N. (2025). Therapeutic Potential of Food-Derived Rutin Phytosome Nanoparticles: Anti-Tumor, Antioxidant, and Anti-Inflammatory Activity in Ehrlich Ascites Carcinoma. Pharmaceuticals, 18(9), 1410. https://doi.org/10.3390/ph18091410







