Inflammation-Driven Genomic Instability: A Pathway to Cancer Development and Therapy Resistance
Abstract
1. Introduction
2. Inflammation-Induced DNA Damage Mechanisms
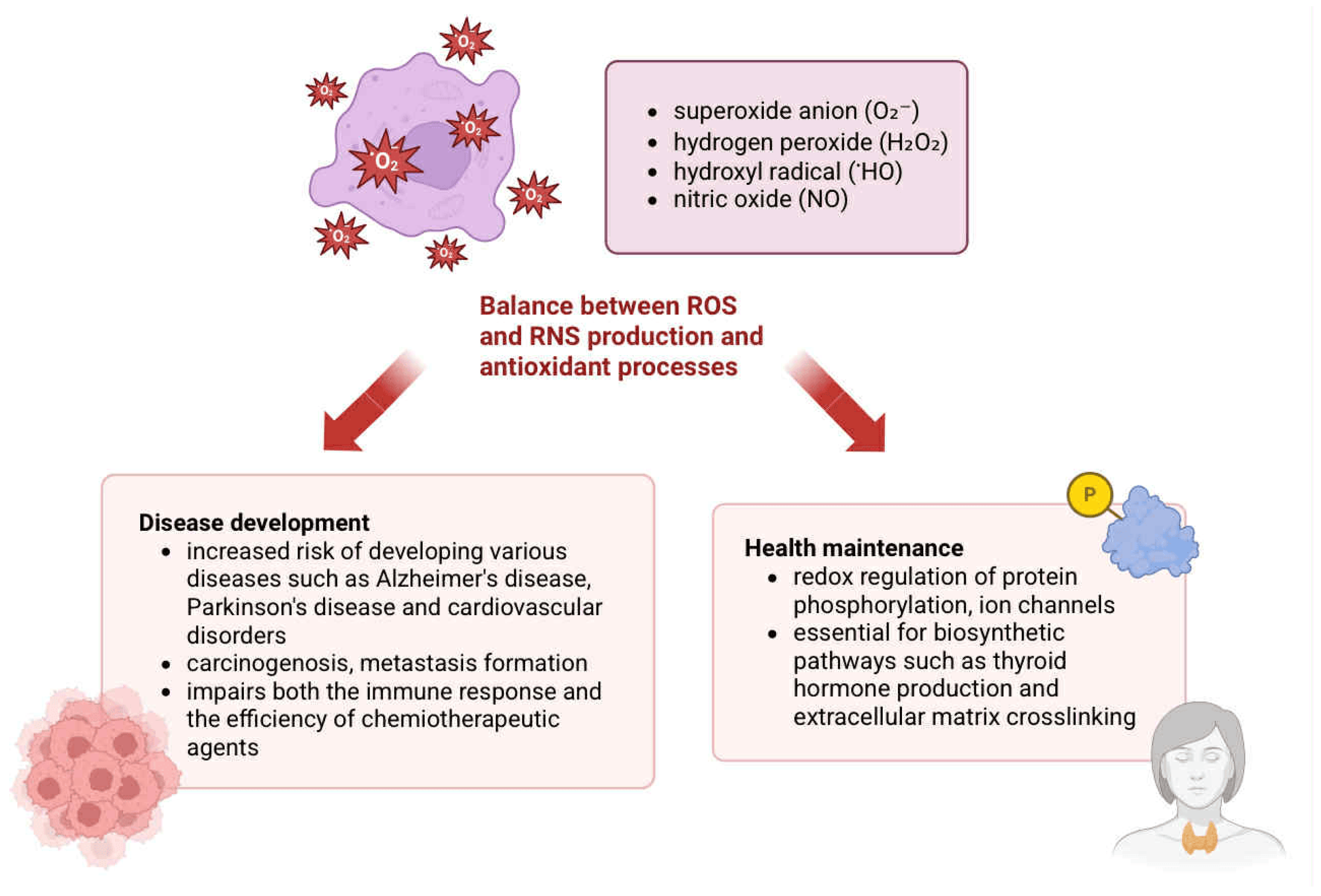
2.1. Generation and Physiological Role of Reactive Species
2.2. Oxidative and Nitrosative Modifications of Nucleic Acids
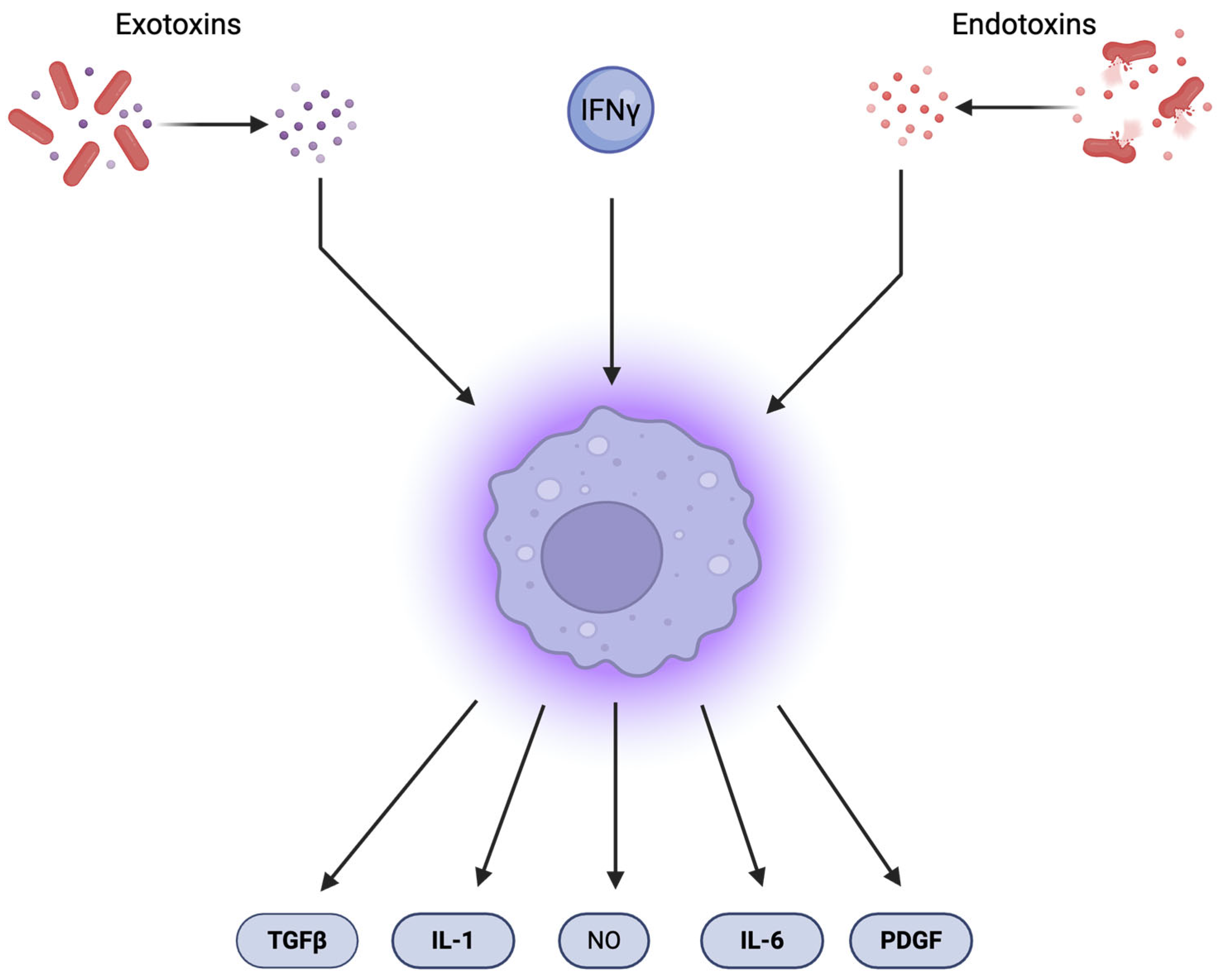
2.3. Immune-Mediated Induction of DNA Damage
3. Influence of Inflammatory Signaling Pathways on DNA Repair
3.1. Inflammatory Mediators and the Modulation of DNA Damage Response
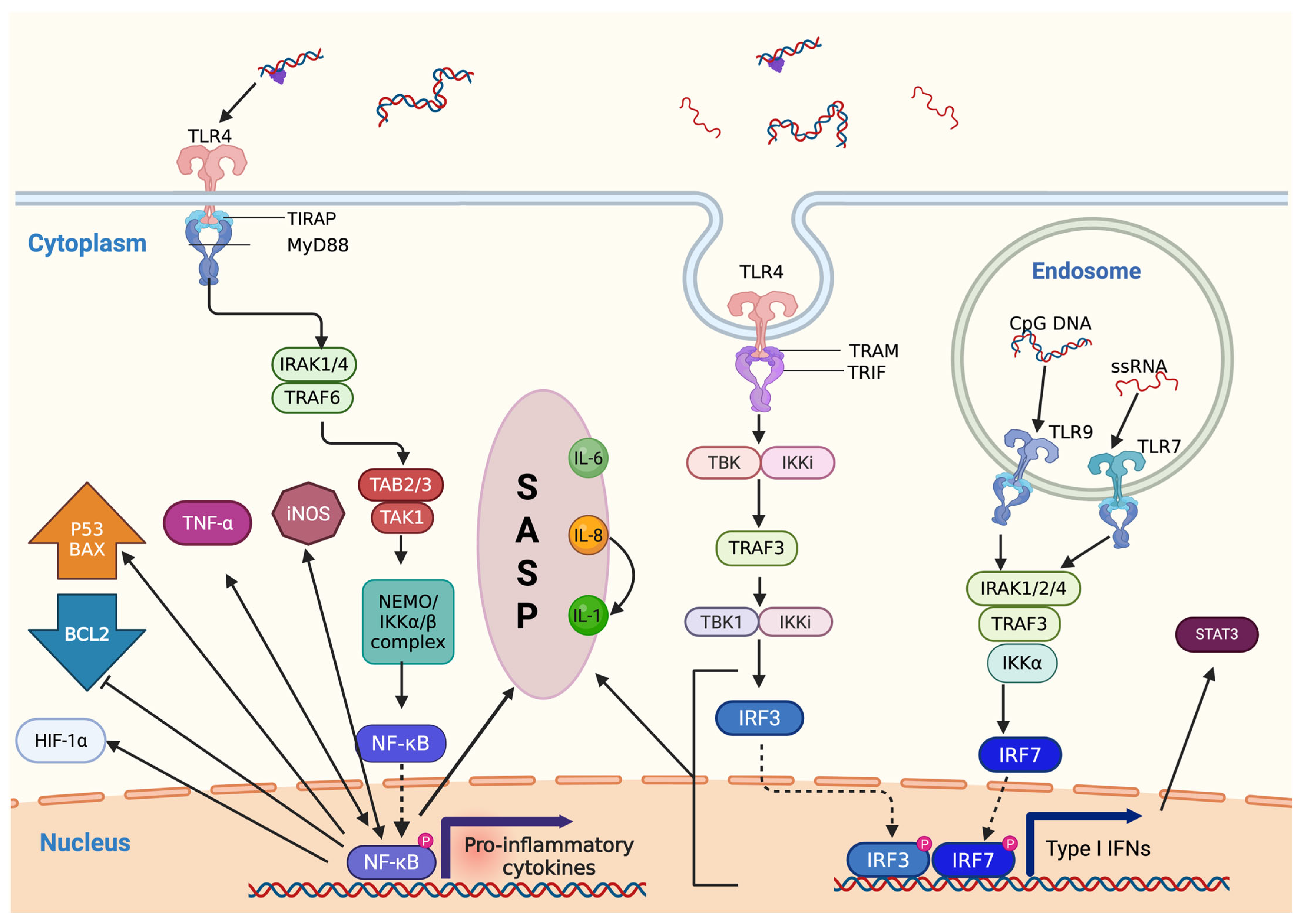
3.2. Activation of NF-κB Signaling and Its Multifaceted Role in DDR
3.3. Senescence-Associated Secretory Phenotype and Cytokine Feedback Loops
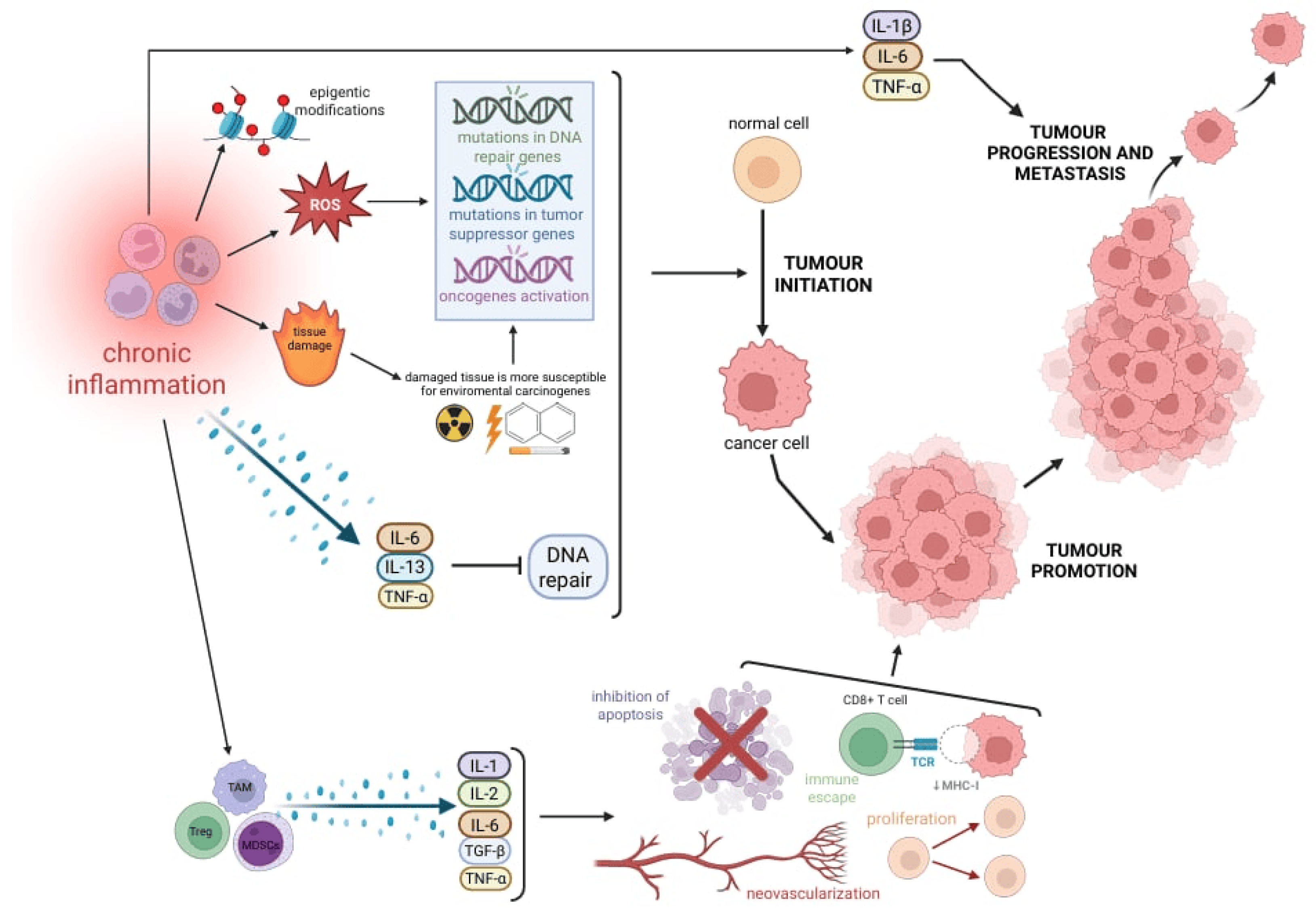
4. Role of Immune Cells in Promoting Tumorigenesis
4.1. Inflammation-Induced Genomic Instability and DNA Repair Suppression
4.2. IRAK-1 and NF-κB Signaling in Immune-Mediated Tumor Promotion
4.3. Epigenetic Modifications, EMT, and Pro-Tumor Immune Cells
5. Therapeutic Strategies to Target Chronic Inflammation in Cancer
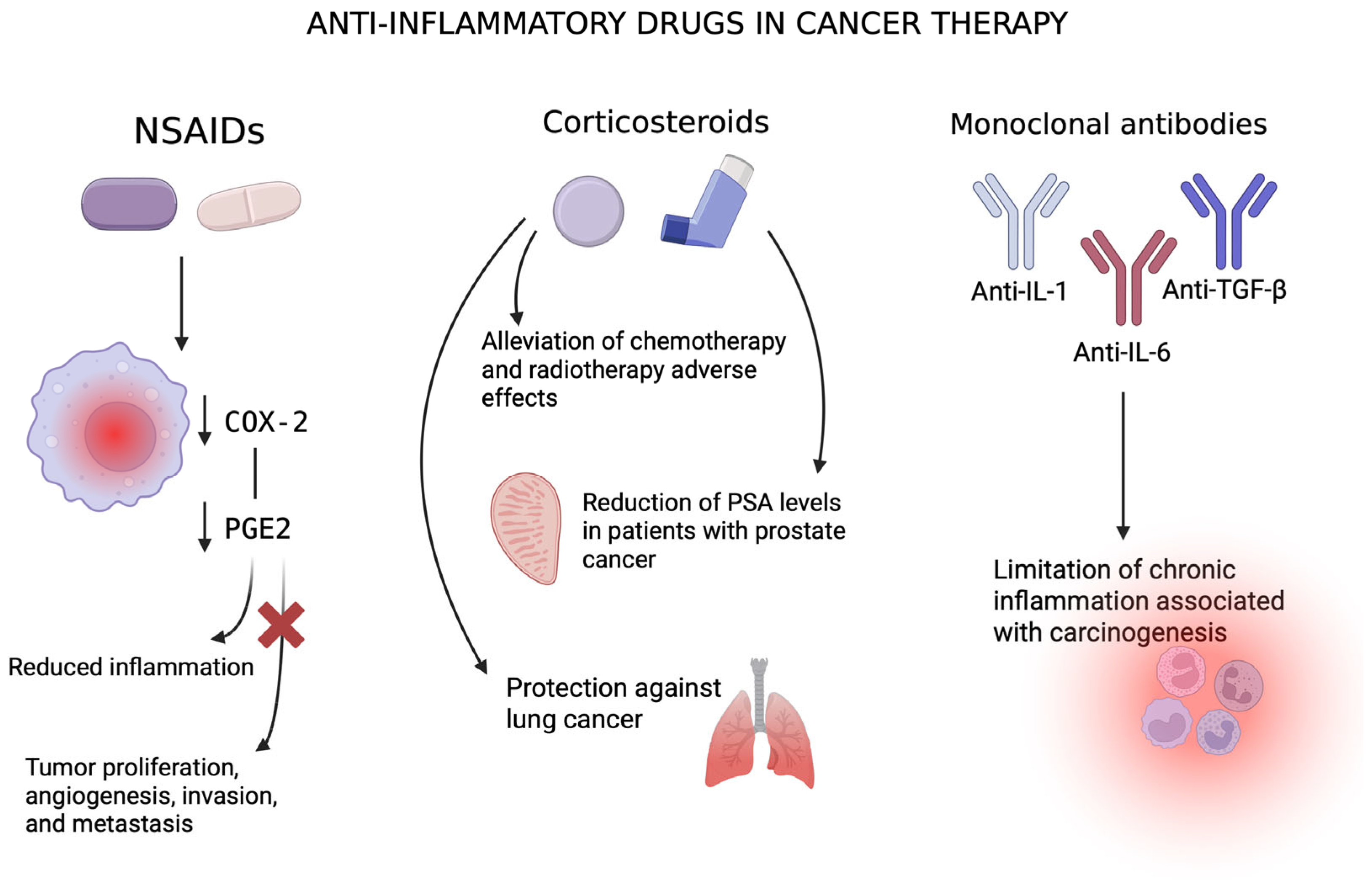
5.1. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
5.2. Corticosteroids
5.3. Monoclonal Antibodies and Anticytokine Therapies
5.4. Challenges and Future Perspectives
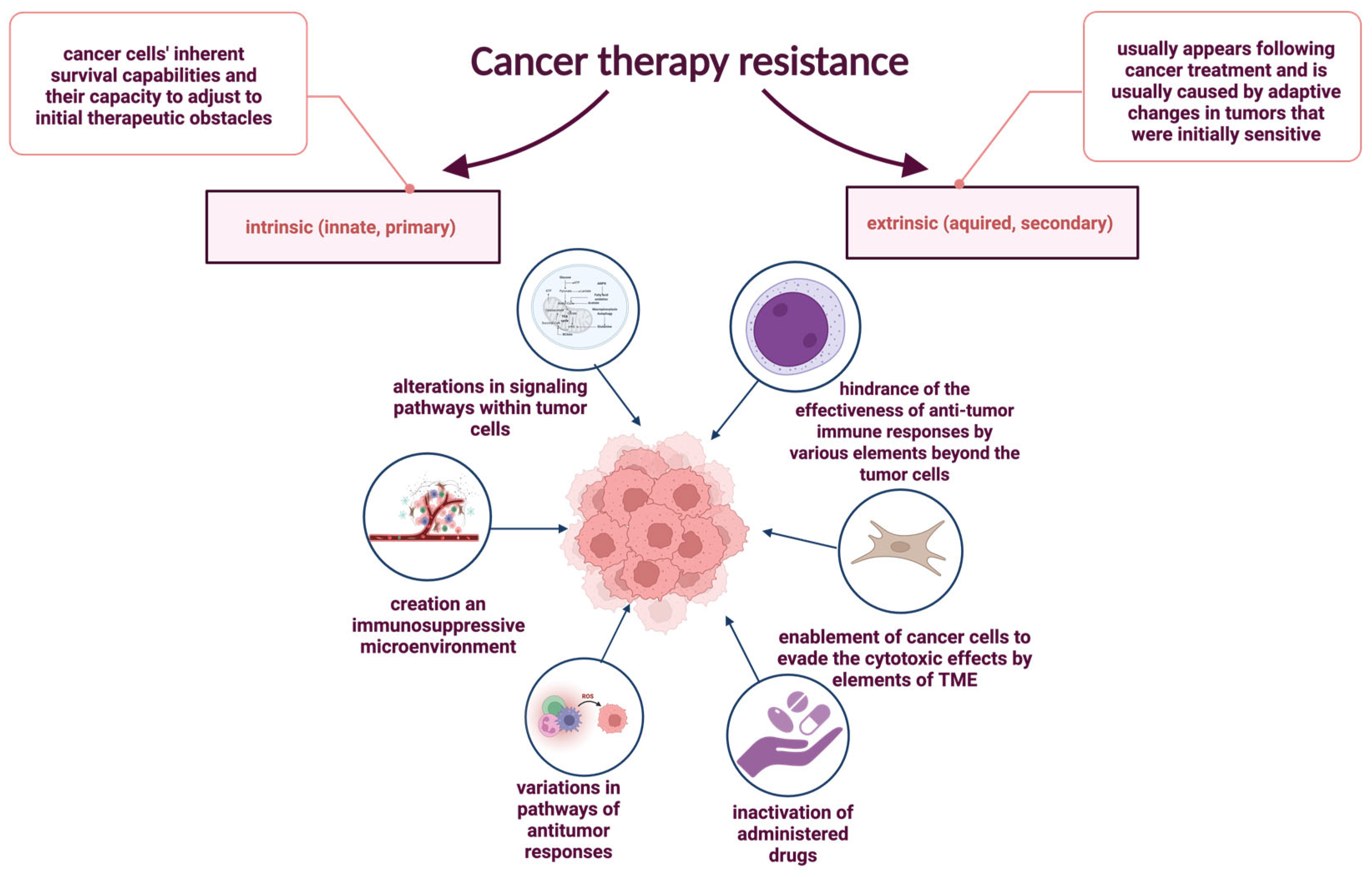
6. Impact of Chronic Inflammation on Cancer Therapy Resistance
6.1. Intrinsic Resistance Mechanisms and Inflammatory Signaling
6.2. Extrinsic Resistance and Tumor Microenvironment (TME) Interactions
6.3. Inflammatory Modulation of Drug Metabolism
6.4. Inflammation-Induced Radioresistance
6.5. Inflammation-Induced Resistance to Immunotherapy
7. Summary, Future Perspectives, and Alternative Hypotheses
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| 8-oxoG | 8-hydroxyguanine |
| AKT | protein kinase B |
| anti-IL-1 | antibody against interleukin-1 |
| anti-IL-1R1 | antibody against interleukin-1 receptor type 1 |
| anti-PD1/L1 | antibody against programmed death receptor 1/ligand 1 |
| ATM | ataxia-telangiectasia mutated |
| BAX | Bcl-2-associated protein X |
| BCL2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra large |
| BER | base excision repair |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| BRCA | breast cancer |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| CAFs | cancer-associated fibroblasts |
| CCL2 | C-C motif chemokine ligand 2 |
| CCL5 | C-C motif chemokine ligand 5 |
| CCR2 | C-C motif chemokine receptor 2 |
| CD40L | CD40 ligand |
| CDK2 | cyclin-dependent kinase 2 |
| CEBP | CCAAT/enhancer-binding protein |
| c-FLIP | cellular FLICE-like inhibitory protein |
| cGAMP | cyclic guanosine monophosphate-adenosine monophosphate |
| cGAS | cyclic GMP-AMP synthase |
| cIAPs | cellular inhibitor of apoptosis proteins |
| c-myc | cellular myelocytomatosis oncogene |
| COPD | chronic obstructive pulmonary disease |
| COX | cyclo-oxygenase |
| CXCL-8 | C-X-C motif chemokine ligand 8 |
| CXCR-2 | C-X-C motif chemokine receptor 2 |
| CYP | cytochrome P450 |
| DDR | DNA damage response |
| DNMTs | DNA methyltransferases |
| DOT1L | disruptor of telomeric silencing 1-like |
| DSBs | DNA double-strand breaks |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| EVs | extracellular vesicles |
| GATA4 | GATA binding protein 4 |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| H2O2 | hydrogen peroxide |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| HO· | hydroxyl radical |
| HPV | human papillomavirus |
| ICB | immune checkpoint blockade |
| ICI | immune checkpoint inhibitor |
| IKK | IκB kinase |
| IL-1 | Interleukin-1 |
| IL-10 | Interleukin-10 |
| IL-12 | Interleukin-12 |
| IL-13 | Interleukin-13 |
| IL-1β | Interleukin-1 beta |
| IL-5 | Interleukin-5 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| INF-γ | interferon-γ |
| iNOS | inducible nitric oxide synthase |
| IRAK-1 | interleukin-1 receptor-associated kinase 1 |
| IRF3 | interferon regulatory factor 3 |
| ISG15 | IFN-stimulated gene 15 |
| JAK | Janus kinase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| MALT | mucosa-associated lymphoid tissue |
| MCL1 | myeloid cell leukemia-1 |
| MCP-1 | monocyte chemoattractant protein-1 |
| MDSCs | myeloid-derived suppressor cells |
| MHC-I | major histocompatibility complex class I |
| miR-146a | microRNA 146a |
| miRNAs | microRNAs |
| MLH1 | MutL homolog 1 |
| MMPs | matrix metalloproteinase |
| MMR | mismatch repair |
| MSH2 | MutL homolog 2 |
| MSH6 | MutL homolog 6 |
| MSI | microsatellite instability |
| MYC | myelocytomatosis oncogene |
| N2O3 | nitrous anhydride |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NEMO | NF-κB essential modulator |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | nitric oxide |
| NRAS | neuroblastoma RAS viral oncogene homolog |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| O2− | superoxide anion |
| OGG | 8-oxoguanine DNA glycosylases |
| p15INK4B | cyclin-dependent kinase inhibitor 2B |
| PAI-1 | plasminogen activator inhibitor-1 |
| PDGF | platelet-derived growth factor |
| PD-L1 | programmed death-ligand 1 |
| PGE2 | prostaglandin E2 |
| PI3K | phosphatidylinositol 3-kinase |
| PTEN | Phosphatase and TENsin homolog deleted on chromosome 10 |
| PXR | pregnane X receptor |
| RAS | rat sarcoma gene |
| RB1 | retinoblastoma transcriptional corepressor 1 |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RXR | retinoid X receptor |
| SASP | senescence-associated secretory phenotype |
| Snail | Snail family transcriptional repressor |
| STAT3 | signal transducer and activator transcription 3 |
| STING | stimulator of interferon genes |
| TAMs | tumor-associated macrophages |
| TGF-β | transforming growth factor beta |
| TKI | tyrosine kinase inhibitor |
| TLR4 | Toll-like receptor 4 |
| TLR5 | Toll-like receptor 5 |
| TLR7 | Toll-like receptor 7 |
| TLR9 | Toll-like receptor 9 |
| TM | thymidylate kinase |
| TME | tumor microenvironment |
| TP53 | tumor protein p53 |
| TRAF6 | tumor necrosis factor receptor-associated factor 6 |
| Tregs | regulatory T cells |
| Wnt | wingless-type MMTV integration site family |
| ZEB | zinc finger E-box binding homeobox |
| β-2M | beta-2-microglobulin |
References
- Whalen, K.; Lerchenfeldt, S. Lippincott Illustrated Reviews: Pharmacology, 8th ed.; Edra Urban & Partner: Wrocław, Poland, 2024. [Google Scholar]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simone, V.; D’Avenia, M.; Argentiero, A.; Felici, C.; Rizzo, F.M.; De Pergola, G.; Silvestris, F. Obesity and Breast Cancer: Molecular Interconnections and Potential Clinical Applications. Oncologist 2016, 21, 404–417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Contaldo, M.; Boccellino, M.; Zannini, G.; Romano, A.; Sciarra, A.; Sacco, A.; Settembre, G.; Coppola, M.; Di Carlo, A.; D’Angelo, L.; et al. Sex Hormones and Inflammation Role in Oral Cancer Progression: A Molecular and Biological Point of View. J. Oncol. 2020, 2020, 9587971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, F.C.; Vázquez-Torres, A. Reactive nitrogen species in host-bacterial interactions. Curr. Opin. Immunol. 2019, 60, 96–102. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Bioenergetics and Reactive Nitrogen Species in Bacteria. Int. J. Mol. Sci. 2022, 23, 7321. [Google Scholar] [CrossRef]
- Jiang, H.; Lin, Q.; Yu, Z.; Wang, C.; Zhang, R. Nanotechnologies for Reactive Oxygen Species “Turn-On” Detection. Front. Bioeng. Biotechnol. 2021, 9, 780032. [Google Scholar] [CrossRef]
- Krause, K.H. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2007, 42, 256–262. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Debeleç Bütüner, B.; Ertunç Hasbal, N.; Işel, E.; Roggenbuck, D.; Korkmaz, K.S. Androgen receptor contributes to repairing DNA damage induced by inflammation and oxidative stress in prostate cancer. Turk. J. Biol. 2023, 47, 325–335. [Google Scholar] [CrossRef]
- Lonkar, P.; Dedon, P.C. Reactive species and DNA damage in chronic inflammation: Reconciling chemical mechanisms and biological fates. Int. J. Cancer 2011, 128, 1999–2009. [Google Scholar] [CrossRef]
- Zappavigna, S.; Cossu, A.M.; Grimaldi, A.; Bocchetti, M.; Ferraro, G.A.; Nicoletti, G.F.; Filosa, R.; Caraglia, M. Anti-Inflammatory Drugs as Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 2605. [Google Scholar] [CrossRef] [PubMed]
- Kloeber, J.A.; Lou, Z. Critical DNA damaging pathways in tumorigenesis. Semin. Cancer Biol. 2022, 85, 164–184. [Google Scholar] [CrossRef] [PubMed]
- Bartek, J. DNA damage response, genetic instability and cancer: From mechanistic insights to personalized treatment. Mol. Oncol. 2011, 5, 303–307. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552 Pt 2, 335–344. [Google Scholar] [CrossRef]
- Helm, J.S.; Rudel, R.A. Adverse outcome pathways for ionizing radiation and breast cancer involve direct and indirect DNA damage, oxidative stress, inflammation, genomic instability, and interaction with hormonal regulation of the breast. Arch. Toxicol. 2020, 94, 1511–1549. [Google Scholar] [CrossRef] [PubMed]
- D’Augustin, O.; Huet, S.; Campalans, A.; Radicella, J.P. Lost in the Crowd: How Does Human 8-Oxoguanine DNA Glycosylase 1 (OGG1) Find 8-Oxoguanine in the Genome? Int. J. Mol. Sci. 2020, 21, 8360. [Google Scholar] [CrossRef]
- Jung, H.; Lee, S. Promutagenic bypass of 7,8-dihydro-8-oxoadenine by translesion synthesis DNA polymerase Dpo4. Biochem. J. 2020, 477, 2859–2871. [Google Scholar] [CrossRef]
- Faucher, F.; Doublié, S.; Jia, Z. 8-Oxoguanine DNA Glycosylases: One Lesion, Three Subfamilies. Int. J. Mol. Sci. 2012, 13, 6711–6729. [Google Scholar] [CrossRef]
- Hirano, T. Repair system of 7, 8-dihydro-8-oxoguanine as a defense line against carcinogenesis. J. Radiat. Res. 2008, 49, 329–340. [Google Scholar] [CrossRef]
- Caulfield, J.L.; Wishnok, J.S.; Tannenbaum, S.R. Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J. Biol. Chem. 1998, 273, 12689–12695. [Google Scholar] [CrossRef]
- Barnes, D.E.; Lindahl, T. Repair and genetic consequences of endogenous DNA base damage in mammalian Cells. Annu. Rev. Genet. 2004, 38, 445–476. [Google Scholar] [CrossRef] [PubMed]
- Meibers, H.E.; Warrick, K.A.; VonHandorf, A.; Vallez, C.N.; Kawarizadeh, K.; Saha, I.; Donmez, O.; Jain, V.G.; Kottyan, L.C.; Weirauch, M.T.; et al. Effector memory T cells induce innate inflammation by triggering DNA damage and a non-canonical STING pathway in dendritic Cells. Cell Rep. 2023, 42, 113180. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.M.; Malkin, D.J.; Camacho, J.; Schiestl, R.H. IL-13 overexpression in mouse lungs triggers systemic genotoxicity in peripheral blood. Mutat. Res. 2014, 769, 100–107. [Google Scholar] [CrossRef]
- Jaiswara, P.K.; Shukla, S.K. Chemotherapy-Mediated Neuronal Aberration. Pharmaceuticals 2023, 16, 1165. [Google Scholar] [CrossRef]
- Ren, X.; St Clair, D.K.; Butterfield, D.A. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol. Res. 2017, 117, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef] [PubMed]
- Ragu, S.; Matos-Rodrigues, G.; Lopez, B.S. Replication Stress, DNA Damage, Inflammatory Cytokines and Innate Immune Response. Genes 2020, 11, 409. [Google Scholar] [CrossRef]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef]
- Xie, Q.W.; Kashiwabara, Y.; Nathan, C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994, 269, 4705–4708. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, L.; Zhang, S.; Tian, X.; De La Cruz, A.; George, A.; Arnoff, T.E.; El-Deiry, W.S. The role of p53 in anti-tumor immunity and response to immunotherapy. Front. Mol. Biosci. 2023, 10, 1148389. [Google Scholar] [CrossRef]
- Moro, R.N.; Biswas, U.; Kharat, S.S.; Duzanic, F.D.; Das, P.; Stavrou, M.; Raso, M.C.; Freire, R.; Chaudhuri, A.R.; Sharan, S.K.; et al. Interferon restores replication fork stability and cell viability in BRCA-defective cells via ISG15. Nat. Commun. 2023, 14, 6140. [Google Scholar] [CrossRef]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Gronke, K.; Hernández, P.P.; Zimmermann, J.; Klose, C.S.N.; Kofoed-Branzk, M.; Guendel, F.; Witkowski, M.; Tizian, C.; Amann, L.; Schumacher, F.; et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 2019, 566, 249–253. [Google Scholar] [CrossRef]
- Jovasevic, V.; Wood, E.M.; Cicvaric, A.; Zhang, H.; Petrovic, Z.; Carboncino, A.; Parker, K.K.; Bassett, T.E.; Moltesen, M.; Yamawaki, N.; et al. Formation of memory assemblies through the DNA-sensing TLR9 pathway. Nature 2024, 628, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Ajay, A.K.; Gasser, M.; Hsiao, L.L.; Böldicke, T.; Waaga-Gasser, A.M. TLR2 and TLR9 Blockade Using Specific Intrabodies Inhibits Inflammation-Mediated Pancreatic Cancer Cell Growth. Antibodies 2024, 13, 11. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Wang, S.; Mao, G. DNA damage response (DDR): A link between cellular senescence and human cytomegalovirus. Virol. J. 2023, 20, 250. [Google Scholar] [CrossRef]
- Qiu, Y.; Hu, X.; Zeng, X.; Wang, H. Triple kill: DDR inhibitors, radiotherapy and immunotherapy leave cancer cells with no escape. Acta Biochim. Biophys. Sin. 2022, 54, 1569–1576. [Google Scholar] [CrossRef]
- Huang, R.X.; Zhou, P.K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World. 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Solano-Altamirano, J.M.; Ledez, K.M. The evils that bubbles do…. In Gas Bubble Dynamics in the Human Body; Academic Press: Cambridge, MA, USA, 2018; pp. 161–185. [Google Scholar]
- Kunachowicz, D.; Tomecka, P.; Sędzik, M.; Kalinin, J.; Kuźnicki, J.; Rembiałkowska, N. Influence of Hypoxia on Tumor Heterogeneity, DNA Repair, and Cancer Therapy: From Molecular Insights to Therapeutic Strategies. Cells 2025, 14, 1057. [Google Scholar] [CrossRef] [PubMed]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA Damage, Mutations and Cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Abbas, T.; Keaton, M.A.; Dutta, A. Genomic Instability in Cancer. Cold Spring Harb. Perspect. Biol. 2013, 5, a012914. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Gerlach, S.U.; Herranz, H. Genomic instability and cancer: Lessons from Drosophila. Open Biol. 2020, 10, 200060. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Xiao, D.; Guo, Y.; Tian, D.; Yun, H.; Chen, D.; Su, M. Chronic inflammation-related DNA damage response: A driving force of gastric cardia carcinogenesis. Oncotarget 2014, 6, 2856–2864. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, C.; Zheng, J.; Tian, D.; Lei, Z.; Chen, D.; Xu, Z.; Su, M. Chronic inflammation-associated genomic instability paves the way for human esophageal carcinogenesis. Oncotarget 2016, 7, 24564–24571. [Google Scholar] [CrossRef] [PubMed]
- Roessner, A.; Kuester, D.; Malfertheiner, P.; Schneider-Stock, R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol. Res. Pract. 2008, 204, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef]
- Nobre, C.C.G.; de Araújo, J.M.G.; Fernandes, T.A.A.M.; Cobucci, R.N.O.; Lanza, D.C.F.; Andrade, V.S.; Fernandes, J.V. Macrophage Migration Inhibitory Factor (MIF): Biological Activities and Relation with Cancer. Pathol. Oncol. Res. 2017, 23, 235–244. [Google Scholar] [CrossRef]
- Bhat, A.A.; Nisar, S.; Singh, M.; Ashraf, B.; Masoodi, T.; Prasad, C.P.; Sharma, A.; Maacha, S.; Karedath, T.; Hashem, S.; et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun. 2022, 42, 689–715. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, Y.; Zhang, C.; Yang, X.; Gao, Y.; Li, M.; Yang, H.; Liu, T.; Tang, H. Chronic inflammation, cancer development and immunotherapy. Front. Pharmacol. 2022, 13, 1040163. [Google Scholar] [CrossRef] [PubMed]
- de’Angelis, G.L.; Bottarelli, L.; Azzoni, C.; de’Angelis, N.; Leandro, G.; Di Mario, F.; Federica, G.; Francesca, N. Microsatellite instability in colorectal cancer. Acta Biomed. Atenei Parmensis 2018, 89 (Suppl. S9), 97–101. [Google Scholar]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer—The stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Afrăsânie, V.A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Păduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and Microsatellite Instability in Metastatic Colorectal Cancer—Practical Implications for the Clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar] [CrossRef]
- Moon, C.; Gordon, M.; Moon, D.; Reynolds, T. Microsatellite Instability Analysis (MSA) for Bladder Cancer: Past History and Future Directions. Int. J. Mol. Sci. 2021, 22, 12864. [Google Scholar] [CrossRef]
- Riedinger, C.J.; Esnakula, A.; Haight, P.J.; Suarez, A.A.; Chen, W.; Gillespie, J.; Villacres, A.; Chassen, A.; Cohn, D.E.; Goodfellow, P.J. Characterization of mismatch repair (MMR)/microsatellite instability (MSI) discordant endometrial cancers. Cancer 2024, 130, 385–399. [Google Scholar] [CrossRef]
- Ilango, S.; Paital, B.; Jayachandran, P.; Padma, P.R.; Nirmaladevi, R. Epigenetic alterations in cancer. Front. Biosci. Landmark 2020, 25, 1058–1109. [Google Scholar]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, F.; Qin, Z. TNF Receptor 2 Makes Tumor Necrosis Factor a Friend of Tumors. Front. Immunol. 2018, 9, 1170. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhang, Y.M.; Zhang, L.Y.; Zhou, T.; Li, Y.Y.; Zhou, G.C.; Miao, Z.M.; Shang, M.; He, J.P.; Dong, N.; et al. Duality of Interactions Between TGF-β and TNF-α During Tumor Formation. Front. Immunol. 2022, 12, 810286. [Google Scholar] [CrossRef]
- Gelfo, V.; Romaniello, D.; Mazzeschi, M.; Sgarzi, M.; Grilli, G.; Morselli, A.; Manzan, B.; Rihawi, K.; Lauriola, M. Roles of IL-1 in Cancer: From Tumor Progression to Resistance to Targeted Therapies. Int. J. Mol. Sci. 2020, 21, 6009. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial–mesenchymal transition and its transcription factors. Biosci. Rep. 2021, 42, BSR20211754. [Google Scholar] [CrossRef]
- Zhang, Y.; Weinberg, R.A. Epithelial-to-mesenchymal transition in cancer: Complexity and opportunities. Front. Med. 2018, 12, 361–373. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Ray, I.; Michael, A.; Meira, L.B.; Ellis, P.E. The Role of Cytokines in Epithelial-Mesenchymal Transition in Gynaecological Cancers: A Systematic Review. Cells 2023, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Martins-Lima, C.; Chianese, U.; Benedetti, R.; Altucci, L.; Jerónimo, C.; Correia, M.P. Tumor microenvironment and epithelial-mesenchymal transition in bladder cancer: Cytokines in the game? Front. Mol. Biosci. 2022, 9, 1070383. [Google Scholar] [CrossRef]
- Khandia, R.; Munjal, A. Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 2020, 119, 199–245. [Google Scholar]
- Pereg, D.; Lishner, M. Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. J. Intern. Med. 2005, 258, 115–123. [Google Scholar] [CrossRef]
- Pelly, V.S.; Moeini, A.; Roelofsen, L.M.; Bonavita, E.; Bell, C.R.; Hutton, C.; Blanco-Gomez, A.; Banyard, A.; Bromley, C.P.; Flanagan, E.; et al. Anti-Inflammatory Drugs Remodel the Tumor Immune Environment to Enhance Immune Checkpoint Blockade Efficacy. Cancer Discov. 2021, 11, 2602–2619. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.R.; Kashfi, K. NSAIDs and Cancer Resolution: New Paradigms beyond Cyclooxygenase. Int. J. Mol. Sci. 2022, 23, 1432. [Google Scholar] [CrossRef]
- Yu, C.; Li, W.B.; Liu, J.B.; Lu, J.W.; Feng, J.F. Autophagy: Novel applications of nonsteroidal anti-inflammatory drugs for primary cancer. Cancer Med. 2018, 7, 471–484. [Google Scholar] [CrossRef]
- Zaman, F.Y.; Orchard, S.G.; Haydon, A.; Zalcberg, J.R. Non-aspirin non-steroidal anti-inflammatory drugs in colorectal cancer: A review of clinical studies. Br. J. Cancer 2022, 127, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Waddell, W.R.; Ganser, G.F.; Cerise, E.J.; Loughry, R.W. Sulindac for polyposis of the colon. Am. J. Surg. 1989, 157, 175–179. [Google Scholar] [CrossRef]
- Kübler, H. Corticosteroids in the management of advanced prostate cancer. Urol. A 2017, 56, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Seijo, L.M.; Peces-Barba, G. Inhaled Corticosteroids and Lung Cancer in COPD. Arch. Bronconeumol. 2019, 55, 407–408. [Google Scholar] [CrossRef]
- Goodman, R.S.; Johnson, D.B.; Balko, J.M. Corticosteroids and Cancer Immunotherapy. Clin. Cancer Res. 2023, 29, 2580–2587. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; He, X.; Yuan, Y.; Wei, X. Targeting inflammation as cancer therapy. J. Hematol. Oncol. 2024, 17, 13. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Gulce-Iz, S.; Biray-Avci, C. Monoclonal antibodies in cancer immunotherapy. Mol. Biol. Rep. 2018, 45, 2935–2940. [Google Scholar] [CrossRef]
- Delgado, M.; Garcia-Sanz, J.A. Therapeutic Monoclonal Antibodies against Cancer: Present and Future. Cells 2023, 12, 2837. [Google Scholar] [CrossRef]
- Andreakos, E.; Taylor, P.C.; Feldmann, M. Monoclonal antibodies in immune and inflammatory diseases. Curr. Opin. Biotechnol. 2002, 13, 615–620. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Thase, M.E.; Pillinger, T. Treatment resistance in psychiatry: State of the art and new directions. Mol. Psychiatry 2022, 27, 58–72. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria-A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef] [PubMed]
- Kurt Yilmaz, N.; Schiffer, C.A. Introduction: Drug Resistance. Chem. Rev. 2021, 121, 3235–3237. [Google Scholar] [CrossRef]
- Dhanyamraju, P.K. Drug resistance mechanisms in cancers: Execution of pro-survival strategies. J. Biomed. Res. 2024, 38, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, P.; Lin, J.; Chen, K.; Shen, J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist. 2023, 6, 390–415. [Google Scholar] [CrossRef] [PubMed]
- Kelderman, S.; Schumacher, T.N.M.; Haanen, J.B.A.G. Acquired and intrinsic resistance in cancer immunotherapy. Mol. Oncol. 2014, 8, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, Z. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef]
- Mikubo, M.; Inoue, Y.; Liu, G.; Tsao, M.S. Mechanism of Drug Tolerant Persister Cancer Cells: The Landscape and Clinical Implication for Therapy. J. Thorac. Oncol. 2021, 16, 1798–1809. [Google Scholar] [CrossRef]
- Mancarella, D.; Plass, C. Epigenetic signatures in cancer: Proper controls, current challenges and the potential for clinical translation. Genome Med. 2021, 13, 23. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Rekiel, K.; Urbanowicz, P.; Mamala, M.; Marczuk, K.; Wojtaszek, M.; Żywica, M.; Radzevičiūtė-Valčiukė, E.; Novickij, V.; Kulbacka, J. Epigenetic Dysregulation in Cancer: Implications for Gene Expression and DNA Repair-Associated Pathways. Int. J. Mol. Sci. 2025, 26, 6531. [Google Scholar] [CrossRef]
- Cooks, T.; Pateras, I.S.; Tarcic, O.; Solomon, H.; Schetter, A.J.; Wilder, S.; Lozano, G.; Pikarsky, E.; Forshew, T.; Rozenfeld, N.; et al. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell 2013, 23, 634–646. [Google Scholar] [CrossRef]
- Wörmann, S.M.; Song, L.; Ai, J.; Diakopoulos, K.N.; Kurkowski, M.U.; Görgülü, K.; Ruess, D.; Campbell, A.; Doglioni, C.; Jodrell, D.; et al. Loss of P53 Function Activates JAK2-STAT3 Signaling to Promote Pancreatic Tumor Growth, Stroma Modification, and Gemcitabine Resistance in Mice and Is Associated with Patient Survival. Gastroenterology 2016, 151, 180–193.e12. [Google Scholar] [CrossRef]
- Mortezaee, K.; Majidpoor, J. Mechanisms of CD8+ T cell exclusion and dysfunction in cancer resistance to anti-PD-(L)1. Biomed. Pharmacother. 2023, 163, 114824. [Google Scholar] [CrossRef] [PubMed]
- Wellenstein, M.D.; de Visser, K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018, 48, 399–416. [Google Scholar] [CrossRef]
- Soucek, L.; Lawlor, E.R.; Soto, D.; Shchors, K.; Swigart, L.B.; Evan, G.I. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat. Med. 2007, 13, 1211–1218. [Google Scholar] [CrossRef]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and Cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Casagrande, N.; Borghese, C.; Visser, L.; Mongiat, M.; Colombatti, A.; Aldinucci, D. CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica 2019, 104, 564–575. [Google Scholar] [CrossRef]
- Zi, J.; Yuan, S.; Qiao, J.; Zhao, K.; Xu, L.; Qi, K.; Xu, K.; Zeng, L. Treatment with the C-C chemokine receptor type 5 (CCR5)-inhibitor maraviroc suppresses growth and induces apoptosis of acute lymphoblastic leukemia Cells. Am. J. Cancer Res. 2017, 7, 869–880. [Google Scholar]
- Abe, M.; Hiura, K.; Wilde, J.; Moriyama, K.; Hashimoto, T.; Ozaki, S.; Wakatsuki, S.; Kosaka, M.; Kido, S.; Inoue, D.; et al. Role for macrophage inflammatory protein (MIP)-1α and MIP-1β in the development of osteolytic lesions in multiple myeloma. Blood 2002, 100, 2195–2202. [Google Scholar] [CrossRef]
- Zhong, F.L.; Mamaï, O.; Sborgi, L.; Boussofara, L.; Hopkins, R.; Robinson, K.; Szeverényi, I.; Takeichi, T.; Balaji, R.; Lau, A.; et al. Germline NLRP1 Mutations Cause Skin Inflammatory and Cancer Susceptibility Syndromes via Inflammasome Activation. Cell 2016, 167, 187–202.e17. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva-Posocco, O.; Dzutsev, A.; Posocco, D.F.; Hou, V.; Yuan, W.; Thovarai, V.; Mufazalov, I.A.; Gunzer, M.; Shilovskiy, I.P.; Khaitov, M.R.; et al. Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity 2019, 50, 166–180.e7. [Google Scholar] [CrossRef] [PubMed]
- Matanić, D.; Beg-Zec, Z.; Stojanović, D.; Matakorić, N.; Flego, V.; Milevoj-Ribić, F. Cytokines in patients with lung cancer. Scand. J. Immunol. 2003, 57, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Yuan, R.Q.; Fuchs, A.; Yao, Y.; Joseph, A.; Schwall, R.; Schnitt, S.J.; Guida, A.; Hastings, H.M.; Andres, J.; et al. Expression of interleukin-1β in human breast carcinoma. Cancer 1997, 80, 421–434. [Google Scholar]
- Li, Y.; VandenBoom, T.G.; Wang, Z.; Kong, D.; Ali, S.; Philip, P.A.; Sarkar, F.H. miR-146a Suppresses Invasion of Pancreatic Cancer Cells. Cancer Res. 2010, 70, 1486–1495. [Google Scholar]
- Nussinov, R.; Tsai, C.J.; Jang, H. Anticancer drug resistance: An update and perspective. Drug Resist. Updat. 2021, 59, 100796. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Senthebane, D.A.; Thomford, N.E.; Rowe, A.; Dandara, C.; Parker, M.I. Not Everyone Fits the Mold: Intratumor and Intertumor Heterogeneity and Innovative Cancer Drug Design and Development. OMICS 2018, 22, 17–34. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Yang, W.; Zhou, M.; Liu, F. Acquired resistance for immune checkpoint inhibitors in cancer immunotherapy: Challenges and prospects. Aging 2022, 14, 1048–1064. [Google Scholar] [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Smyth, M.J.; Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 2016, 16, 121–126. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. Treg-mediated acquired resistance to immune checkpoint inhibitors. Cancer Lett. 2019, 457, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Hossam Abdelmonem, B.; Abdelaal, N.M.; Anwer, E.K.E.; Rashwan, A.A.; Hussein, M.A.; Ahmed, Y.F.; Khashana, R.; Hanna, M.M.; Abdelnaser, A. Decoding the Role of CYP450 Enzymes in Metabolism and Disease: A Comprehensive Review. Biomedicines 2024, 12, 1467. [Google Scholar] [CrossRef]
- Darakjian, L.; Deodhar, M.; Turgeon, J.; Michaud, V. Chronic Inflammatory Status Observed in Patients with Type 2 Diabetes Induces Modulation of Cytochrome P450 Expression and Activity. Int. J. Mol. Sci. 2021, 22, 4967. [Google Scholar] [CrossRef]
- Wang, X.; Rao, J.; Tan, Z.; Xun, T.; Zhao, J.; Yang, X. Inflammatory signaling on cytochrome P450-mediated drug metabolism in hepatocytes. Front. Pharmacol. 2022, 13, 1043836. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schulz, A.; Meyer, F.; Dubrovska, A.; Borgmann, K. Cancer Stem Cells and Radioresistance: DNA Repair and Beyond. Cancers 2019, 11, 862. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcone, S.; Buckley, A.; Ryan, C.J.; McCabe, M.; Lynam-Lennon, N.; Matallanas, D.; O’Sullivan, J.; Kennedy, S. Proteomic signatures of radioresistance: Alteration of inflammation, angiogenesis and metabolism-related factors in radioresistant oesophageal adenocarcinoma. Cancer Treat. Res. Commun. 2021, 27, 100376. [Google Scholar] [CrossRef] [PubMed]
- Bochet, L.; Meulle, A.; Imbert, S.; Salles, B.; Valet, P.; Muller, C. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem. Biophys. Res. Commun. 2011, 411, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Galeaz, C.; Totis, C.; Bisio, A. Radiation Resistance: A Matter of Transcription Factors. Front. Oncol. 2021, 11, 662840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, L.; Dong, J.; Wang, L.; Xia, Q.; Zhang, D.; Kim, H.; Yin, T.; Fan, S.; Shen, Q. Activation of STAT3 and Bcl-2 and reduction of reactive oxygen species (ROS) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide. Oncogene 2018, 37, 5292–5304. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.P.; Ming, L.C.; Dhaliwal, J.S.; Gupta, M.; Ardianto, C.; Goh, K.W.; Hussain, Z.; Shafqat, N. Role of Immunotherapy in the Treatment of Cancer: A Systematic Review. Cancers 2022, 14, 5205. [Google Scholar] [CrossRef] [PubMed]
- Lalani, A.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. Immunother. Cancer 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, C.; Talukder, A.; Savage, N.M.; Singh, N.; Liu, K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology 2017, 6, e1291106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rieth, J.; Subramanian, S. Mechanisms of Intrinsic Tumor Resistance to Immunotherapy. Int. J. Mol. Sci. 2018, 19, 1340. [Google Scholar] [CrossRef]
- Karin, M.; Shalapour, S. Regulation of antitumor immunity by inflammation-induced epigenetic alterations. Cell. Mol. Immunol. 2022, 19, 59–66. [Google Scholar] [CrossRef] [PubMed]
- McClellan, B.L.; Haase, S.; Nunez, F.J.; Alghamri, M.S.; Dabaja, A.A.; Lowenstein, P.R.; Castro, M.G. Impact of epigenetic reprogramming on antitumor immune responses in glioma. J. Clin. Investig. 2023, 133, e163450. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Araszkiewicz, A.F.; Jańczak, K.; Wójcik, P.; Białecki, B.; Kubiak, S.; Szczechowski, M.; Januszkiewicz-Lewandowska, D. MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications—A Review. Genes 2025, 16, 441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrone, I.; Bernardo, P.S.; dos Santos, E.C.; Abdelhay, E. MTHFR C677T and A1298C Polymorphisms in Breast Cancer, Gliomas and Gastric Cancer: A Review. Genes 2021, 12, 587. [Google Scholar] [CrossRef]
- Focaccetti, C.; Nardozi, D.; Benvenuto, M.; Lucarini, V.; Angiolini, V.; Carrano, R.; Scimeca, M.; Servadei, F.; Mauriello, A.; Mancini, P.; et al. Bisphenol-A in Drinking Water Accelerates Mammary Cancerogenesis and Favors an Immunosuppressive Tumor Microenvironment in BALB-neuT Mice. Int. J. Mol. Sci. 2024, 25, 6259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.Y.; Ho, Y.C.; Lee, S.S.; Li, Y.C.; Lai, M.Y.; Kuan, Y.H. Cytotoxicity and Apoptotic Mechanism of 2-Hydroxyethyl Methacrylate via Genotoxicity and the Mitochondrial-Dependent Intrinsic Caspase Pathway and Intracellular Reactive Oxygen Species Accumulation in Macrophages. Polymers 2022, 14, 3378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Romo-Huerta, M.J.; Cervantes-Urenda, A.D.R.; Velasco-Neri, J.; Torres-Bugarín, O.; Valdivia, A.D.C.M. Genotoxicity Associated with Residual Monomers in Restorative Dentistry: A Systematic Review. Oral Health Prev. Dent. 2021, 19, 471–480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baima, G.; Minoli, M.; Michaud, D.S.; Aimetti, M.; Sanz, M.; Loos, B.G.; Romandini, M. Periodontitis and risk of cancer: Mechanistic evidence. Periodontol 2000. 2024, 96, 83–94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yangyanqiu, W.; Shuwen, H. Bacterial DNA involvement in carcinogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 996778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhuang, H.; Yu, B.; Tao, D.; Xu, X.; Xu, Y.; Wang, J.; Jiao, Y.; Wang, L. The role of m6A methylation in therapy resistance in cancer. Mol. Cancer 2023, 22, 91. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Mechanism | Mediators/Molecules | Type of DNA Damage | Biological Consequences | References |
|---|---|---|---|---|
| Oxidative damage | ROS: O2−, H2O2, HO• | Base oxidation (e.g., 8-oxoG), single- and double-strand breaks (SSBs, DSBs) | Mutagenesis, replication errors, genomic instability | [15,17,21,24] |
| Nitrosative deamination | NO, N2O3 | Deamination of cytosine, adenine, guanine, 5-methylcytosine → uracil, hypoxanthine, xanthine, thymine | Transition mutations, mispairing, inadequate repair | [28,30] |
| Lipid peroxidation byproducts | 4-HNE, MDA (from PUFA oxidation) | Formation of DNA adducts | Interference with replication and transcription | [23] |
| Protein nitration and DNA lesions | IL-13, RNS (NO-derived) | Protein nitration, micronucleus formation | Chromosomal aberrations, DNA fragmentation | [31] |
| Double-strand breaks (DSBs) | ROS, ATM activation, H2A.X phosphorylation | DNA strand scission | Cell cycle arrest, apoptosis, senescence | [16] |
| Immune-cell-induced damage | Mitochondrial ROS from CD4+ T cells; STING–TRAF6–NF-κB axis | Indirect DNA damage via cytokine release (IL-1β, IL-6) and DSBs in dendritic cells | Local immunopathology, activation of inflammatory signaling | [30] |
| Mediator/Pathway | Mechanism of Action | Effect on DDR and Genome Stability | References |
|---|---|---|---|
| TNF-α | Activates NF-κB via TNFR signaling | Induces transcription of inflammatory genes and oxidative stress responses | [32,33] |
| NF-κB | Activated by TLRs, thymidylate kinase (TM)/NEMO/IKK, cGAS-STING, p38MAPKα, or GATA4 | Regulates expression of iNOS, HIF-1α, SASP components, and apoptotic genes | [34,35,36,37,38] |
| Interferons (IFNs) | Triggered by TLR7/9 and cGAS-STING, promote STAT3 activation and ISG15 expression | Enhance DNA repair capacity, promote replication fork stability | [39,40] |
| ISG15 | Upregulated by IFN signaling | Supports replication fork stabilization in BRCA-defective breast cancer cells | [40] |
| IL-6, IL-8 (SASP cytokines) | Maintain senescence signaling via STAT3, CXCR2, and C/EBP transcription factors | Reinforce cell cycle arrest and DNA repair in low-damage conditions | [41] |
| IL-1 | Enhances NF-κB and C/EBPβ activity, sustaining the SASP | Promotes persistent inflammatory signaling and modulates DDR | [41] |
| IL-22 | Activates ATM transcription through STAT3 | Prevents accumulation of DNA mutations via enhanced DDR | [42] |
| cGAS–STING pathway | Detects cytosolic DNA, synthesizes cGAMP, activates IRF3 and NF-κB | Promotes IFN responses and links inflammation with DDR | [43,44] |
| TLRs (e.g., TLR4, TLR7, TLR9) | Recognize extracellular DNA or pathogens, trigger downstream NF-κB and IFN pathways | Indirectly modulate DDR via inflammatory cytokine production | [43,45] |
| iNOS (inducible nitric oxide synthase) | Upregulated by NF-κB signaling | Increases nitrosative stress, enhancing DNA damage and DDR activation | [35,37] |
| Drug Class | Representative Agents | Mechanism of Action | Cancer Types Studied | Limitations |
|---|---|---|---|---|
| NSAIDs | Aspirin, Sulindac, Celecoxib | COX-2 inhibition, ↓ PGE2, immune modulation | Colorectal, Breast, Pancreatic | GI bleeding, CV risk, renal toxicity |
| Corticosteroids | Dexamethasone, Prednisone | Broad immunosuppression, NF-κB/PI3K inhibition | Prostate, Lung (COPD-related) | Immunosuppression, ↓ immunotherapy response |
| Anti-cytokine mAbs | Canakinumab, Anakinra, Anti-IL-6 | IL-1β/IL-6/TGF-β blockade, ↓ immune evasion | Lung, Myeloma, Prostate, Ovarian | Infection risk, incomplete clinical validation |
| Chemokine Inhibitors | Anti-CCL2/CCR2 mAbs | Blocks immune cell recruitment, ↓ TME suppression | Breast, Lung, Pancreatic (experimental) | Early-stage trials, immunologic compensation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rembiałkowska, N.; Kocik, Z.; Kłosińska, A.; Kübler, M.; Pałkiewicz, A.; Rozmus, W.; Sędzik, M.; Wojciechowska, H.; Gajewska-Naryniecka, A. Inflammation-Driven Genomic Instability: A Pathway to Cancer Development and Therapy Resistance. Pharmaceuticals 2025, 18, 1406. https://doi.org/10.3390/ph18091406
Rembiałkowska N, Kocik Z, Kłosińska A, Kübler M, Pałkiewicz A, Rozmus W, Sędzik M, Wojciechowska H, Gajewska-Naryniecka A. Inflammation-Driven Genomic Instability: A Pathway to Cancer Development and Therapy Resistance. Pharmaceuticals. 2025; 18(9):1406. https://doi.org/10.3390/ph18091406
Chicago/Turabian StyleRembiałkowska, Nina, Zofia Kocik, Amelia Kłosińska, Maja Kübler, Agata Pałkiewicz, Weronika Rozmus, Mikołaj Sędzik, Helena Wojciechowska, and Agnieszka Gajewska-Naryniecka. 2025. "Inflammation-Driven Genomic Instability: A Pathway to Cancer Development and Therapy Resistance" Pharmaceuticals 18, no. 9: 1406. https://doi.org/10.3390/ph18091406
APA StyleRembiałkowska, N., Kocik, Z., Kłosińska, A., Kübler, M., Pałkiewicz, A., Rozmus, W., Sędzik, M., Wojciechowska, H., & Gajewska-Naryniecka, A. (2025). Inflammation-Driven Genomic Instability: A Pathway to Cancer Development and Therapy Resistance. Pharmaceuticals, 18(9), 1406. https://doi.org/10.3390/ph18091406





