Design, Synthesis, and Biological Evaluation of Novel Multitarget 7-Alcoxyamino-3-(1,2,3-triazole)-coumarins as Potent Acetylcholinesterase Inhibitors
Abstract
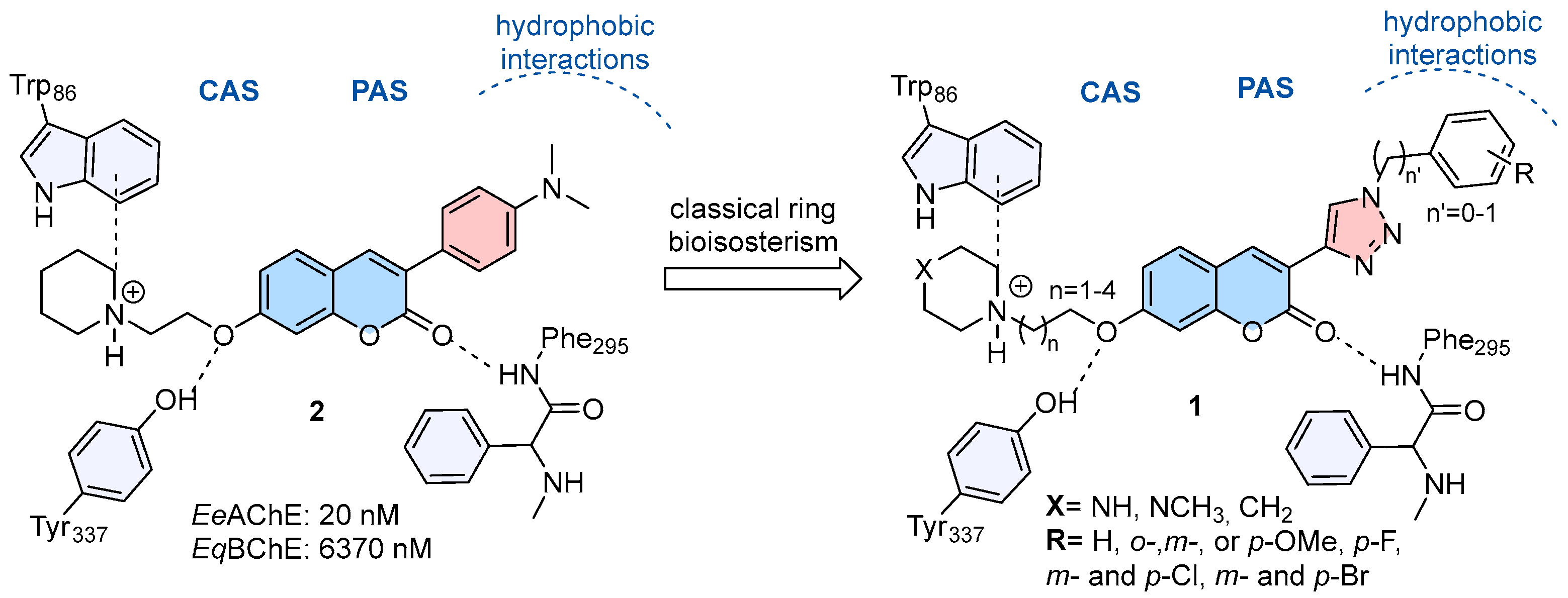
1. Introduction
2. Results
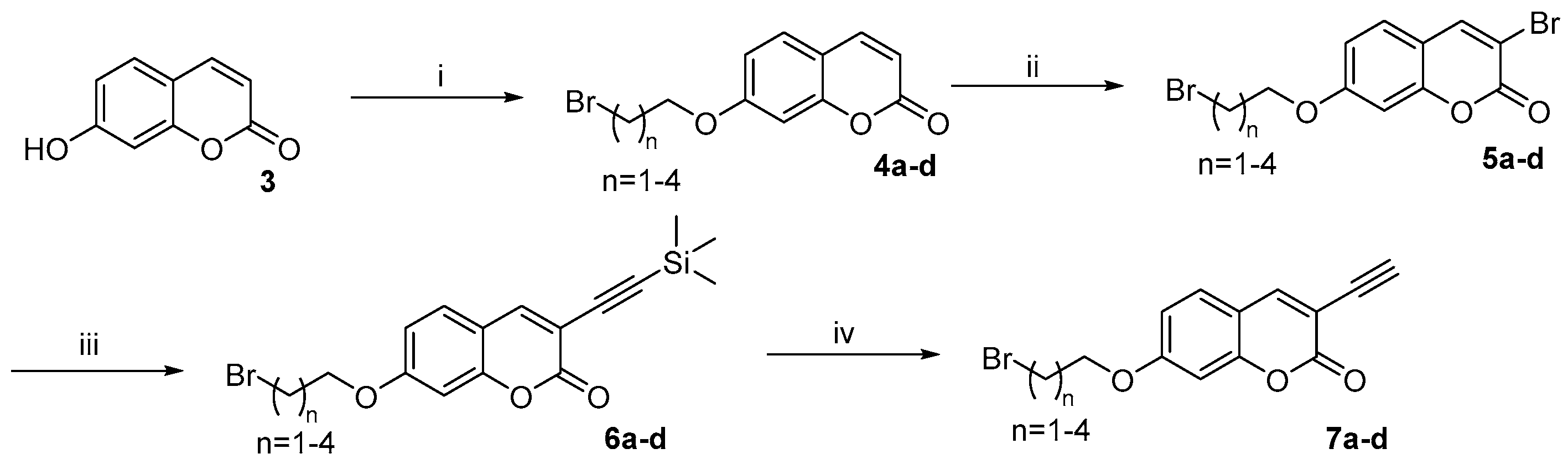
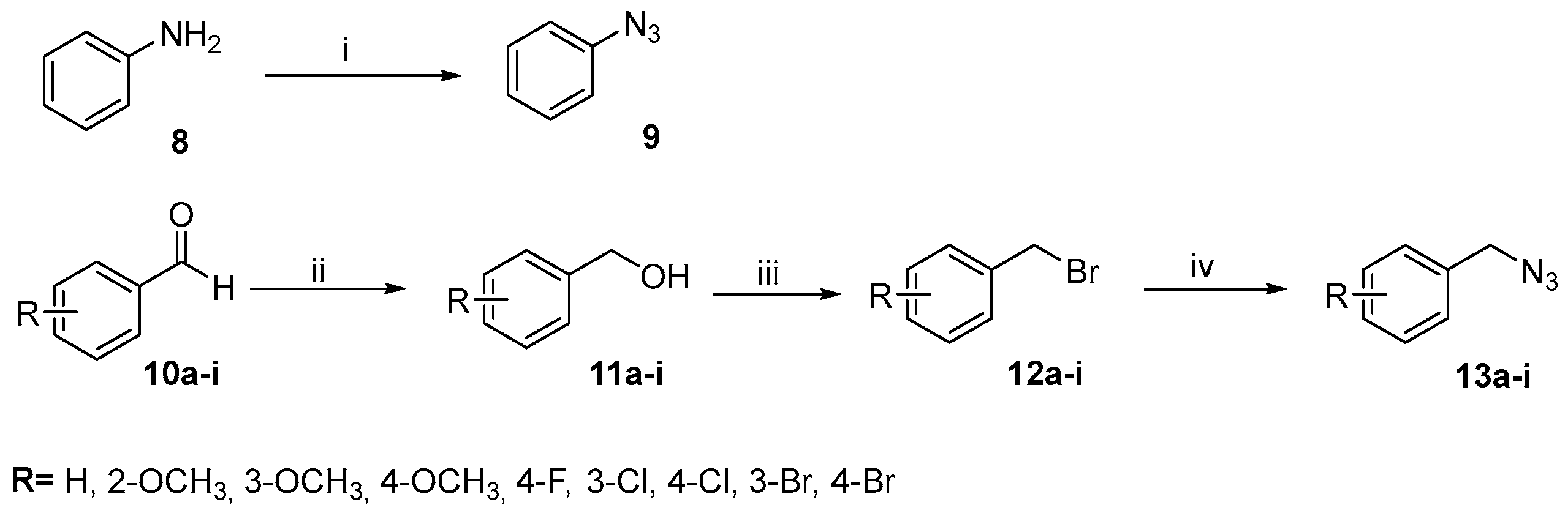
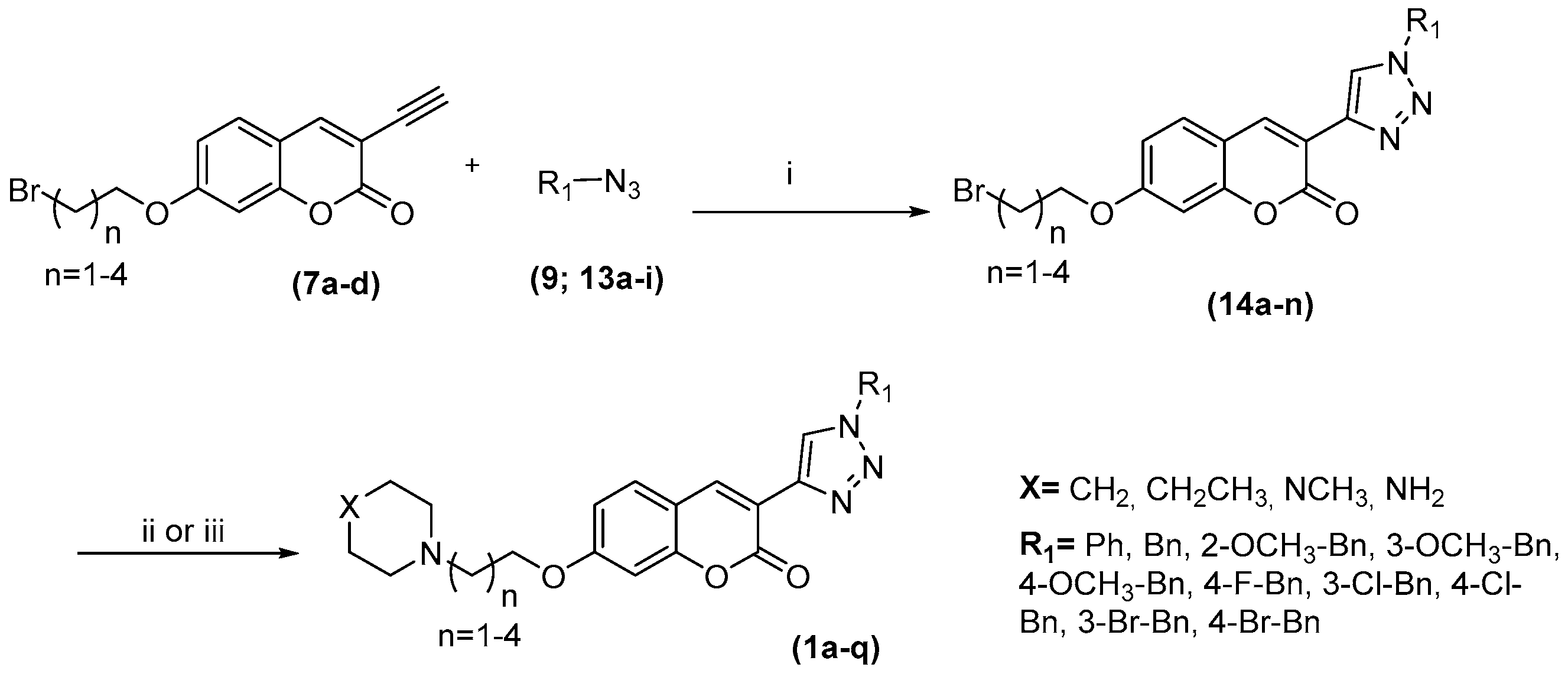
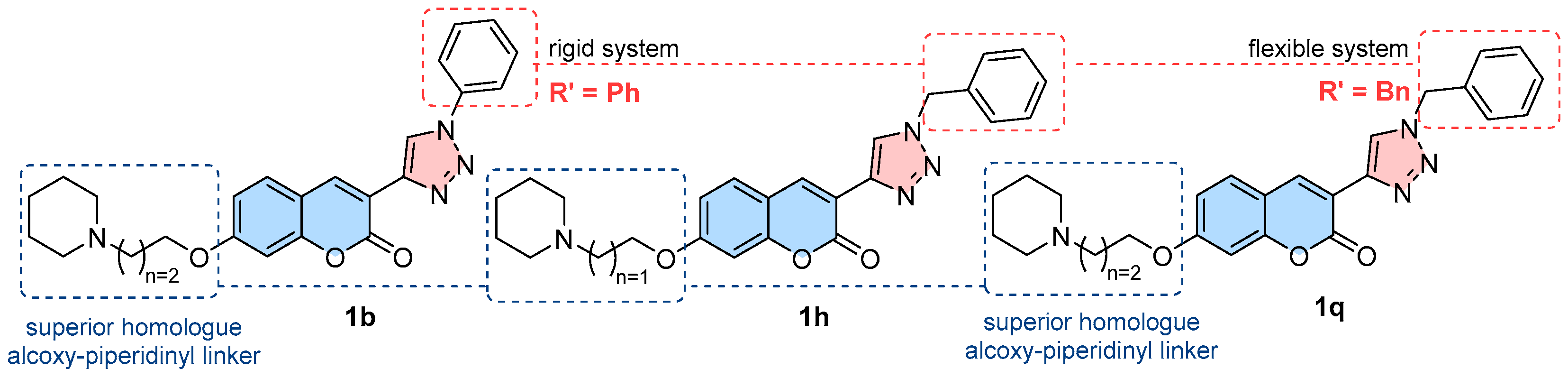
2.1. Compounds Synthesis
2.2. Evaluation of Anticholinesterase Activity
2.3. Mechanism of Cholinesterase Inhibitions
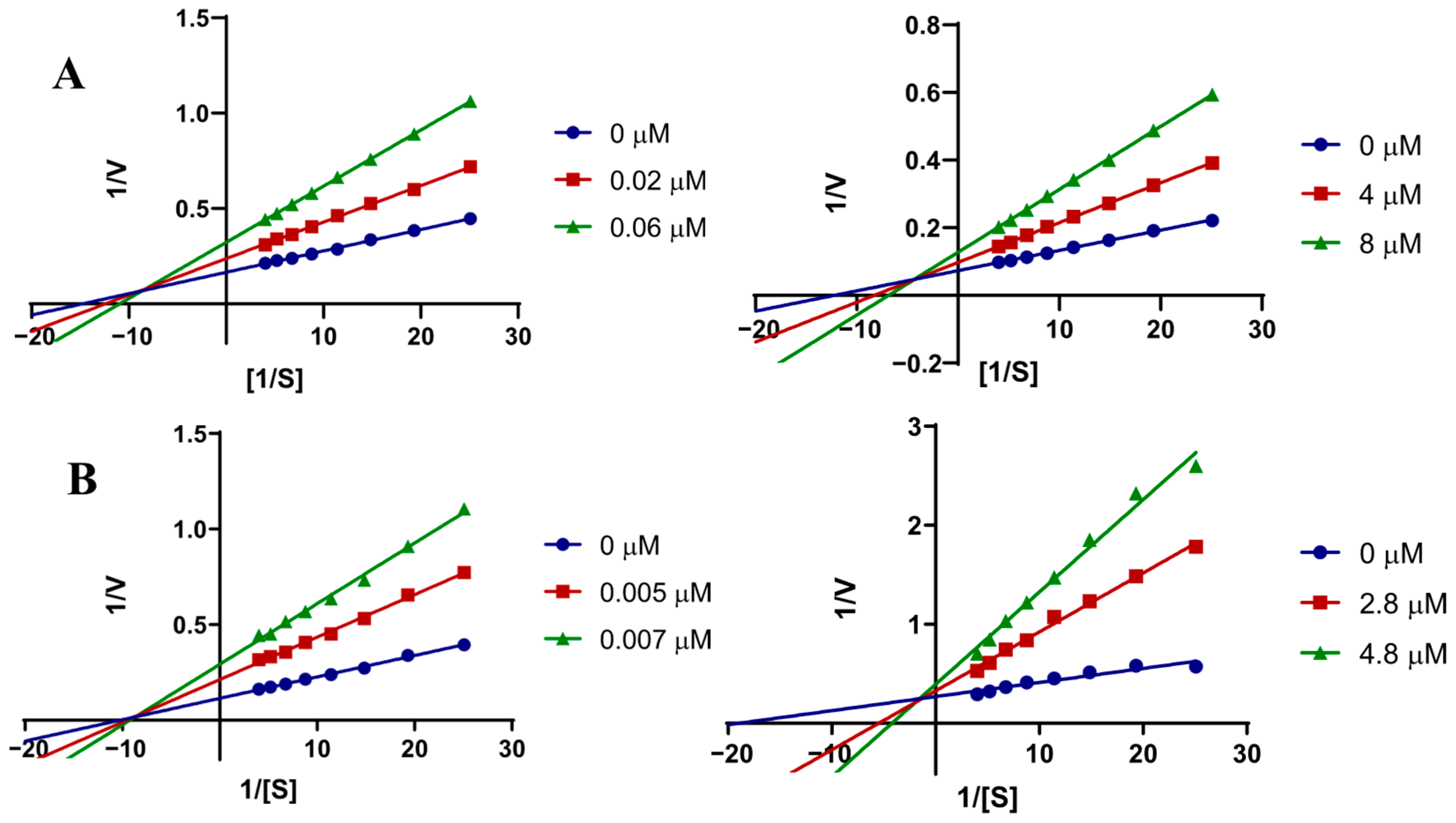
2.3.1. Kinetic Studies
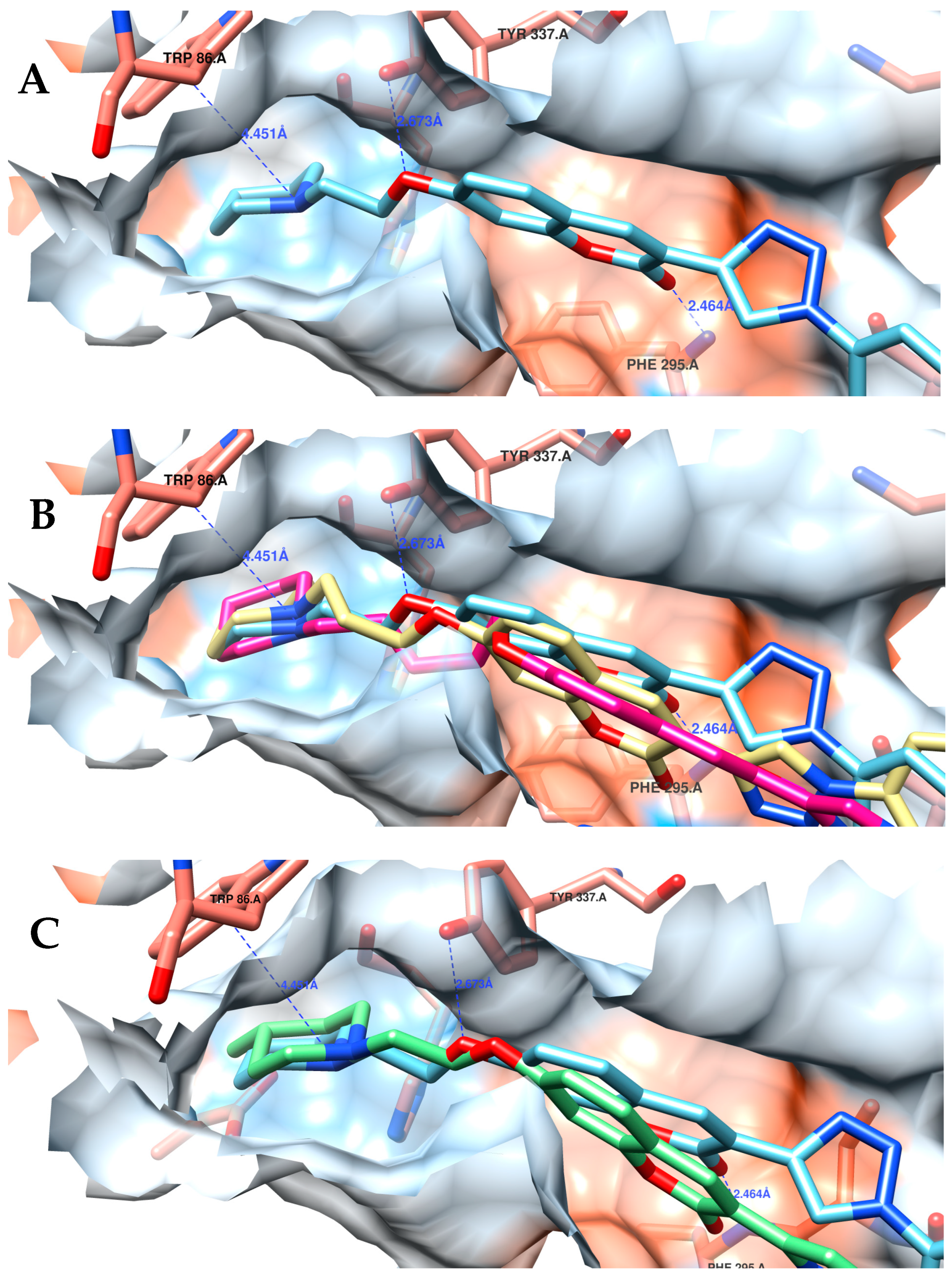
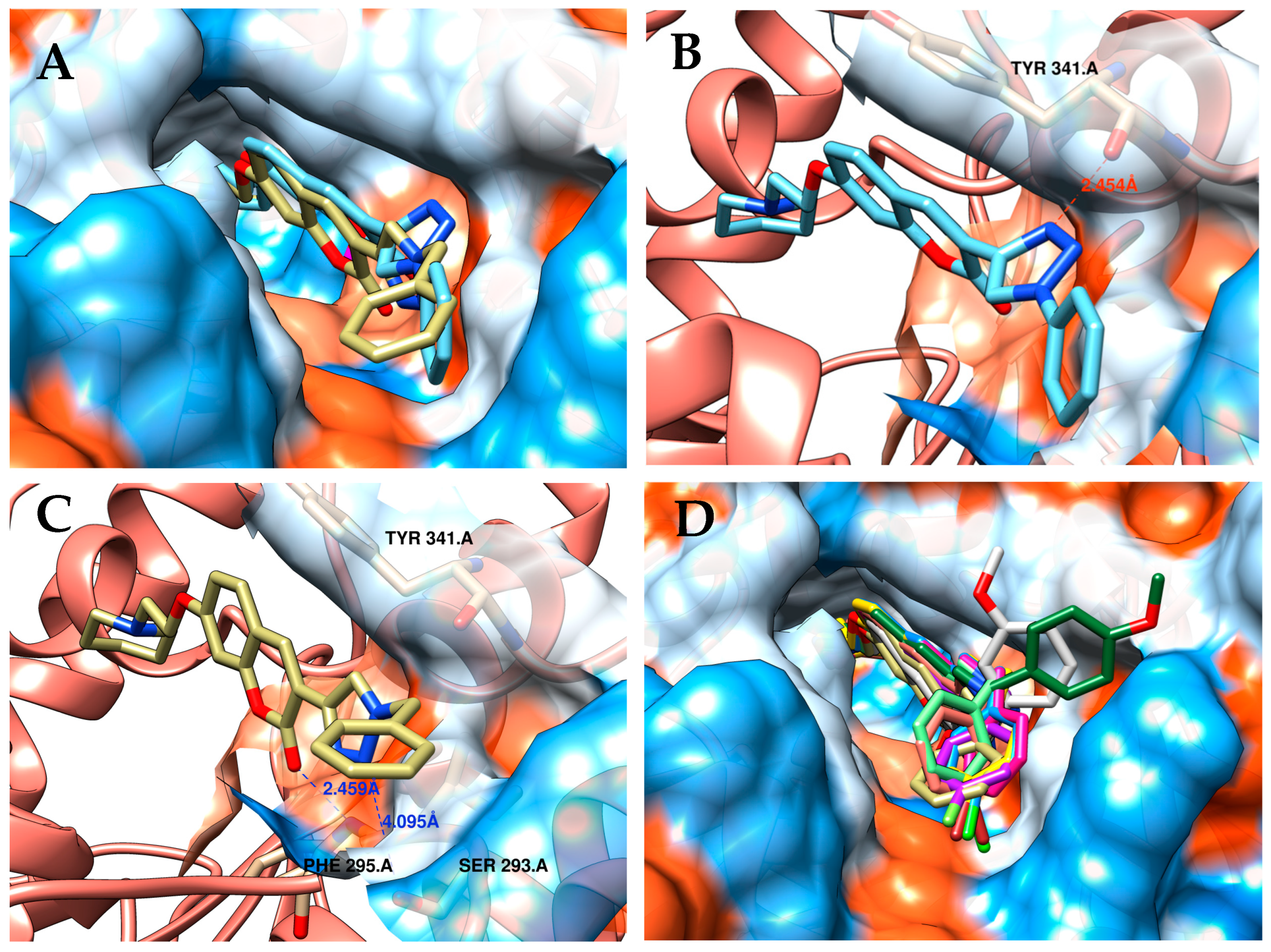
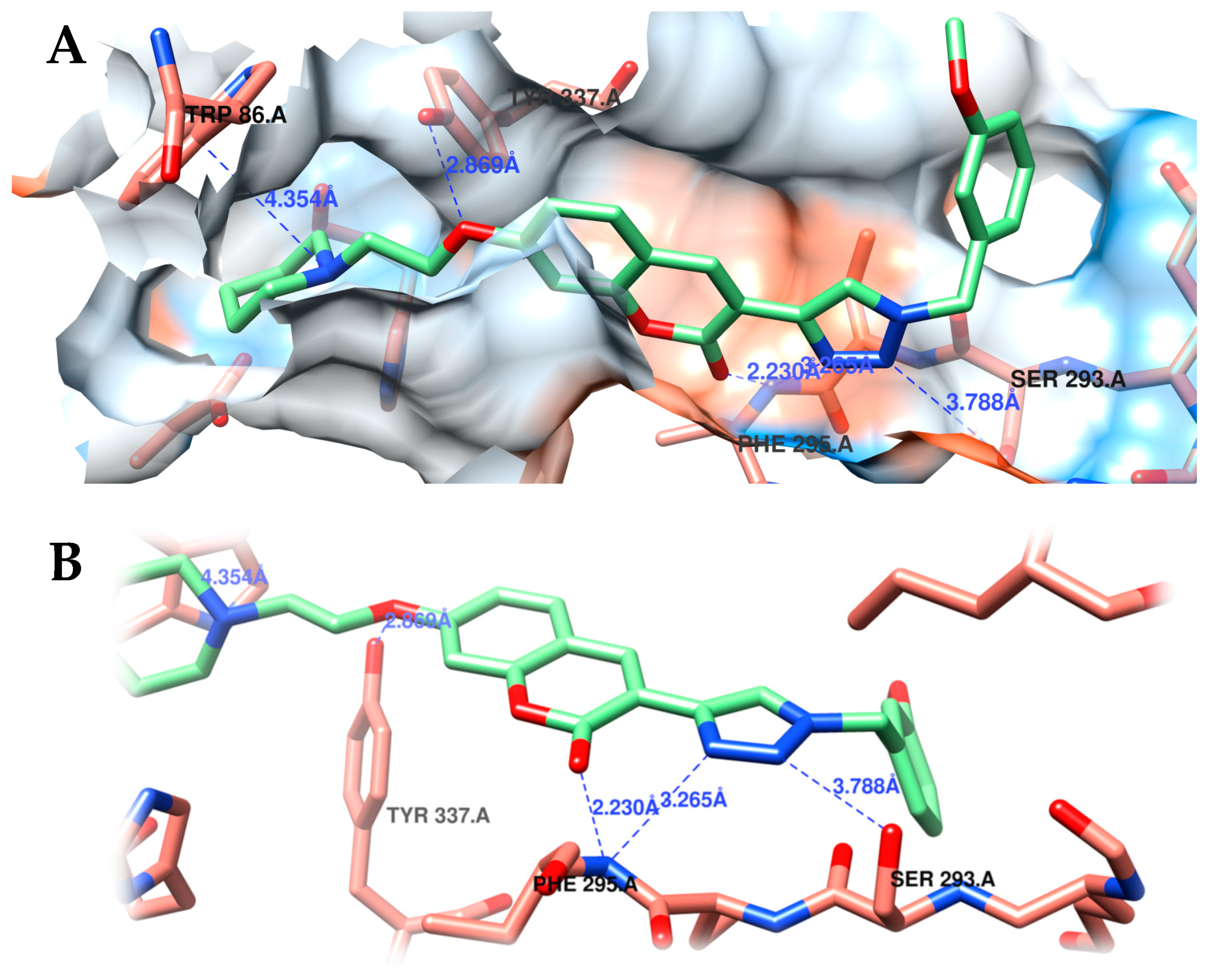
2.3.2. Molecular Docking Studies
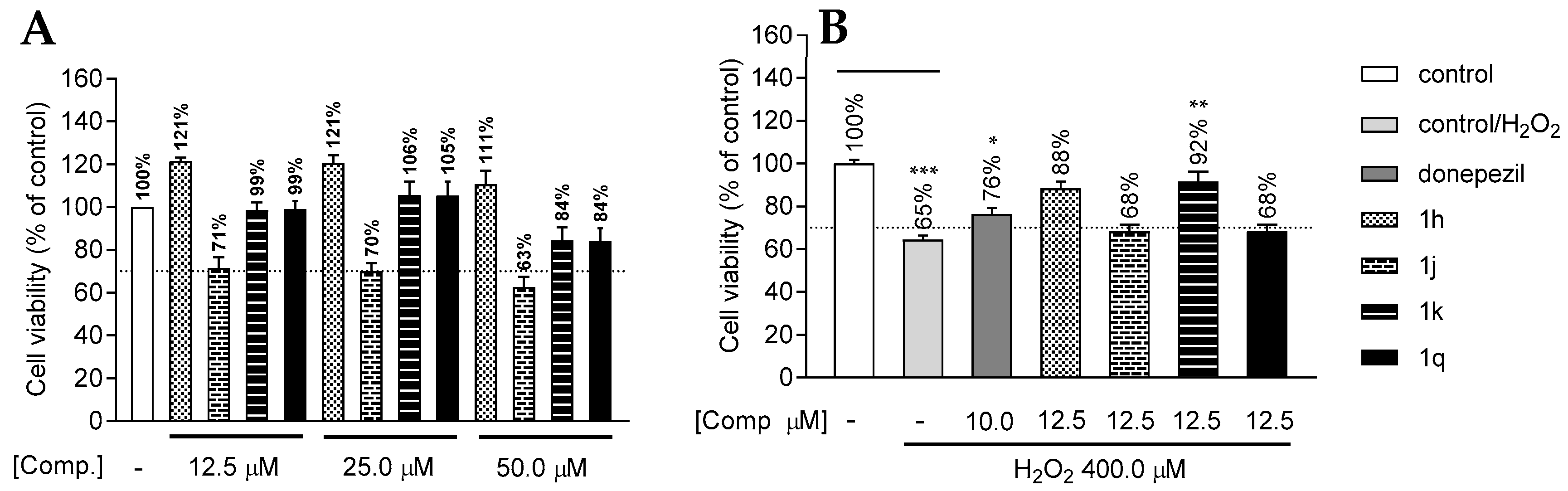
2.4. Cytotoxicity and In Vitro Neuroprotection Assay in SH-SY5Y Neuroblastoma Cells
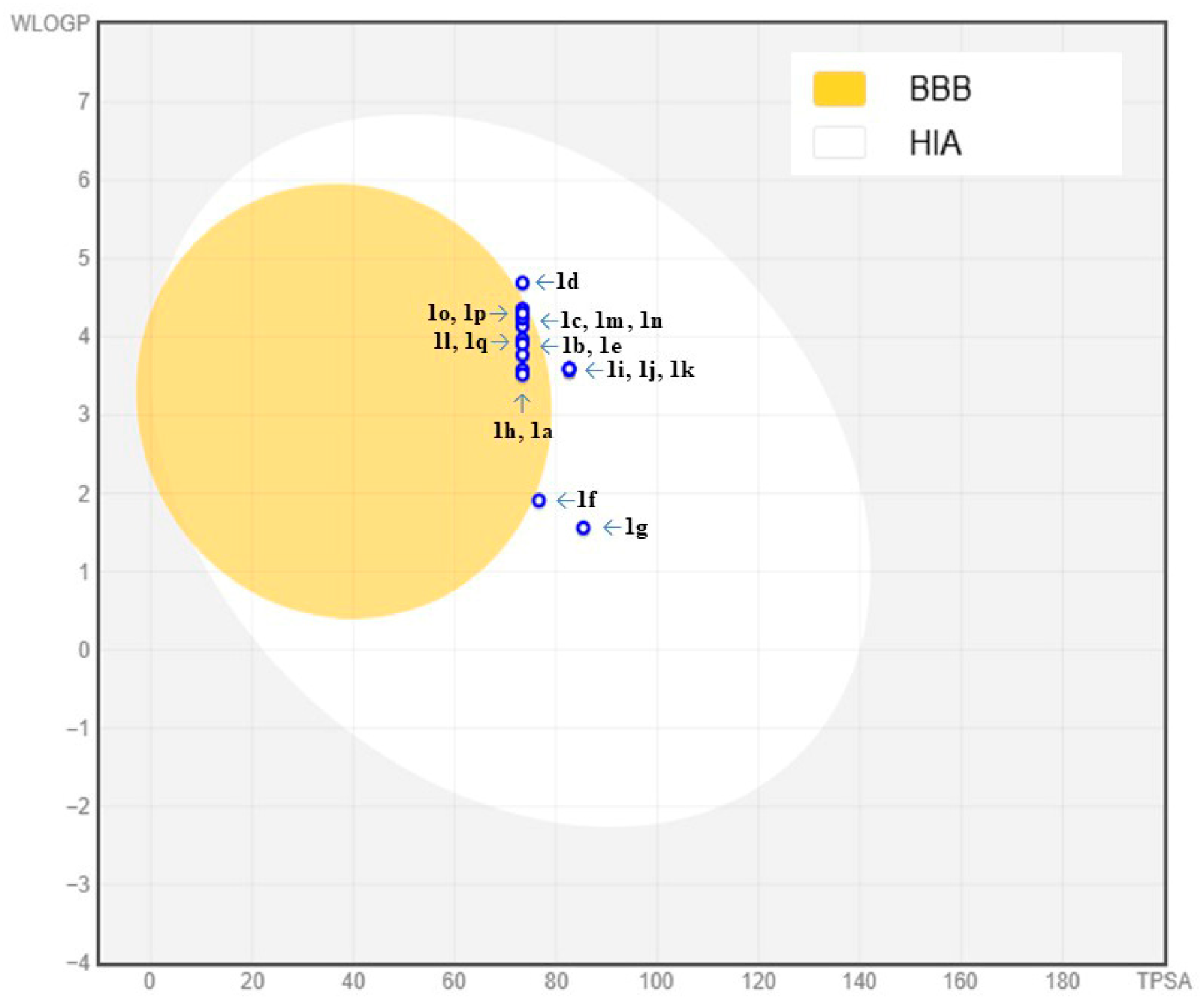
2.5. In Silico ADMET Physicochemical Profile Analysis
| Compound | TPSA (Å2) | LogP a | LogS b | GI Absorption c | BBB Permeant d |
|---|---|---|---|---|---|
| 1a | 73.39 | 3.62 | −4.96 | High | Yes |
| 1b | 73.39 | 3.91 | −5.19 | High | Yes |
| 1c | 73.39 | 4.24 | −5.42 | High | Yes |
| 1d | 73.39 | 4.60 | −5.65 | High | No |
| 1e | 73.39 | 3.87 | −5.31 | High | Yes |
| 1f | 76.63 | 2.70 | −4.39 | High | No |
| 1g | 85.42 | 2.50 | −4.02 | High | No |
| 1h | 73.39 | 3.63 | −4.93 | High | Yes |
| 1i | 82.62 | 3.64 | −5.00 | High | No |
| 1j | 82.62 | 3.63 | −5.00 | High | No |
| 1k | 82.62 | 3.63 | −5.00 | High | No |
| 1l | 73.39 | 4.04 | −5.09 | High | Yes |
| 1m | 73.39 | 4.22 | −5.52 | High | Yes |
| 1n | 73.39 | 4.19 | −5.52 | High | Yes |
| 1o | 73.39 | 4.30 | −5.84 | High | Yes |
| 1p | 73.39 | 4.27 | −5.84 | High | Yes |
| 1q | 73.39 | 3.99 | −5.15 | High | Yes |
2.6. Evaluation of Compounds as MTDLs: hH3R Ligands and MAO-B Inhibitors
3. Materials and Methods
3.1. Chemicals, Reagents, and Equipment
3.2. Synthesis of the New Compounds
3.2.1. Synthetic Procedures and Characterization Data for Trimethylsilyl Ethynyl (6a–d)
3.2.2. Synthesis Procedure for Deprotection (7a–d)
3.2.3. Synthesis Procedures for Phenyl Azide (9)
3.2.4. Synthetic Procedures for Benzyl Azides (13a–i)
3.2.5. Synthetic Procedures for Triazoles (14a–m)
3.2.6. Synthetic Procedures for Amines (1a–q)
3.3. Biological In Vitro Assays
3.3.1. Anticholinesterase Activity Assays
3.3.2. Enzymatic Kinetic Study
3.3.3. Evaluation of H3R Affinity
3.3.4. Monoamine Oxidase A/B Inhibitory Activity
3.4. Cytotoxicity in SH-SY5Y Neuroblastoma Cell Line
3.4.1. Culture Conditions for the SH-SY5Y Neuroblastoma Cell Line
3.4.2. Preparation of Samples for the MTT Cytotoxicity Assay
3.4.3. Cytotoxicity Assay Using the MTT Method
3.5. In Vitro Neuroprotection Assay Against SH-SY5Y Neuroblastoma Cell Line
3.6. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.; Iliffe, S. Alzheimer’s Disease. BMJ 2009, 338, b158. [Google Scholar] [CrossRef]
- Jellinger, K.A. Alzheimer 100—Highlights in the History of Alzheimer Research. J. Neural Transm. 2006, 113, 1603–1623. [Google Scholar] [CrossRef]
- Citron, M. Alzheimer’s Disease: Strategies for Disease Modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Ali, N.; Sayeed, U.; Shahid, S.M.A.; Akhtar, S.; Khan, M.K.A. Molecular Mechanisms and Biomarkers in Neurodegenerative Disorders: A Comprehensive Review. Mol. Biol. Rep. 2025, 52, 337. [Google Scholar] [CrossRef]
- Añazco, T.; Werner, T.; Torres, M.J.; Hornos-Carneiro, M.F.; Fernández, J.; Zivkovic, A.; Salas, C.O.; Castro-Álvarez, A.; Gutiérrez, M.; Stark, H.; et al. First in Class Pyrrolo[2,3-d]Pyrimidine Derivatives Fused to Fluorobenzylpiperidines as Dual Ligands of Acetylcholinesterase and Histamine H3 Receptor. Arch. Der Pharm. 2025, 358, e2400387. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, P.; Cui, W.; Yan, X.; Zhang, B.; He, S. Recent Advances in Multi-Target Anti-Alzheimer Disease Compounds (2013 Up to the Present). Curr. Med. Chem. 2019, 26, 5684–5710. [Google Scholar] [CrossRef]
- Han, X.; He, G. Toward a Rational Design to Regulate β-Amyloid Fibrillation for Alzheimer’s Disease Treatment. ACS Chem. Neurosci. 2018, 9, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wang, Y.; Li, X.; Wang, S.; Wang, Z. Recent Advance on Carbamate-Based Cholinesterase Inhibitors as Potential Multifunctional Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2022, 240, 114606. [Google Scholar] [CrossRef] [PubMed]
- De Boer, D.; Nguyen, N.; Mao, J.; Moore, J.; Sorin, E.J. A Comprehensive Review of Cholinesterase Modeling and Simulation. Biomolecules 2021, 11, 580. [Google Scholar] [CrossRef]
- Peitzika, S.-C.; Pontiki, E. A Review on Recent Approaches on Molecular Docking Studies of Novel Compounds Targeting Acetylcholinesterase in Alzheimer Disease. Molecules 2023, 28, 1084. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Singh, V.K.; Ahmad, I.; Agrawal, M.; Singh, R.K. Design, Synthesis, Molecular Docking, DFT Analysis, Dynamics Simulation and Cytotoxicity Evaluation of Coumarin Derivatives as Acetylcholinesterase (AChE) Inhibitors against Alzheimer’s Disease. J. Mol. Struct. 2025, 1329, 141436. [Google Scholar] [CrossRef]
- Pereira, T.M.; Franco, D.P.; Vitorio, F.; Kummerle, A.E. Coumarin Compounds in Medicinal Chemistry: Some Important Examples from the Last Years. Curr. Top. Med. Chem. 2018, 18, 124–148. [Google Scholar] [CrossRef]
- Joshi, R. Coumarin Derivatives: Mechanistic Insights and Promising Applications as Potential Alzheimer’s Disease (AD) Therapeutic Agents. ChemistrySelect 2023, 8, e202303861. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Y.; Zhu, L.; Wang, S.; Ji, J.; Rakesh, K.P. Triazole Derivatives as Inhibitors of Alzheimer’s Disease: Current Developments and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 180, 656–672. [Google Scholar] [CrossRef]
- de Freitas Silva, M.; Tardelli Lima, E.; Pruccoli, L.; Castro, N.G.; Guimarães, M.J.R.; da Silva, F.M.R.; Fonseca Nadur, N.; de Azevedo, L.L.; Kümmerle, A.E.; Guedes, I.A.; et al. Design, Synthesis and Biological Evaluation of Novel Triazole N-Acylhydrazone Hybrids for Alzheimer’s Disease. Molecules 2020, 25, 3165. [Google Scholar] [CrossRef]
- Michalska, B.; Dzięgielewski, M.; Godyń, J.; Werner, T.; Bajda, M.; Karcz, T.; Szczepańska, K.; Stark, H.; Więckowska, A.; Walczyński, K.; et al. 4-Oxypiperidine Ethers as Multiple Targeting Ligands at Histamine H3 Receptors and Cholinesterases. ACS Chem. Neurosci. 2024, 15, 1206–1218. [Google Scholar] [CrossRef]
- Singh, Y.P.; Tej, G.N.V.C.; Pandey, A.; Priya, K.; Pandey, P.; Shankar, G.; Nayak, P.K.; Rai, G.; Chittiboyina, A.G.; Doerksen, R.J.; et al. Design, Synthesis and Biological Evaluation of Novel Naturally-Inspired Multifunctional Molecules for the Management of Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 198, 112257. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Parambi, D.G.T.; Mathew, G.E.; Uddin, M.S.; Inasu, S.T.; Kim, H.; Marathakam, A.; Unnikrishnan, M.K.; Carradori, S. Emerging Therapeutic Potentials of Dual-Acting MAO and AChE Inhibitors in Alzheimer’s and Parkinson’s Diseases. Arch. Pharm. 2019, 352, 1900177. [Google Scholar] [CrossRef]
- Zagórska, A.; Jaromin, A. Perspectives for New and More Efficient Multifunctional Ligands for Alzheimer′s Disease Therapy. Molecules 2020, 25, 3337. [Google Scholar] [CrossRef]
- Silva, M.A.; Kiametis, A.S.; Treptow, W. Donepezil Inhibits Acetylcholinesterase via Multiple Binding Modes at Room Temperature. J. Chem. Inf. Model. 2020, 60, 3463–3471. [Google Scholar] [CrossRef]
- de Souza, G.A.; da Silva, S.J.; Del Cistia, C.D.N.; Pitasse-Santos, P.; Pires, L.D.O.; Passos, Y.M.; Cordeiro, Y.; Cardoso, C.M.; Castro, R.N.; Sant’Anna, C.M.R.; et al. Discovery of Novel Dual-Active 3-(4-(Dimethylamino)Phenyl)-7-Aminoalcoxy-Coumarin as Potent and Selective Acetylcholinesterase Inhibitor and Antioxidant. J. Enzym. Inhib. Med. Chem. 2019, 34, 631–637. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Jia, W.; Wang, J.; Yang, Z.; Liu, Y.; Huang, D.; Mei, X.; Xiong, X.; Shi, J.; Tang, Y.; et al. Design, Synthesis, and Biological Evaluation of Novel Donepezil-Tacrine Hybrids as Multi-Functional Agents with Low Neurotoxicity against Alzheimer’s Disease. Bioorganic Chem. 2024, 143, 107010. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Paletta, S.; Maffini, M.; Colasanti, A.; Dragogna, F.; Di Pace, C.; Altamura, A.C. Clinical Pharmacology of Atypical Antipsychotics: An Update. EXCLI J. 2014, 13, 1163–1191. [Google Scholar]
- Santos, S.N.; de Souza, G.A.; Pereira, T.M.; Franco, D.P.; Cistia, C.D.N.D.; Sant’Anna, C.M.R.; Lacerda, R.B.; Kümmerle, A.E. Regioselective Microwave Synthesis and Derivatization of 1,5-Diaryl-3-Amino-1,2,4-Triazoles and a Study of Their Cholinesterase Inhibition Properties. RSC Adv. 2019, 9, 20356–20369. [Google Scholar] [CrossRef] [PubMed]
- De Conto, V.; Cheung, V.; Maubon, G.; Souguir, Z.; Maubon, N.; Vandenhaute, E.; Bérézowski, V. In Vitro Differentiation Modifies the Neurotoxic Response of SH-SY5Y Cells. Toxicol. Vitr. 2021, 77, 105235. [Google Scholar] [CrossRef]
- Das, J.R.; Tizabi, Y. Additive Protective Effects of Donepezil and Nicotine Against Salsolinol-Induced Cytotoxicity in SH-SY5Y Cells. Neurotox. Res. 2009, 16, 194–204. [Google Scholar] [CrossRef]
- Queda, F.; Calò, S.; Gwizdala, K.; Magalhães, J.D.; Cardoso, S.M.; Chaves, S.; Piemontese, L.; Santos, M.A. Novel Donepezil–Arylsulfonamide Hybrids as Multitarget-Directed Ligands for Potential Treatment of Alzheimer’s Disease. Molecules 2021, 26, 1658. [Google Scholar] [CrossRef]
- Lingappa, S.; Shivakumar, M.S.; Manivasagam, T.; Somasundaram, S.T.; Seedevi, P. Neuroprotective Effect of Epalrestat on Hydrogen Peroxide-Induced Neurodegeneration in SH-SY5Y Cellular Model. J. Microbiol. Biotechnol. 2021, 31, 867–874. [Google Scholar] [CrossRef]
- Leonel Silva Sousa, G.; da Silva Honório, T.; de Souza Furtado, P.; Simon, A.; Mendes Cabral, L.; Rodrigues Coutinho Pereira, G.; Emanuel Ferreira Alves, J.; Mônica Vitalino de Almeida, S.; Rodrigues Silva, V.; de Souza Santos, L.; et al. Design, Synthesis and Antiproliferative Evaluation of New Acridine-Thiosemicarbazone Derivatives as Topoisomerase IIα Inhibitors. Results Chem. 2024, 7, 101371. [Google Scholar] [CrossRef]
- Belenichev, I.; Bukhtiyarova, N.; Ryzhenko, V.; Makyeyeva, L.; Morozova, O.; Oksenych, V.; Kamyshnyi, O. Methodological Approaches to Experimental Evaluation of Neuroprotective Action of Potential Drugs. Int. J. Mol. Sci. 2024, 25, 10475. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Didziapetris, R.; Japertas, P.; Avdeef, A.; Petrauskas, A. Classification Analysis of P-Glycoprotein Substrate Specificity. J. Drug Target. 2003, 11, 391–406. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Wingen, K.; Stark, H. Scaffold Variations in Amine Warhead of Histamine H3 Receptor Antagonists. Drug Discov. Today Technol. 2013, 10, e483–e489. [Google Scholar] [CrossRef]
- Rullo, M.; Benzi, A.; Bianchi, L.; Maccagno, M.; Marcantoni Taddei, G.; Miniero, D.V.; Mangiatordi, G.F.; Lentini, G.; Pisani, L.; Petrillo, G.; et al. In Vitro Evaluation of Novel Furo[3,2-c]Coumarins as Cholinesterases and Monoamine Oxidases Inhibitors. Molecules 2025, 30, 1830. [Google Scholar] [CrossRef]
- Bautista-Aguilera, Ó.M.; Hagenow, S.; Palomino-Antolin, A.; Farré-Alins, V.; Ismaili, L.; Joffrin, P.; Jimeno, M.L.; Soukup, O.; Janočková, J.; Kalinowsky, L.; et al. Multitarget-Directed Ligands Combining Cholinesterase and Monoamine Oxidase Inhibition with Histamine H3 R Antagonism for Neurodegenerative Diseases. Angew. Chem. Int. Ed. 2017, 56, 12765–12769. [Google Scholar] [CrossRef]
- Khanfar, M.A.; Reiner, D.; Hagenow, S.; Stark, H. Design, Synthesis, and Biological Evaluation of Novel Oxadiazole- and Thiazole-Based Histamine H3R Ligands. Bioorganic Med. Chem. 2018, 26, 4034–4046. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.M.; Lira, A.F.; Silva, D.R.; Guzzo, L.M.; Sant’Anna, C.M.R.; Kümmerle, A.E.; Rumjanek, V.M. Structural Insights into Cholinesterases Inhibition by Harmane β-Carbolinium Derivatives: A Kinetics—Molecular Modeling Approach. Phytochemistry 2012, 81, 24–30. [Google Scholar] [CrossRef]
- Kottke, T.; Sander, K.; Weizel, L.; Schneider, E.H.; Seifert, R.; Stark, H. Receptor-Specific Functional Efficacies of Alkyl Imidazoles as Dual Histamine H3/H4 Receptor Ligands. Eur. J. Pharmacol. 2011, 654, 200–208. [Google Scholar] [CrossRef]
- Feng, C.; Luo, T.; Zhang, S.; Liu, K.; Zhang, Y.; Luo, Y.; Ge, P. Lycopene Protects Human SH-SY5Y Neuroblastoma Cells against Hydrogen Peroxide-Induced Death via Inhibition of Oxidative Stress and Mitochondria-Associated Apoptotic Pathways. Mol. Med. Rep. 2016, 13, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Silva Sousa, G.L.; Nadur, N.F.; de Almeida Peixoto Ferreira, L.; da Silva Honório, T.; Simon, A.; Cabral, L.M.; Móra Santos, M.L.; Andrade, B.; de Lima, E.V.; Clarke, J.R.; et al. Discovery of Novel Thiosemicarbazone-Acridine Targeting Butyrylcholinesterase with Antioxidant, Metal Complexing and Neuroprotector Abilities as Potential Treatment of Alzheimer’s Disease: In Vitro, in Vivo, and in Silico Studies. Eur. J. Med. Chem. 2025, 281, 117030. [Google Scholar] [CrossRef]
- Shidore, M.; Machhi, J.; Shingala, K.; Murumkar, P.; Sharma, M.K.; Agrawal, N.; Tripathi, A.; Parikh, Z.; Pillai, P.; Yadav, M.R. Benzylpiperidine-Linked Diarylthiazoles as Potential Anti-Alzheimer’s Agents: Synthesis and Biological Evaluation. J. Med. Chem. 2016, 59, 5823–5846. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Wang, F.-Q.; Xie, J.; Chen, L.; Zhou, X.-L. Design, Synthesis, and Biological Activity of Donepezil: Aromatic Amine Hybrids as Anti-Alzheimerss Drugs. ACS Omega 2023, 8, 21802–21812. [Google Scholar] [CrossRef]
- Bourne, Y.; Grassi, J.; Bougis, P.E.; Marchot, P. Conformational Flexibility of the Acetylcholinesterase Tetramer Suggested by X-Ray Crystallography. J. Biol. Chem. 1999, 274, 30370–30376. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.-Y. Crystal Structures of Human Cholinesterases in Complex with Huprine W and Tacrine: Elements of Specificity for Anti-Alzheimer’s Drugs Targeting Acetyl- and Butyryl-Cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef]
 | |||||||
|---|---|---|---|---|---|---|---|
| IC50(nM) ± SD a | |||||||
| Compound | n | X | n’ | R | AChE b,c | BChE d | SI e |
| 1a | 1 | -CH2 | 0 | Ph | 50.02 ± 2.04 b | 8660 ± 150 | 173 |
| 1b | 2 | -CH2 | 0 | Ph | 1330 ± 45 b | 3970 ± 94 | 3 |
| 1c | 3 | -CH2 | 0 | Ph | 1110 ± 10 b | 2410 ± 29 | ~2 |
| 1d | 4 | -CH2 | 0 | Ph | 4790 ± 290 b | 3540 ± 81 | ~1 |
| 1e | 1 | -CHCH3 | 0 | Ph | 1690 ± 75 b | 11,130 ± 800 | ~7 |
| 1f | 1 | -NCH3 | 0 | Ph | 3160 ± 76 b | 8140 ± 890 | 3 |
| 1g | 1 | -NH | 0 | Ph | 1060 ± 74 b | 9840 ± 130 | ~8 |
| 1h | 1 | -CH2 | 1 | Ph | 6.03 ± 0.62 b/ 115.0 ± 8.1 c | 3790 ± 400 | 632/33 f |
| 1i | 1 | -CH2 | 1 | 2-OCH3-Ph | 15.26 ± 0.25 b | 2810 ± 230 | 184 |
| 1j | 1 | -CH2 | 1 | 3-OCH3-Ph | 4.16 ± 0.11 b/ 12.51 ± 0.55 c | 2880 ± 120 | 686/222 f |
| 1k | 1 | -CH2 | 1 | 4-OCH3-Ph | 4.58 ± 0.62 b | 2080 ± 99 | 443 |
| 1l | 1 | -CH2 | 1 | 4-F-Ph | 23.41 ± 1.77 b | 4120 ± 110 | 176 |
| 1m | 1 | -CH2 | 1 | 3-Cl-Ph | 13.08 ± 0.83 b | 1950 ± 120 | 149 |
| 1n | 1 | -CH2 | 1 | 4-Cl-Ph | 103.8 ± 5.4 b | 2240 ± 60 | 16 |
| 1o | 1 | -CH2 | 1 | 3-Br-Ph | 7.92 ± 0.48 b | 2090 ± 54 | 265 |
| 1p | 1 | -CH2 | 1 | 4-Br-Ph | 12.88 ± 0.96 | 1140 ± 70 | 88 |
| 1q | 2 | -CH2 | 1 | Ph | 1950 ± 86 b/ 1412 ± 64 c | 3560 ± 190 | ~2 e,f |
| Donepezil | - | - | - | - | 7.02 ± 0.24 b/ 89 g | 2390 ± 110 | 341/27 f |
| Conc. (μM) | Vmax ± SD (1 × 103 nM/min) | Km ± SD (1 × 103 nM) | Ki (nM) ± SE a | Ki’ (nM) ± SE b |
|---|---|---|---|---|
| 1a in AChE | ||||
| 0 | 6.10 ± 0.09 | 40.38 ± 1.57 | ||
| 0.02 | 2.78 ± 0.04 | 70.30 ± 1.53 | 30.79 ± 5.38 | 61.59 ± 20.04 |
| 0.06 | 2.06 ± 0.03 | 102.1 ± 0.10 | ||
| 1a in BChE | ||||
| 0 | 13.92 ± 0.09 | 85.46 ± 0.83 | ||
| 4 | 11.69 ± 0.20 | 113.1 ± 0.8 | 3940 ± 230 | 11,400 ± 1100 |
| 8 | 8.24 ± 0.21 | 157.5 ± 1.5 | ||
| 1h in AChE | ||||
| 0 | 8.50 ± 0.17 | 72.19 ± 6.82 | ||
| 0.005 | 4.45 ± 0.02 | 88.07 ± 6.78 | 4.73 ± 0.88 | 7.17 ± 1.48 |
| 0.007 | 3.19 ± 0.010 | 93.73 ± 7.01 | ||
| 1h in BChE | ||||
| 0 | 4.51 ± 0.16 | 88.7 2± 7.61 | ||
| 2.8 | 3.62 ± 0.14 | 234.4 ± 16.2 | 1250 ± 150 | 4830 ± 620 |
| 4.8 | 3.40 ± 0.03 | 355.7 ± 19.2 | ||
| Compound | Ki hH3R [nM] a | hMAO-A IC50 [nM] a | hMAO-B IC50 [nM] a |
|---|---|---|---|
| 1b | 32 [25; 41] | 6.4 ± 0.5 b | 11.4 ± 1.9 b |
| 1h | 558 [337; 925] | 5.2± 1.9 b | 11.3 ± 1.9 b |
| 1q | 151 [71; 325] | 31,055 [11,758; 82,018] | 1688 [1164; 2449] |
| Pitolisant | 6.5 [2.3; 18] | - | - |
| Rasagiline | - | - | 25 ± 7 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadur, N.F.; Ferreira, L.d.A.P.; Franco, D.P.; de Azevedo, L.L.; Caruso, L.; Honório, T.d.S.; Furtado, P.d.S.; Simon, A.; Cabral, L.M.; Werner, T.; et al. Design, Synthesis, and Biological Evaluation of Novel Multitarget 7-Alcoxyamino-3-(1,2,3-triazole)-coumarins as Potent Acetylcholinesterase Inhibitors. Pharmaceuticals 2025, 18, 1398. https://doi.org/10.3390/ph18091398
Nadur NF, Ferreira LdAP, Franco DP, de Azevedo LL, Caruso L, Honório TdS, Furtado PdS, Simon A, Cabral LM, Werner T, et al. Design, Synthesis, and Biological Evaluation of Novel Multitarget 7-Alcoxyamino-3-(1,2,3-triazole)-coumarins as Potent Acetylcholinesterase Inhibitors. Pharmaceuticals. 2025; 18(9):1398. https://doi.org/10.3390/ph18091398
Chicago/Turabian StyleNadur, Nathalia F., Larissa de A. P. Ferreira, Daiana P. Franco, Luciana L. de Azevedo, Lucas Caruso, Thiago da S. Honório, Priscila de S. Furtado, Alice Simon, Lucio M. Cabral, Tobias Werner, and et al. 2025. "Design, Synthesis, and Biological Evaluation of Novel Multitarget 7-Alcoxyamino-3-(1,2,3-triazole)-coumarins as Potent Acetylcholinesterase Inhibitors" Pharmaceuticals 18, no. 9: 1398. https://doi.org/10.3390/ph18091398
APA StyleNadur, N. F., Ferreira, L. d. A. P., Franco, D. P., de Azevedo, L. L., Caruso, L., Honório, T. d. S., Furtado, P. d. S., Simon, A., Cabral, L. M., Werner, T., Stark, H., & Kümmerle, A. E. (2025). Design, Synthesis, and Biological Evaluation of Novel Multitarget 7-Alcoxyamino-3-(1,2,3-triazole)-coumarins as Potent Acetylcholinesterase Inhibitors. Pharmaceuticals, 18(9), 1398. https://doi.org/10.3390/ph18091398







