The Senotherapeutic Effects of APPA (Apocynin [AP] and Paeonol [PA]) on Senescent Human Chondrocytes
Abstract
1. Introduction
2. Results
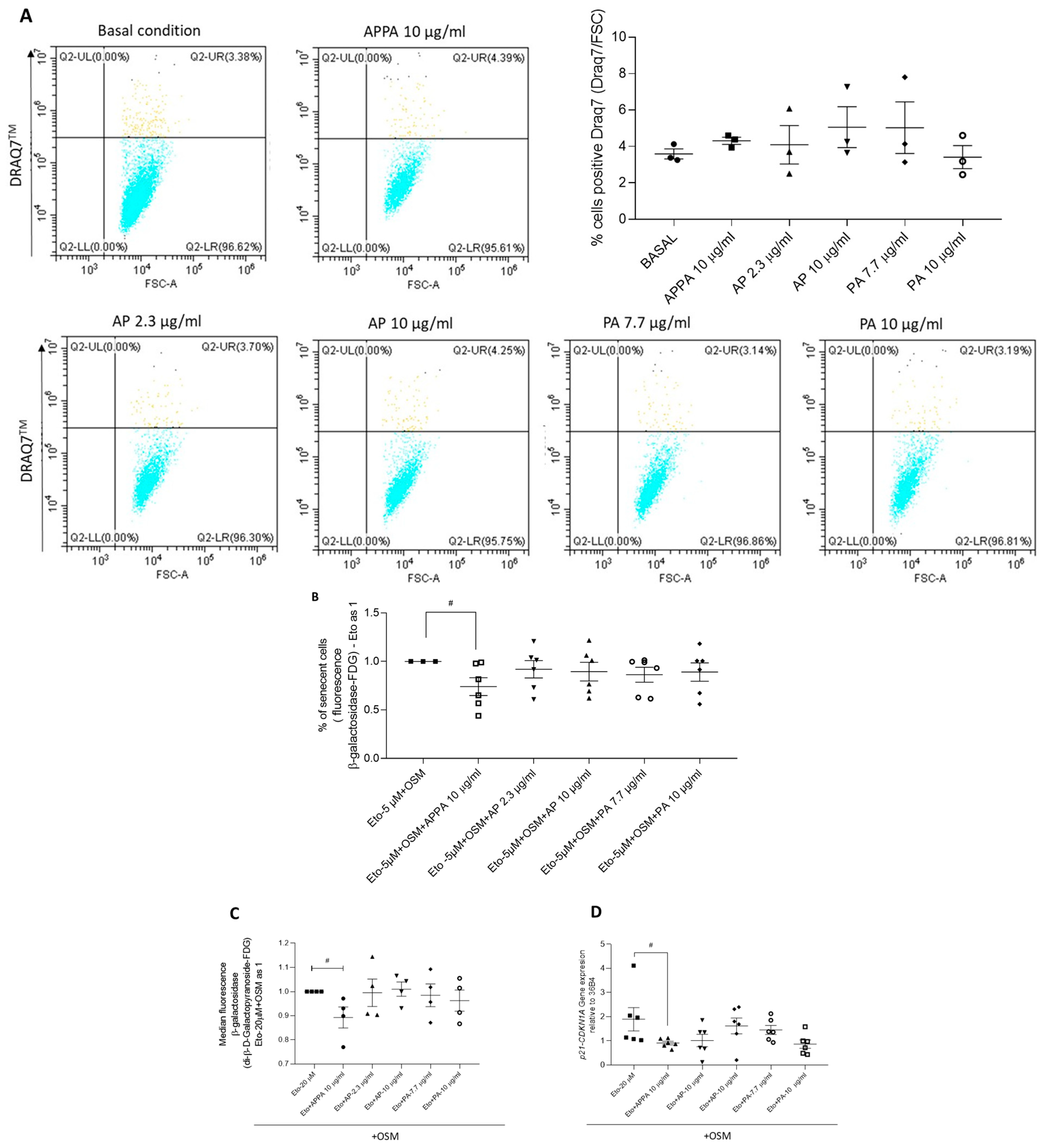
2.1. Evaluation of Stimuli to Induce Senescence
2.2. Effect of APPA and Its Components on Senescent T/C28a2 Chondrocytes
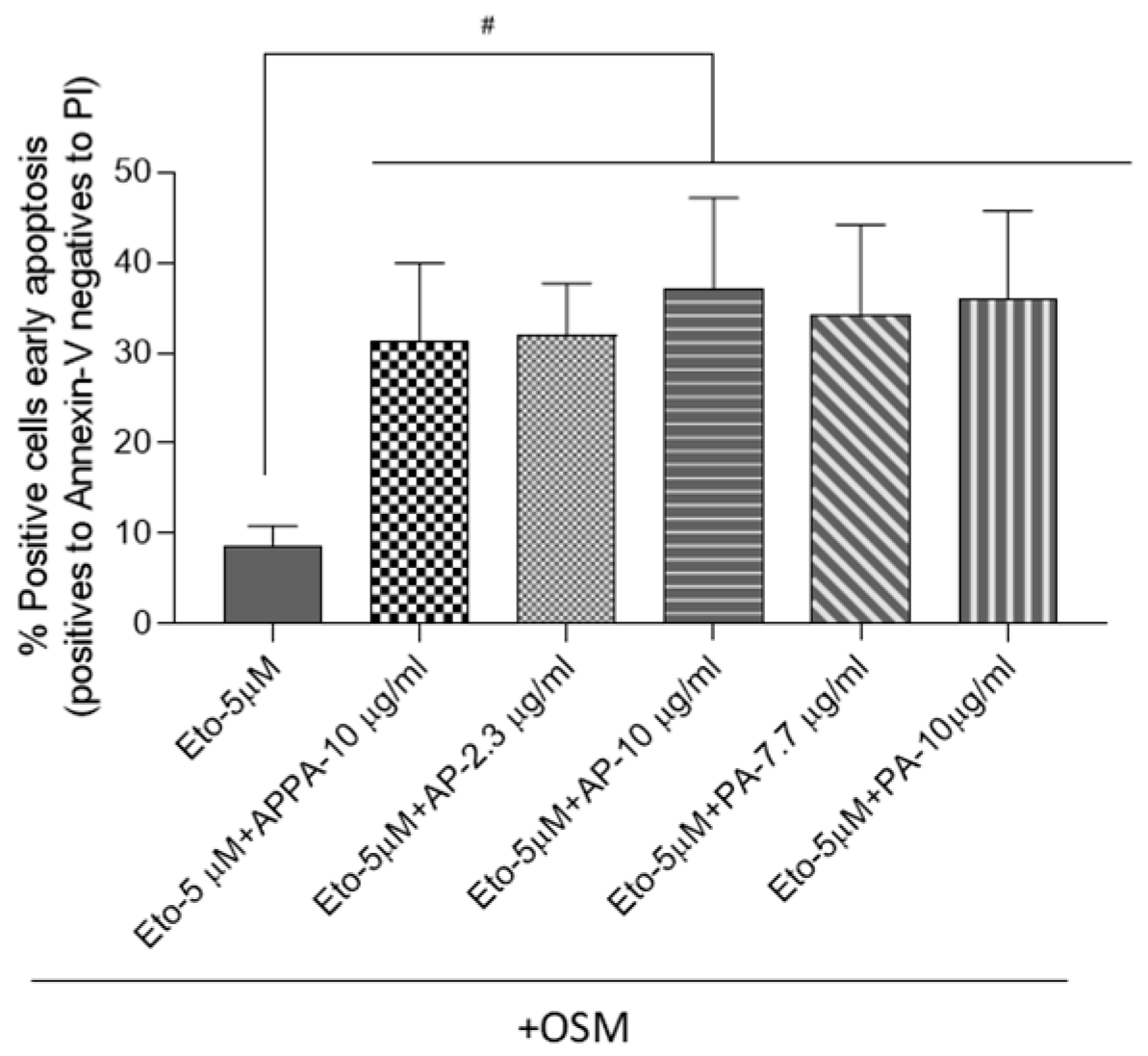
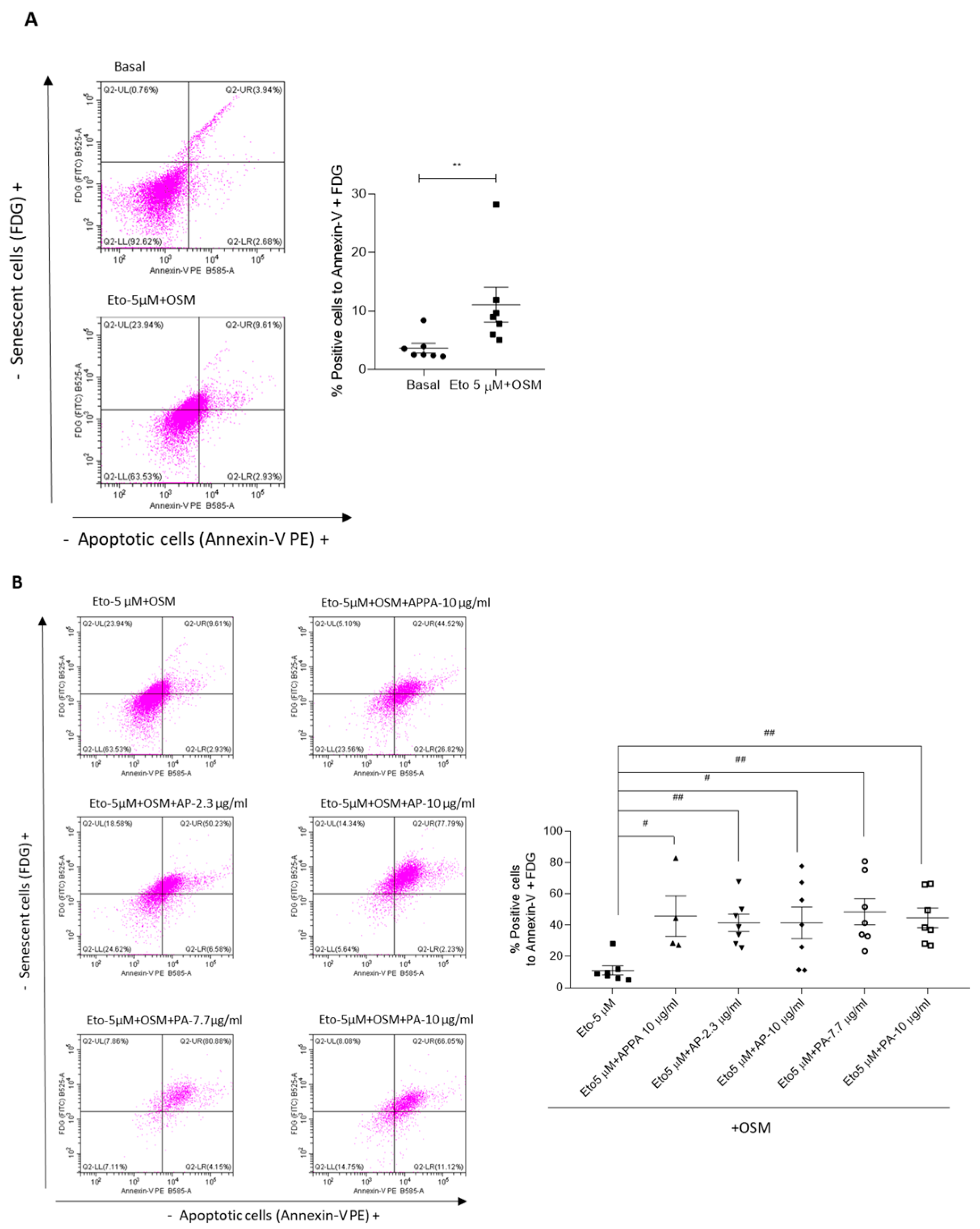
2.3. APPA Increased the Number of T/C28a2 Apoptotic Cells
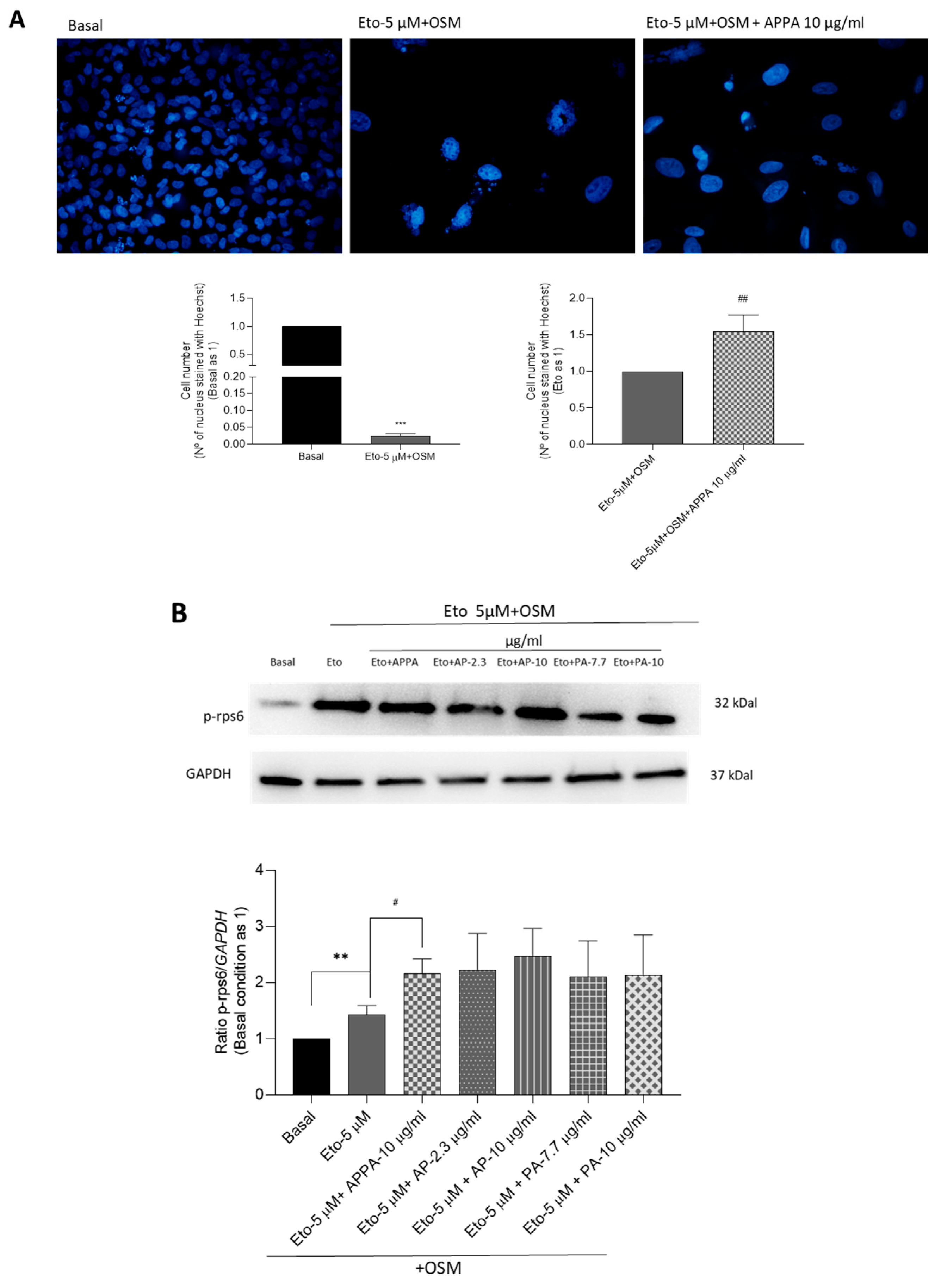
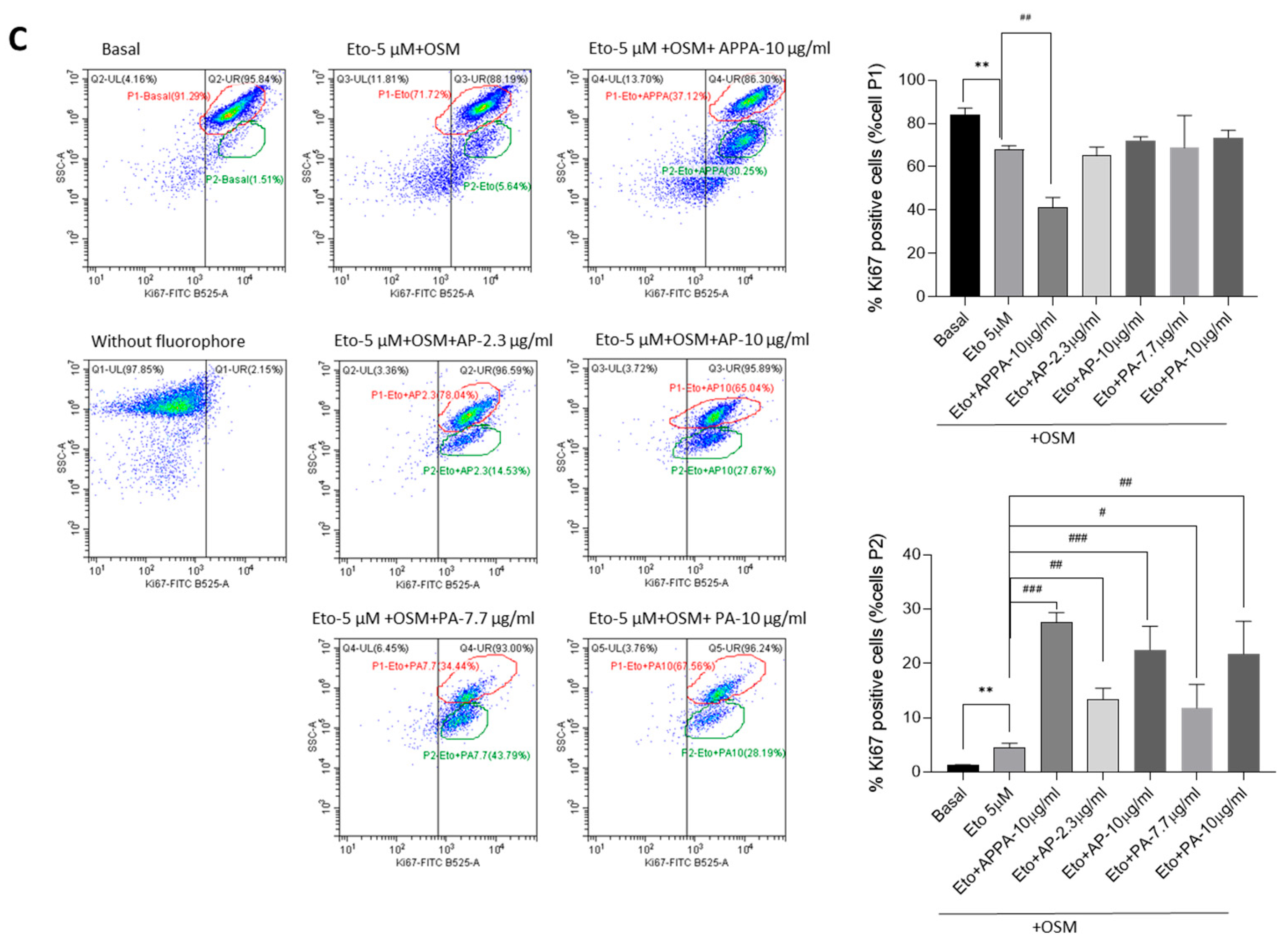
2.4. Evaluation APPA Effect on Cell Proliferation
3. Discussion
4. Material and Methods
4.1. Drug Preparation
4.2. Chondrocytes Culture
4.2.1. Chondrocytes from Human OA Hip Cartilage
4.2.2. T/C-28a2 Chondrocytes Cell Line
4.2.3. Cell Culture Human Articular Chondrocytes and T/C-28a2 Cell Line
4.2.4. Induction of Senescence
4.3. Assay for Measuring Viability in Presence of AP and PA by DRAQ7TM Staining
4.4. Determination of Senotherapeutic Activity
4.4.1. Senescence Determination
Flow Cytometry
Gene Expression by Quantitative Real-Time PCR (qRT-PCR)
4.5. Apoptotic Cell Determination
4.6. Senescence and Apoptotic Cell Determination at the Same Time
4.7. Number of Cells in Cell Culture
4.8. Proliferative Cells Determination by Ki-67 Analysis
4.9. Western Blotting (WB)
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. New York Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Ding, S.; Zhang, M. Cross-talk of inflammation and cellular senescence: A new insight into the occurrence and progression of osteoarthritis. Bone Res. 2024, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Walker, C. Osteoarthritis and inflammation: A serious disease with overlapping phenotypic patterns. Postgrad. Med. 2020, 132, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Dell’Isola, A.; Steultjens, M. Classification of patients with knee osteoarthritis in clinical phenotypes: Data from the osteoarthritis initiative. PLoS ONE 2018, 13, e0191045. [Google Scholar] [CrossRef]
- Deveza, L.A.; Melo, L.; Yamato, T.P.; Mills, K.; Ravi, V.; Hunter, D.J. Knee osteoarthritis phenotypes and their relevance for outcomes: A systematic review. Osteoarthr. Cartil. 2017, 25, 1926–1941. [Google Scholar] [CrossRef]
- Mobasheri, A.; van Spil, W.E.; Budd, E.; Uzieliene, I.; Bernotiene, E.; Bay-Jensen, A.C.; Larkin, J.; Levesque, M.C.; Gualillo, O.; Henrotin, Y. Molecular taxonomy of osteoarthritis for patient stratification, disease management and drug development: Biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr. Opin. Rheumatol. 2019, 31, 80–89. [Google Scholar] [CrossRef]
- Nelson, F.R.T. The Value of Phenotypes in Knee Osteoarthritis Research. Open Orthop J. 2018, 12, 105–114. [Google Scholar] [CrossRef]
- van der Esch, M.; Knoop, J.; van der Leeden, M.; Roorda, L.D.; Lems, W.F.; Knol, D.L.; Dekker, J. Clinical phenotypes in patients with knee osteoarthritis: A study in the Amsterdam osteoarthritis cohort. Osteoarthr. Cartil. 2015, 23, 544–549. [Google Scholar] [CrossRef]
- Goldring, M.B.; Berenbaum, F. Emerging targets in osteoarthritis therapy. Curr. Opin. Pharmacol. 2015, 22, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Vincent, T.L.; Alliston, T.; Kapoor, M.; Loeser, R.F.; Troeberg, L.; Little, C.B. Osteoarthritis Pathophysiology: Therapeutic Target Discovery may Require a Multifaceted Approach. Clin. Geriatr. Med. 2022, 38, 193–219. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Nogueira-Recalde, U.; Lorenzo-Gómez, I.; Blanco, F.J.; Loza, M.I.; Grassi, D.; Shirinsky, V.; Shirinsky, I.; Lotz, M.; Robbins, P.D.; Domínguez, E.; et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 2019, 45, 588–605. [Google Scholar] [CrossRef] [PubMed]
- Caramés, B.; Hasegawa, A.; Taniguchi, N.; Miyaki, S.; Blanco, F.J.; Lotz, M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann. Rheum. Dis. 2012, 71, 575–581. [Google Scholar] [CrossRef]
- Diekman, B.O.; Loeser, R.F. Aging and the emerging role of cellular senescence in osteoarthritis. Osteoarthr. Cartil. 2024, 32, 365–371. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, T.; Xu, H.; Zhang, H. Senescence in osteoarthritis: From mechanism to potential treatment. Arthritis Res. Ther. 2022, 24, 174. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Fuhrmann-Stroissnigg, H.; Dai, H.M.; Ling, Y.Y.; Stout, M.B.; Pirtskhalava, T.; Giorgadze, N.; Johnson, K.O.; Giles, C.B.; et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016, 15, 428–435. [Google Scholar] [CrossRef]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Fernández-Moreno, M.; Hermida-Gómez, T.; Larkins, N.; Reynolds, A.; Blanco, F.J. Anti-Inflammatory Activity of APPA (Apocynin and Paeonol) in Human Articular Chondrocytes. Pharmaceuticals 2024, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moreno, M.; Hermida Gómez, T.; Larkins, N.; Reynolds, A.; Blanco-Garcia, F. Effect of APPA (combination of apocynin and paeonol) compound on cellular senescence using human articular chondrocytes. Osteoarthr. Cartil. 2023, 31, S401–S402. [Google Scholar] [CrossRef]
- Ansari, M.M.; Ghosh, M.; Lee, D.S.; Son, Y.O. Senolytic therapeutics: An emerging treatment modality for osteoarthritis. Ageing Res. Rev. 2024, 96, 102275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y. Advances in targeted therapies for age-related osteoarthritis: A comprehensive review of current research. Biomed. Pharmacother. 2024, 179, 117314. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Hagen, K.B.; Bijlsma, J.W.; Andreassen, O.; Christensen, P.; Conaghan, P.G.; Doherty, M.; Geenen, R.; Hammond, A.; Kjeken, I.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013, 72, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Perruccio, A.V.; Young, J.J.; Wilfong, J.M.; Denise Power, J.; Canizares, M.; Badley, E.M. Osteoarthritis year in review 2023: Epidemiology & therapy. Osteoarthr. Cartil. 2024, 32, 159–165. [Google Scholar]
- Brandt, M.D.; Malone, J.B.; Kean, T.J. Advances and Challenges in the Pursuit of Disease-Modifying Osteoarthritis Drugs: A Review of 2010–2024 Clinical Trials. Biomedicines 2025, 13, 355. [Google Scholar] [CrossRef]
- Latourte, A.; Kloppenburg, M.; Richette, P. Emerging pharmaceutical therapies for osteoarthritis. Nat. Rev. Rheumatol. 2020, 16, 673–688. [Google Scholar] [CrossRef]
- Oo, W.M. Prospects of Disease-Modifying Osteoarthritis Drugs. Rheum. Dis. Clin. N. Am. 2024, 50, 483–518. [Google Scholar] [CrossRef]
- Boshtam, M.; Kouhpayeh, S.; Amini, F.; Azizi, Y.; Najaflu, M.; Shariati, L.; Khanahmad, H. Anti-inflammatory effects of apocynin: A narrative review of the evidence. All Life 2021, 14, 997–1010. [Google Scholar] [CrossRef]
- Qi, J.-H.; Dong, F.-X.; Wang, X.-L. Exploring targets and signaling pathways of paeonol involved in relieving inflammation based on modern technology. Mol. Divers. 2022, 26, 1731–1742. [Google Scholar] [CrossRef]
- Savla, S.R.; Laddha, A.P.; Kulkarni, Y.A. Pharmacology of apocynin: A natural acetophenone. Drug Metab. Rev. 2021, 53, 542–562. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Cuzzocrea, S. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem. Pharmacol. 2011, 81, 636–648. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Mazzon, E.; Esposito, E.; Paterniti, I.; Bramanti, P.; Cuzzocrea, S. Effect of Apocynin, an inhibitor of NADPH oxidase, in the inflammatory process induced by an experimental model of spinal cord injury. Free Radic. Res. 2011, 45, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, J.; Pawliczak, R. Apocynin: Molecular aptitudes. Mediators Inflamm. 2008, 2008, 106507. [Google Scholar] [CrossRef]
- Bravo-Sanchez, E.; Pena-Montes, D.; Sanchez-Duarte, S.; Saavedra-Molina, A.; Sanchez-Duarte, E.; Montoya-Perez, R. Effects of Apocynin on Heart Muscle Oxidative Stress of Rats with Experimental Diabetes: Implications for Mitochondria. Antioxidants 2021, 10, 335. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, S.; Kong, R.; Shao, H.; Wang, C.; Piao, H.; Lv, W.; Chu, X.; Zhao, Y. Paeonol alleviates interleukin-1β-induced inflammatory responses in chondrocytes during osteoarthritis. Biomed. Pharmacother. 2017, 95, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Wang, C.; Tang, Q.; Zheng, W.; Feng, Z.; Yu, X.; Guo, X.; Wang, J. Paeonol Inhibits IL-1β-Induced Inflammation via PI3K/Akt/NF-κB Pathways: In Vivo and Vitro Studies. Inflammation 2017, 40, 1698–1706. [Google Scholar] [CrossRef]
- Cao, S.; Wei, Y.; Xiong, A.; Yue, Y.; Yang, J.; Wang, D.; Liu, X.; Zeng, H.; Shi, D.; Li, Y. Paeonol inhibits ACSL4 to protect chondrocytes from ferroptosis and ameliorates osteoarthritis progression. J. Orthop. Transl. 2025, 50, 1–13. [Google Scholar] [CrossRef]
- Hougee, S.; Hartog, A.; Sanders, A.; Graus, Y.M.; Hoijer, M.A.; Garssen, J.; van den Berg, W.B.; van Beuningen, H.M.; Smit, H.F. Oral administration of the NADPH-oxidase inhibitor apocynin partially restores diminished cartilage proteoglycan synthesis and reduces inflammation in mice. Eur. J. Pharmacol. 2006, 531, 264–269. [Google Scholar] [CrossRef]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef]
- Glasson, S.; Bendele, A.; Larkins, N. APPA provides disease modification in preclinical osteoarthritis. Osteoarthr. Cartil. 2012, 20, S72–S73. [Google Scholar] [CrossRef]
- Glasson, S.; Larkins, N. APPA provides symptom relief in clinical canine osteoarthritis. Osteoarthr. Cartil. 2012, 20, S287. [Google Scholar] [CrossRef]
- Larkins, N.I.; King, C. Appa compared against Meloxicam in canine OA. Osteoarthr. Cartil. 2017, 25, S176. [Google Scholar] [CrossRef]
- Larkins, N.; King, C. Effectiveness of apocynin-paeonol (APPA) for the management of osteoarthritis in dogs: Comparisons with placebo and meloxicam in client-owned dogs. Matters 2017. [Google Scholar] [CrossRef]
- Bihlet, A.R.; Byrjalsen, I.; Andersen, J.R.; Reynolds, A.; Larkins, N.; Alexandersen, P.; Rovsing, H.; Moots, R.; Conaghan, P.G. The efficacy and safety of a fixed-dose combination of apocynin and paeonol, APPA, in symptomatic knee OA: A double-blind, randomized, placebo-controlled, clinical trial. Osteoarthr. Cartil. 2024, 32, 952–962. [Google Scholar] [CrossRef]
- Cross, A.L.; Hawkes, J.; Wright, H.L.; Moots, R.J.; Edwards, S.W. APPA (apocynin and paeonol) modulates pathological aspects of human neutrophil function, without supressing antimicrobial ability, and inhibits TNFα expression and signalling. Inflammopharmacology 2020, 28, 1223–1235. [Google Scholar] [CrossRef]
- Price, J.S.; Waters, J.G.; Darrah, C.; Pennington, C.; Edwards, D.R.; Donell, S.T.; Clark, I.M. The role of chondrocyte senescence in osteoarthritis. Aging Cell 2002, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Cha, B.H.; Kim, J.S.; Ahn, J.; Han, I.; Park, H.; Lee, S.-H. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr. Cartil. 2016, 24, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, S.; Hu, D.; Zhang, L.; Shi, S. ALKBH5 regulates etoposide-induced cellular senescence and osteogenic differentiation in osteoporosis through mediating the m(6)A modification of VDAC3. Sci. Rep. 2024, 14, 23461. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.N.; Chang, H.C.; Chao, Y.Y.; Cheng, H.L.; Lien, W.C.; Wang, C.Y. Etoposide Triggers Cellular Senescence by Inducing Multiple Centrosomes and Primary Cilia in Adrenocortical Tumor Cells. Cells 2021, 10, 1466. [Google Scholar] [CrossRef]
- Miao, Y.; Fan, L.; Li, J.Y. Potential Treatments for COVID-19 Related Cytokine Storm—Beyond Corticosteroids. Front. Immunol. 2020, 11, 1445. [Google Scholar] [CrossRef]
- Aoyagi, T.; Sato, Y.; Baba, H.; Shiga, T.; Seike, I.; Niitsuma Sugaya, I.; Takei, K.; Iwasaki, Y.; Oshima, K.; Kanamori, H.; et al. Case Report: Successful Treatment of Five Critically Ill Coronavirus Disease 2019 Patients Using Combination Therapy with Etoposide and Corticosteroids. Front. Med. 2021, 8, 718641. [Google Scholar] [CrossRef]
- Kolachana, S.; Malik, A.; Nanjudappa, A.; Iding, J.; Bhansali, D.; Haas, C.J. Haemophagocytic lymphocytic histiocytosis/macrophage activation syndrome with acute inflammatory gastroenteritis. BMJ Case Rep. 2022, 15, e250809. [Google Scholar] [CrossRef]
- Bailly, C. Etoposide: A rider on the cytokine storm. Cytokine 2023, 168, 156234. [Google Scholar] [CrossRef]
- Henter, J.I.; von Bahr Greenwood, T. Etoposide Therapy of Cytokine Storm Syndromes. In Cytokine Storm Syndrome; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2024; Volume 1448, pp. 525–551. [Google Scholar]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Doolittle, M.L.; Saul, D.; Kaur, J.; Rowsey, J.L.; Vos, S.J.; Pavelko, K.D.; Farr, J.N.; Monroe, D.G.; Khosla, S. Multiparametric senescent cell phenotyping reveals targets of senolytic therapy in the aged murine skeleton. Nat. Commun. 2023, 14, 4587. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Grandi, F.C.; Bhutani, N. A single-cell mass cytometry platform to map the effects of preclinical drugs on cartilage homeostasis. JCI Insight 2022, 7, e160702. [Google Scholar] [CrossRef] [PubMed]
- Matta, C.; Takács, R.; Dvir-Ginzberg, M.; Richardson, S.M.; Pelttari, K.; Pattappa, G.; Risbud, M.V.; Mobasheri, A. Insights into chondrocyte populations in cartilaginous tissues at the single-cell level. Nat. Rev. Rheumatol. 2025, 21, 465–477. [Google Scholar] [CrossRef]
- Imb, M.; Véghelyi, Z.; Maurer, M.; Kühnel, H. Exploring Senolytic and Senomorphic Properties of Medicinal Plants for Anti-Aging Therapies. Int. J. Mol. Sci. 2024, 25, 10419. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Aprile, D.; Cappabianca, S.; Peluso, G.; Di Bernardo, G.; Galderisi, U. Different Stages of Quiescence, Senescence, and Cell Stress Identified by Molecular Algorithm Based on the Expression of Ki67, RPS6, and Beta-Galactosidase Activity. Int. J. Mol. Sci. 2021, 22, 3102. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: Terminology for TOR-driven aging. Aging 2012, 4, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, Z.N.; Blagosklonny, M.V. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle 2008, 7, 3355–3361. [Google Scholar] [CrossRef]
- Olascoaga-Del Angel, K.S.; Gutierrez, H.; Königsberg, M.; Pérez-Villanueva, J.; López-Diazguerrero, N.E. Exploring the fuzzy border between senolytics and senomorphics with chemoinformatics and systems pharmacology. Biogerontology 2022, 23, 453–471. [Google Scholar] [CrossRef]
- Maneiro, E.; Martín, M.A.; de Andres, M.C.; López-Armada, M.J.; Fernández-Sueiro, J.L.; del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Mitochondrial respiratory activity is altered in osteoarthritic human articular chondrocytes. Arthritis Rheum. 2003, 48, 700–708. [Google Scholar] [CrossRef]
- Goldring, M.B.; Birkhead, J.R.; Suen, L.F.; Yamin, R.; Mizuno, S.; Glowacki, J.; Arbiser, J.L.; Apperley, J.F. Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J. Clin. Investig. 1994, 94, 2307–2316. [Google Scholar] [CrossRef]
- Georget, M.; Defois, A.; Guiho, R.; Bon, N.; Allain, S.; Boyer, C.; Halgand, B.; Waast, D.; Grimandi, G.; Fouasson-Chailloux, A.; et al. Development of a DNA damage-induced senescence model in osteoarthritic chondrocytes. Aging 2023, 15, 8576–8593. [Google Scholar] [CrossRef]
- Dalmao-Fernández, A.; Hermida-Gómez, T.; Nogueira-Recalde, U.; Rego-Pérez, I.; Blanco-Garcia, F.J.; Fernández-Moreno, M. Mitochondrial Role on Cellular Apoptosis, Autophagy, and Senescence during Osteoarthritis Pathogenesis. Cells 2024, 13, 976. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Moreno, M.; Hermida-Gómez, T.; Vaamonde-Garcia, C.; Paniagua-Barro, S.; Larkins, N.; Reynolds, A.; Blanco, F.J. The Senotherapeutic Effects of APPA (Apocynin [AP] and Paeonol [PA]) on Senescent Human Chondrocytes. Pharmaceuticals 2025, 18, 1386. https://doi.org/10.3390/ph18091386
Fernández-Moreno M, Hermida-Gómez T, Vaamonde-Garcia C, Paniagua-Barro S, Larkins N, Reynolds A, Blanco FJ. The Senotherapeutic Effects of APPA (Apocynin [AP] and Paeonol [PA]) on Senescent Human Chondrocytes. Pharmaceuticals. 2025; 18(9):1386. https://doi.org/10.3390/ph18091386
Chicago/Turabian StyleFernández-Moreno, Mercedes, Tamara Hermida-Gómez, Carlos Vaamonde-Garcia, Sara Paniagua-Barro, Nicholas Larkins, Alan Reynolds, and Francisco J. Blanco. 2025. "The Senotherapeutic Effects of APPA (Apocynin [AP] and Paeonol [PA]) on Senescent Human Chondrocytes" Pharmaceuticals 18, no. 9: 1386. https://doi.org/10.3390/ph18091386
APA StyleFernández-Moreno, M., Hermida-Gómez, T., Vaamonde-Garcia, C., Paniagua-Barro, S., Larkins, N., Reynolds, A., & Blanco, F. J. (2025). The Senotherapeutic Effects of APPA (Apocynin [AP] and Paeonol [PA]) on Senescent Human Chondrocytes. Pharmaceuticals, 18(9), 1386. https://doi.org/10.3390/ph18091386






