1. Introduction
Heterocycles constitute a broad class of molecules found in many biologically active compounds in the human body [
1]. Their natural abundance, structural complexity, and ability to form hydrogen bonds make heterocycles particularly attractive for pharmaceutical applications [
2]. Particularly, their use falls under a wide set of applications in multitargeting strategies by adopting both heterocycles and/or chimeric moieties [
3,
4,
5]. Briefly, a multitarget strategy involves designing drugs that can interact with multiple biological targets to treat complex diseases [
3,
6,
7]. This approach is an alternative to traditional “one drug, one target” therapies [
6], offering potential advantages in efficacy and safety for multifactorial conditions [
7]. This strategy involves combining pharmacophores from different bioactive molecules into a single chemical entity [
3,
4,
5,
6,
7]. Among the various applications, this can be achieved either by linking two distinct pharmacophores with an appropriate spacer or linker or by merging the active pharmacophores into a single structural framework, consequently generating a unified scaffold (chimeric scaffold) [
7]. These compounds have garnered significant interest due to their capacity to interact with multiple enzymes and modulate their activity.
The study of heterocycles as inhibitors of human carbonic anhydrase (hCA) activity has emerged as a promising strategy for therapeutic intervention [
8,
9]. hCAs constitute a family of drug targets characterized by multiple isoforms with distinct cellular localizations and physiological roles, which can vary significantly across tissues and pathological conditions [
10]. The 12 catalytically active isoforms exhibit a central role in processes such as pH homeostasis, respiration, and CO
2/HCO
3− transport [
10]. They also contribute to key biosynthetic pathways, including gluconeogenesis, lipogenesis, and ureagenesis, all of which are essential for cellular metabolism [
11,
12,
13]. Dysregulation of hCAs has been linked to a wide spectrum of disorders, including glaucoma, inflammatory conditions, hypoxic tumors, and neurodegenerative diseases [
14]. As a consequence, over time, the inhibition of hCA isoforms has become an important therapeutic approach, offering promising avenues for the treatment of diverse diseases [
11,
12,
13,
14].
Despite the large number of reviews published in the carbonic anhydrase field in recent years [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20], this review summarizes, for the first time, the role of hybrid and chimeric heterocycles in carbonic anhydrase inhibition produced in the last 10 years, focusing on their use as scaffolds, linkers, and multitarget-directed moieties to enhance CA binding.
4. Heterocycles Used as Linkers for the Design of Carbonic Anhydrase Inhibitors
Using heterocyclic rings as linkers between two pharmacophoric units is a simple and highly versatile synthetic strategy in medicinal chemistry. The availability of robust synthetic protocols, coupled with the rapid and flexible incorporation of nitrogen- or oxygen-containing heterocycles as linkers, enables the efficient generation of pharmacophore hybrids. These heterocycles can contribute to conformational rigidity and overall molecular polarity and direct interactions with biological targets, thereby enhancing binding affinity and selectivity. Furthermore, the modular nature of heterocyclic scaffolds enables variation in both substituents and ring systems, thus facilitating the modification of physicochemical and target interaction properties with minimal synthetic effort. Overall, heterocycles used as linkers offer synthetic accessibility and structural diversity while actively participating in binding mechanisms, making them an ideal platform for dual-pharmacophore drug design [
38,
39,
40].
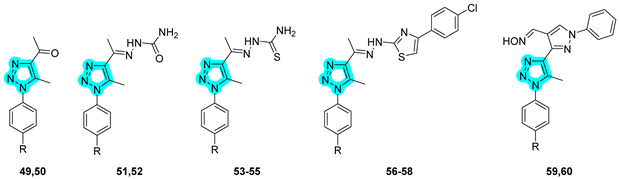
In 2020, Elzahhar et al. reported, for the first time, a novel series of multitarget-directed compounds designed to simultaneously inhibit cyclooxygenase-2, 15-lipoxygenase (15-LOX), and tumor-associated carbonic anhydrases to develop new anticancer agents [
41]. The researchers employed a 1,2,3-triazole linker to conjugate distinct pharmacophoric moieties, yielding the hybrid structures depicted in
Table 12. Compounds
49–
60 were subsequently evaluated in vitro for their inhibitory activity against COX-1; COX-2; 15-LOX; and hCA isoforms I, II, IX, and XII (
Table 12).
Among them, compounds
57–
60 exhibited superior COX-2 inhibition compared to the reference drug celecoxib, as well as enhanced COX-1/COX-2 selectivity indices. The entire series demonstrated moderate 15-LOX inhibition, and compounds
57 and
59 showed potent inhibition of the tumor-associated CA isoforms hCA IX and XII. It is noteworthy that the activity of the compounds toward COX-1 and COX-2 is generally comparable within members of the same subseries, suggesting a limited influence of the CAI moiety on COX binding. Conversely, regarding CA inhibition, a sulfonamide group was present, as the CAI moiety usually results in stronger inhibition, an effect that is particularly pronounced against CA I and II. However, exceptions such as derivative 60 highlight that binding to the CA active site is primarily driven by the CAI moiety but is also strongly influenced by the overall molecular architecture. The antiproliferative activity of these compounds was also assessed in vitro against human cancer cell lines, including lung (A549), liver (HepG2), and breast (MCF-7) cells [
41]. Notably,
57 exhibited moderate activity against A549 cells (IC
50 = 28.5 μM), whereas
59 displayed significant inhibitory activity against MCF-7 cells (IC
50 = 3.2 μM). Importantly, all compounds demonstrated a favorable safety profile, showing limited cytotoxicity against normal human lung fibroblasts (WI-38). Mechanistic investigations revealed that the antitumor activity of
57 was associated with G2/M cell cycle arrest and apoptosis induction, evidenced by upregulation of caspase-9 and Bax expression and concurrent downregulation of Bcl-2 levels. In vivo studies on a xenograft mouse model further confirmed the therapeutic potential of
59, which significantly reduced tumor volume following treatment. Finally, in silico docking studies supported the experimental findings, highlighting the dual role of the triazole linker: beyond its structural bridging function, it actively participates in target binding by engaging in key interactions within the active sites [
41].
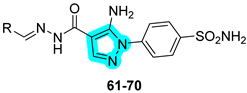
The same research group in 2023 further explored the same type of molecular hybrids by applying bioisosteric modifications to celecoxib (compounds
61–
70,
Table 13) and polmacoxib (compounds
71–
80,
Table 14) to develop novel anti-inflammatory agents [
42,
43]. As in their previous study, the compounds were evaluated in vitro for their inhibitory activities against the primary targets, COX-2, 15-LOX, and hCAs IX and XII, as well as against relevant off-targets, COX-1 and the cytosolic isoforms hCAs I and II. Within the first series, compounds
61,
62, and
70 emerged as the most potent dual COX-2/15-LOX inhibitors, exhibiting IC
50 values in the low micromolar range. In the second series, compounds
71,
75, and
76 demonstrated the most promising activity profiles. All compounds across both series showed potent inhibition of hCA IX and XII, with IC
50 values in the nanomolar range [
42,
43].
Compounds
61 and
62, which displayed the most favorable in vitro inhibition profiles, were further assessed in vivo to evaluate their anti-inflammatory potential. Both compounds significantly reduced the number of writhing responses in the acetic acid-induced writhing test and demonstrated efficacy in a carrageenan-induced rat paw edema model. Additionally, ELISA assays revealed a marked decrease in serum levels of key pro-inflammatory cytokines, including TNF-α and IL-1β. Similarly, compounds
75 and
76 from the second series also underwent in vivo evaluation and showed promising analgesic and anti-inflammatory effects, confirming their potential as lead candidates for further preclinical development [
42,
43].
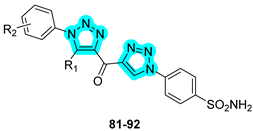
Recently, the research group of Sharma employed heterocyclic linkers, specifically triazole-based structures, for the development of novel anticancer agents targeting tumor-associated carbonic anhydrases (hCAs IX and XII) and cathepsin B [
44]. In 2023, a panel of 28 keto-bridged dual-triazole-containing benzenesulfonamides was synthesized and biologically evaluated for inhibitory activity against hCA isoforms and cathepsin B. Selected compounds with the most promising in vitro biological profiles are presented in
Table 15. Of these, compound
87 demonstrated the most favorable inhibition profile toward hCAs IX and XII, displaying K
I values lower than those of the standard inhibitors
AAZ and
SLC-0111. In contrast, compound
89 emerged as the most potent cathepsin B inhibitor within the series but only displayed activity in the millimolar range. In silico molecular docking studies were conducted to explore the binding interactions of the most active derivatives. The results highlighted the key role of the triazole linker in mediating direct target engagement. In the case of cathepsin B, the triazole ring was found to form a specific hydrogen bond with the side chain of His199 within the active site, contributing significantly to binding affinity. In contrast, within the active sites of CAs, the triazole moiety primarily acted as a structural element critical to correctly orient the terminal tail of the molecule, thereby optimizing its interactions with key residues lining the binding cavity [
44].
In the following year, Sharma and co-workers continued to refine this dual-inhibitor strategy by incorporating a 1,2,4-triazole linker, resulting in the design and synthesis of 22 novel derivatives [
45]. The most active compounds from this new series are shown in
Table 16. As observed, the new derivatives retained strong inhibitory activity against hCA IX and XII, with compound
102 exhibiting the most promising profile, although with slightly weaker K
I values compared to compound
87 from the previous series. However, the second-generation compounds showed significantly enhanced cathepsin B inhibition, reaching activity in the submicromolar range (10
−7 M), thus marking a substantial improvement in dual-target efficacy [
45].
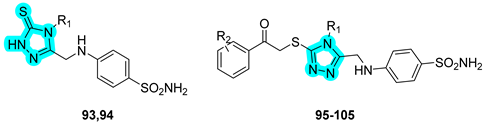
In 2024, Abbas et al. applied a sugar-tail approach utilizing a 1,2,3-triazole linker to conjugate a benzenesulfonamide moiety with glycosidic fragments, aiming to develop dual inhibitors of tumor-associated carbonic anhydrases (hCAs IX and XII) and vascular endothelial growth factor receptor 2 (VEGFR-2) as novel anticancer agents [
46]. The designed and synthesized compounds are presented in
Table 17 and were evaluated in vitro for their inhibitory activity against VEGFR-2, hCA IX, and hCA XII, yielding results comparable to those of the standard inhibitors sorafenib and
SLC-0111 [
46].
These were further screened for antiproliferative activity against various human cancer cell lines, including lung (A549), liver (HepG2), breast (MCF-7), and colorectal (HCT-116) cancer cells. Both compounds showed notable potency against HepG2 cells (IC
50 = 10.45 μM for compound
106 and 8.39 μM for compound
107) and MCF-7 cells (IC
50 = 20.31 μM for
106 and 21.15 μM for
107), with activity values comparable to those of doxorubicin (IC
50 = 13.76 μM and 17.44 μM, respectively). Moreover, molecular docking studies were performed to investigate the binding mechanisms of compounds
106 and
107 within the active sites of VEGFR-2, hCA IX, and hCA XII. These analyses highlighted the direct involvement of the triazole linker in specific interactions with key active site residues, in addition to its structural role as a pharmacophore bridge. Furthermore, the difference in the glycidic moiety between compounds
106 and
107 reduces steric hindrance in the latter, potentially explaining the improved profile observed in the in vitro studies [
46].
Collectively, these studies underscore the critical importance of linker selection in the design of hybrid compounds incorporating two pharmacophores. Beyond controlling spatial orientation and inter-pharmacophore distance, appropriately designed linkers, particularly those incorporating heteroatoms, can directly contribute to target binding. As emphasized throughout this review, heteroatom-rich linkers such as triazoles may expand the interaction landscape via hydrogen bond and polar contacts, thus enhancing both affinity and selectivity of the resulting molecular hybrids.
5. Heterocycles Used as Second Pharmacophores in Multitargeting Carbonic Anhydrase Inhibitors
In the design of dual-target carbonic anhydrase inhibitors (CAIs), various heterocyclic scaffolds have been effectively used as secondary pharmacophores to enhance isoform selectivity and biological potency.
In 2020, Ceni et al. designed and synthesized a novel series of dual-acting compounds as adenosine A
2A receptor (hA
2A AR) antagonists and hCA IX and XII inhibitors to develop new anticancer agents [
47]. The core scaffold used in this study was based on an 8-amino-6-aryl-2-phenyl-1,2,4-triazolo [4,3-a]pyrazin-3-one structure, originally derived from a known hA
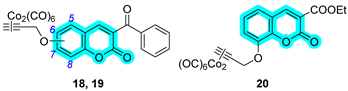
2A AR antagonist, which was conjugated with various hCA-inhibiting moieties through different types of linkers. Representative compounds from the series are shown in
Table 18. Many of the synthesized derivatives demonstrated nanomolar affinity for hA
2A AR, with excellent selectivity over other adenosine receptor subtypes. These derivatives exhibited superior affinity to the reference ligands 5-(N-ethyl-carboxamido)adenosine (NECA) and 2-chloro-N6-cyclopentyladenosine (CCPA). Regarding CA inhibition, most compounds displayed moderate activity, with K
I values in the micromolar range. Among them, compound
114 exhibited the most promising inhibition profile toward hCAs IX and XII. Considering the dual activity profile, compounds
114 and
120 emerged as the most promising candidates for further pharmacological evaluation [
47].
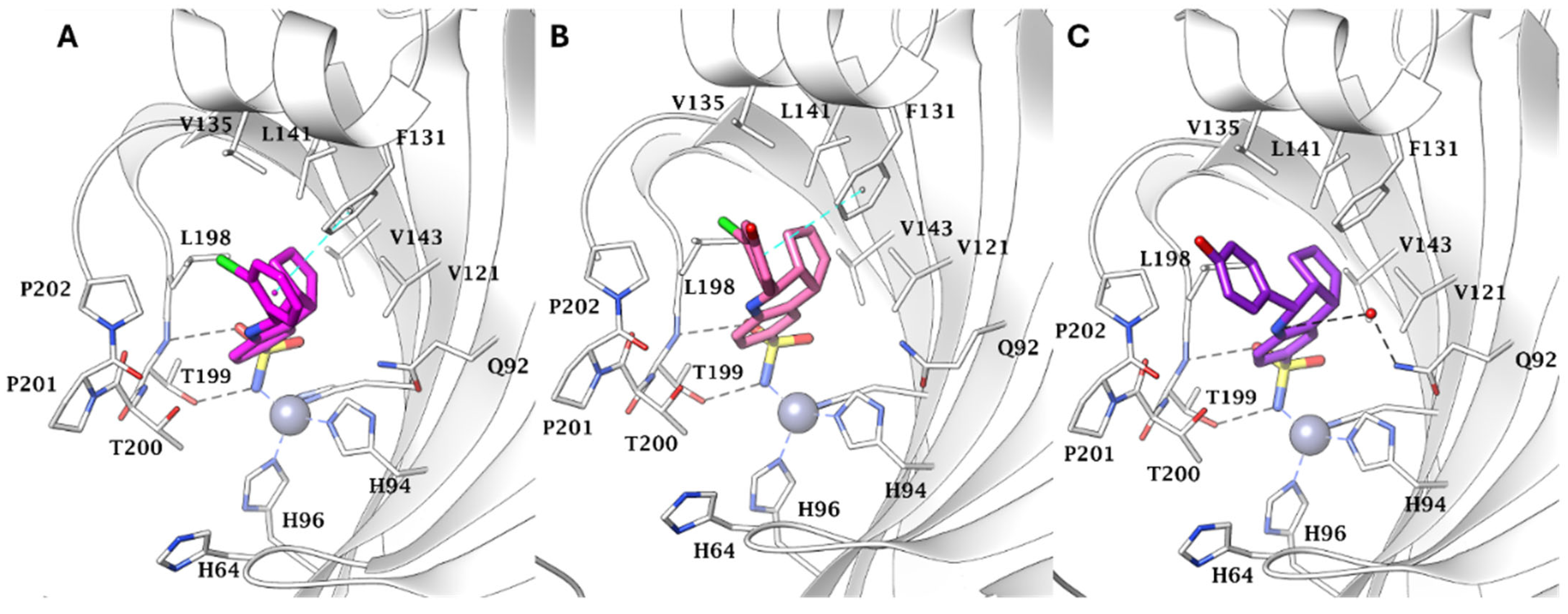
Molecular docking studies were conducted to elucidate the binding modes of selected derivatives within both targets. Interestingly, although the CAI moiety does not contribute to interactions with the hA
2A receptor, the triazolopyrazinone core was found to be directly involved in binding to the outer region of the hCA active sites. Specifically, the nitrogen atom of the pyrazine ring forms a hydrogen bond with the hydroxyl group of Ser132 in hCA XII [
47].
These findings highlight the importance of rational design in dual-target agents, where both pharmacophoric units must be considered active contributors to binding. The optimal spatial and electronic complementarity of both moieties is essential to maximize interactions across the distinct binding environments of the two targets.
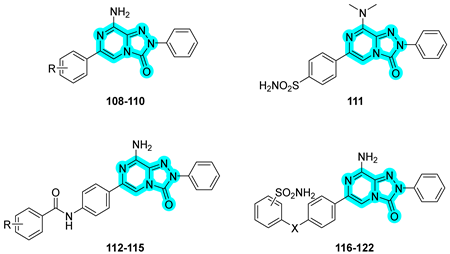
In the field of anticancer drug discovery, Zhang and co-workers developed a series of dual inhibitors targeting both the epidermal growth factor receptor (EGFR) and tumor-associated hCA IX by combining quinazoline-based derivatives with benzenesulfonamide moieties [
48]. The synthesized compounds were evaluated in vitro for their inhibitory activity against wild-type EGFR (EGFR
WT), where compound
123 demonstrated superior potency compared to the reference drug gefitinib. Against the EGFR
T790M mutant, compound
124 exhibited significantly improved inhibition, achieving activity levels comparable to osimertinib (
Table 19). Additionally, the compounds were tested for their inhibitory activity against hCA II and hCA IX, with compound
124 again emerging as the most potent and selective inhibitor of the tumor-associated isoform hCA IX.
The antiproliferative activity of the compounds was assessed in vitro against various human cancer cell lines, including epidermoid carcinoma (A431) and non-small cell lung cancer (A549 and H1975). Notably, compound
124 exhibited comparable potency to osimertinib in H1975 cells (IC
50 = 1.94 μM vs. 0.98 μM) and even outperformed the reference drug under hypoxic conditions (IC
50 = 1.05 μM vs. 2.08 μM). Western blot analysis revealed that
124 significantly suppressed the expression of phosphorylated EGFR (p-EGFR) and its downstream signaling proteins p-AKT and p-ERK in H1975 cells. Furthermore, under hypoxic conditions, it also inhibited the expression of hCA IX and its upstream regulator HIF-1α, confirming its dual mechanism of action. Finally, molecular docking studies provided further insight into the binding interactions of compound
124 with hCA IX, EGFR
WT, and EGFR
T790M. In EGFR
WT, the sulfonamide moiety forms a key hydrogen bond with Lys745. In the EGFR
T790M mutant, the altered binding pocket architecture allows for two additional hydrogen bonds between the sulfonamide group and residues Arg841 and Asp855. These enhanced interactions may explain the potent inhibitory activity of
124 against the resistant EGFR
T790M kinase variant [
48].
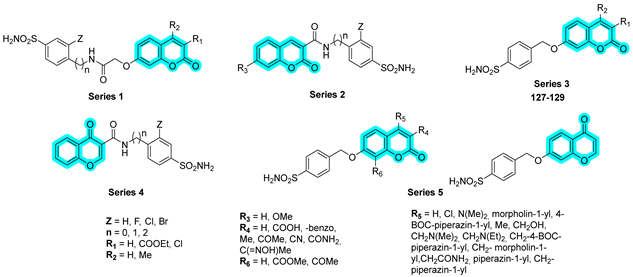
In 2024, Giovannuzzi et al. designed and synthesized a series of dual inhibitors targeting brain hCAs and Monoamine Oxidase B (MAO-B), with the aim of modulating multiple pathological pathways implicated in Alzheimer’s disease and preventing β-amyloid (Aβ-42)-associated neurotoxicity [
49]. The authors combined a chromone or coumarin scaffold, known for its reversible inhibition of MAO-B, with a benzenesulfonamide moiety to target hCAs. Initially, four distinct series of hybrid compounds (Series 1–4,
Table 20) were developed and evaluated for their inhibitory activity against hCA isoforms I, II, IV, VA, VB, VII, and XII, as well as hMAO-A and hMAO-B. The results were benchmarked against standard drugs including methazolamide (
MTZ), acetazolamide (
AAZ), clorgyline (
CLO), and selegiline (
SEL). Among the tested compounds, derivatives from Series 3 demonstrated the most favorable dual-target profile, showing potent inhibition of both hCA isoforms and hMAO-B, with K
I values in the low nanomolar range [
49].
Encouraged by these findings, the authors expanded the library with a fifth series (Series 5), although no further improvement in inhibitory potency was observed. Nevertheless, the most promising multitarget compounds from the earlier series effectively mitigated Aβ-42-induced toxicity, significantly reducing reactive oxygen species (ROS) levels and restoring mitochondrial function in SH-SY5Y neuroblastoma cells [
49].
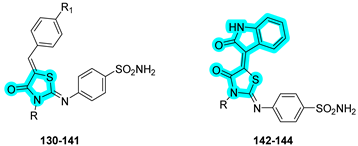
Gamal and coworkers designed and synthesized a series of benzenesulfonamide–thiazolidinone hybrids as novel multitarget agents for the management of type 2 diabetes mellitus [
50]. These agents simultaneously inhibit key enzymes, such as α-glucosidase (targeted by the thiazolidinone moiety) and hCA II (targeted by the benzenesulfonamide moiety). All synthesized derivatives exhibited notable α-glucosidase inhibitory activity, with compounds
131,
133,
134, and
140 showing comparable potency to the reference drug acarbose. Among these, compound
134 emerged as the most potent, displaying higher inhibitory activity than acarbose. Regarding carbonic anhydrase inhibition, compound
133 demonstrated the strongest inhibitory effect against the target hCA II, surpassing the standard inhibitor
AAZ. In addition, the compounds were tested against the tumor-associated hCA isoforms IX and XII, with compound
144 showing the most promising inhibitory profile in this subset (
Table 21).
Given the encouraging in vitro results, compound
133 was selected for in vivo glucose tolerance testing to evaluate its hypoglycemic effect on diabetic mice. Interestingly, compound
133 significantly reduced blood glucose levels compared to acarbose, supporting its potential as an antidiabetic agent [
50].
Furthermore, the antiproliferative activity of the most active hCA IX/XII inhibitors, compounds
140,
141, and
144, was assessed in MCF-7 breast cancer cells under both normoxic and hypoxic conditions. Compound
144 exhibited the highest efficacy under normoxic conditions (IC
50 = 7.95 µM), while compound
140 was more effective under hypoxia (IC
50 = 14.83 µM). However, both compounds were less potent than the reference drug doxorubicin, which showed IC
50 values of 3.84 µM and 9.43 µM, respectively [
50].
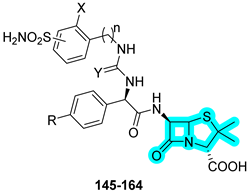
In 2024, Bonardi et al. designed and synthesized a novel series of dual-acting antibiotics in response to the growing challenge of antibiotic resistance [
51]. The strategy involved conjugating a benzenesulfonamide-based CAI moiety to well-known β-lactam antibiotics such as ampicillin and amoxicillin. The synthesized hybrids were initially evaluated in vitro for their inhibitory activity against three bacterial CA isoforms, CynT2 (β-CA from
Escherichia coli), EcoCA (γ-CA from
E. coli), and NgCA (α-CA from
Neisseria gonorrhoeae), as well as against the human off-target isoforms hCA I and hCA II. Most compounds demonstrated strong inhibition of the bacterial CAs, consistently outperforming the reference inhibitor
AAZ. Specifically, compounds
145,
146, and
158 were the most potent against CynT2;
148,
149, and
151 were the most potent against EcoCA; and
146,
158, and
163 were the most potent against NgCA (
Table 22).
Since NgCA was identified as the most selectively inhibited bacterial isoform, the authors further investigated the antibacterial activity of the compounds against clinical strains of
N. gonorrhoeae, including multidrug-resistant strains, both β-lactam-sensitive and resistant to ceftriaxone and/or azithromycin. Notably, compounds
145 and
161 showed 4- to 8-fold enhanced activity compared to ampicillin and amoxicillin against the
N. gonorrhoeae FA1090 strain, with MIC values of 0.03 µM for both
145 and
161, compared to 0.125 µM and 0.250 µM for ampicillin and amoxicillin, respectively. Finally, the binding potential of the β-lactam portion for penicillin-binding proteins (PBPs) was assessed via in silico covalent docking studies, which suggested that, in several compounds, the benzenesulfonamide moiety also contributed directly to interactions with active site residues of PBPs [
51].
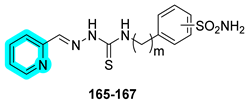
In 2025, Lopez’s research group developed a novel class of sulfonamide–thiosemicarbazone hybrids designed to combine metal chelation and hCA inhibition as a dual approach for anticancer therapy [
52]. The compounds were evaluated for their inhibitory activity against hCAs I, II, IX, and XII, showing good potency and notable selectivity toward the tumor-associated isoforms hCAs IX and XII (
Table 23).
Within this series of compounds,
166 emerged as the most potent dual inhibitor of hCAs IX and XII, underscoring that the positioning of the sulfonamide group (meta rather than para) exerts a greater influence than the linker length. Given its promising profile, its metal-chelating properties were investigated further against a range of metal ions, including Na
+, K
+, Fe
2+, Fe
3+, Zn
2+, and Cu
2+. Compound
166 showed no affinity for monovalent cations but demonstrated a strong ability to chelate Cu
2+, suggesting potential for disrupting metal-dependent tumor processes. The antiproliferative activity of derivatives was then assessed in vitro against a panel of cancer cell lines, including lung (A549, SW1573), breast (HBL-100, T-47D), cervical (HeLa), and colon (WiDr) cells. Notably, compound
166 exhibited the most potent antiproliferative effects, with GI
50 values ranging from 4.5 to 10 µM, reinforcing its potential as a multifunctional anticancer agent [
52].
In the same year, Elkotamy and co-workers designed and synthesized a series of pyrazolo[1,5-a]pyrimidine derivatives bearing zinc-binding groups to achieve dual inhibition of tumor-associated carbonic anhydrase isoforms IX and XII, as well as cyclin-dependent kinase 6 (CDK6), targeting critical pathways in non-small-cell lung cancer (NSCLC) [
53].
The synthesized derivatives, reported in
Table 24, were evaluated in vitro for their inhibitory activity against hCAs I, II, IX, and XII. The majority of compounds displayed potent inhibition and marked selectivity toward hCAs IX and XII, with compounds
170,
178,
180, and
183 emerging as the most selective toward the tumor-associated isoforms over the cytosolic hCA I and hCA II. The results indicate that CA inhibition is primarily influenced by the type of CA inhibitor (R
1), with sulfonamide derivatives showing superior activity compared to their acidic counterparts. Substituents at positions R
2 and R
3 exert variable effects across the four isoforms. In general, the presence of fluorine at R
2 enhances inhibitory activity against all four tested isoforms, whereas the methoxy group (OCH
3) appears to be optimal for improving the selectivity of the target isoforms CA IX and CA XII. The influence of R
3 is more limited; however, the hydroxyl group enhances inhibition of CA I while reducing activity against CA II, IX, and XII compared to the methyl substituent.
The compounds were subsequently assessed for their cytotoxic effects on NSCLC cell lines. Notably, compounds 170, 171, 176, and 181 exhibited superior potency compared to the reference CDK inhibitor roscovitine. Specifically, the following IC50 values were recorded:
In A549 cells: 1.90 μM (170), 5.98 μM (171), 4.91 μM (176), and 2.39 μM (181) versus 15.91 μM for roscovitine.
In NCI-H1734 cells: 5.45 μM (170), 0.75 μM (171), 2.02 μM (176), and 7.92 μM (181) versus 9.10 μM for roscovitine.
The same four compounds were further evaluated for CDK4/6 inhibition, revealing preferential activity toward CDK6. Moreover, mechanistic studies demonstrated that the compounds induced cell cycle arrest at the G1 phase and promoted apoptosis. Compound
171 increased the G1 phase population to 91.02% in NCI-H1734, while
181 achieved 83.66% in A549 cells. In cytotoxicity assays against normal WI-38 fibroblasts, compound
171 showed an IC
50 of 30.80 μM, and
181 exhibited 58.87 μM, both showing acceptable safety margins when compared to staurosporine (IC
50 = 19.59 μM) [
53].
6. Conclusions
The integration of heterocycles into multitarget carbonic anhydrase inhibitors (CAIs) represents a recent but dynamic and rapidly evolving strategy in drug discovery. Heterocyclic motifs, owing to their inherent versatility, can act as core inhibitory chemotypes, structural linkers, or complementary pharmacophores, thus offering multiple entry points for rational molecular design. By enabling the simultaneous modulation of different biological targets, including multiple hCA isoforms, these compounds address the inherent complexity of multifactorial diseases such as cancer, glaucoma, inflammatory disorders, and neurodegenerative conditions. The multitarget approach is especially helpful for diseases caused by connected biochemical pathways because it can lower the need for many drugs, improve treatment results, and reduce side effects by using one treatment that works on multiple parts.
In this context, the use of (sulfo)coumarin cores as CAIs represents the most used strategy to selectively target the cancer-associated hCAs IX and XII, avoiding inhibition of the cytosolic and off-target hCAs I and II. Moreover, many of these (sulfo)coumarin-based hybrids were assessed for their antiproliferative effects, showing favorable results, as demonstrated by derivatives
11–
17, targeting both hCAs IX and XII and telomerase [
25]. Furthermore, several publications have also investigated coumarin-related anti-inflammatory activity [
26,
27], as discussed for compounds
20–
27. Among these,
23–
27 exhibited consistent pain-relieving properties in an in vivo model [
27]. However, the heterocycle’s role in hCA inhibition has also been widely explored, particularly as a linker, because of its ability to establish various interactions with surrounding amino acid residues (e.g., hydrogen bonds and π-π interactions) and for its facile and rapid synthesis. A notable example is Cu(I)-catalysed Huisgen azide–alkyne cycloaddition, which forms the 1,2,3-triazole core. This approach has been extensively adopted to connect two active pharmacophores in a multitargeting strategy [
24,
28,
41,
44,
46].
In general, hybrid and chimeric heterocyclic designs have proven especially valuable, allowing for major physicochemical properties, optimizing binding affinities, and enhancing isoform selectivity. Advances in computational chemistry, high-throughput screening, and structure-guided drug design are now promoting the identification of promising heterocyclic scaffolds, as well as the prediction of off-target interactions and potential toxicity. Accordingly, Tinivella et al. used in silico studies of the ligand-based and structure-based multitarget repurposing strategy to discover new chimeric inhibitors,
46–
48 [
35]. They were able to effectively modulate both hCAs and estrogen receptors, confirming the valuable role of computational chemistry in the design of consistent inhibitors. Future progress will rely on the integration of medicinal chemistry with structural biology and fragment-based screening to better understand target networks and refine ligand design.
Ultimately, the rational exploitation of heterocycles in multitarget paradigms holds the potential to generate next-generation therapeutics with enhanced efficacy and improved patient compliance, thereby paving the way for innovative treatments of complex diseases.