miR-25-3p Modulates Tumor Aggressiveness and Ferroptosis Escape in T24 Bladder Cancer Cells In Vitro
Abstract
1. Introduction
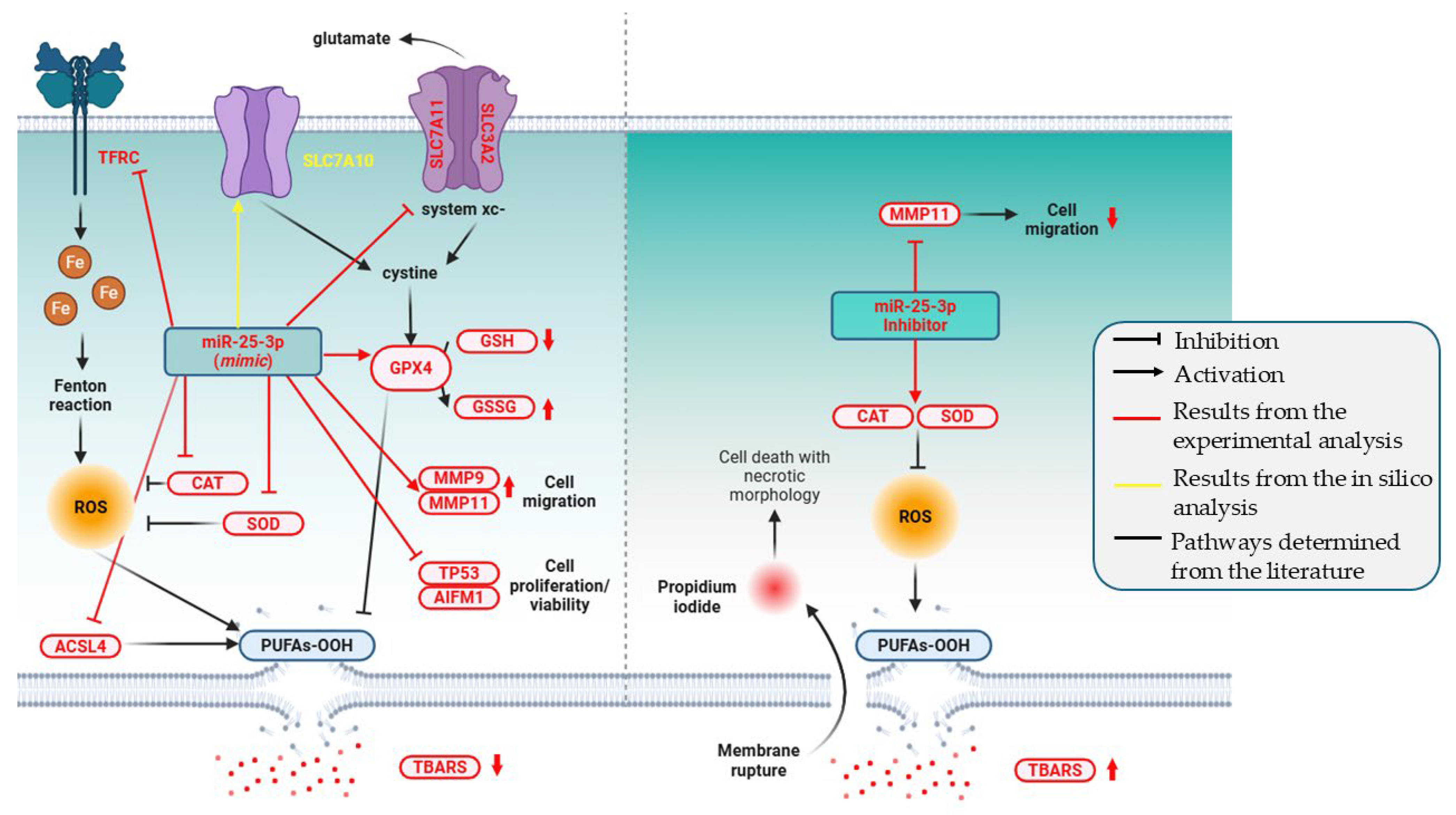
2. Results
2.1. In Silico Analysis
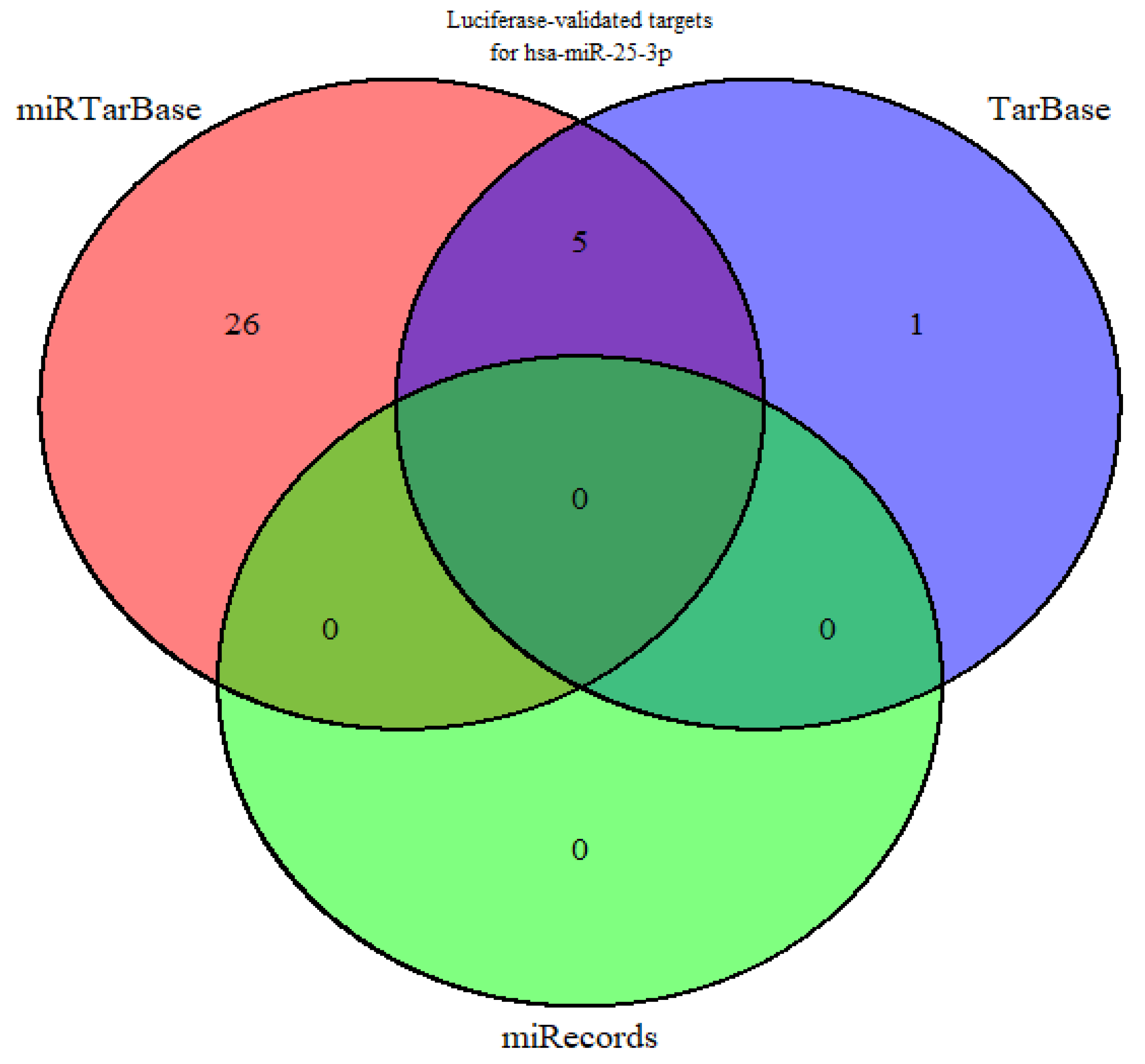
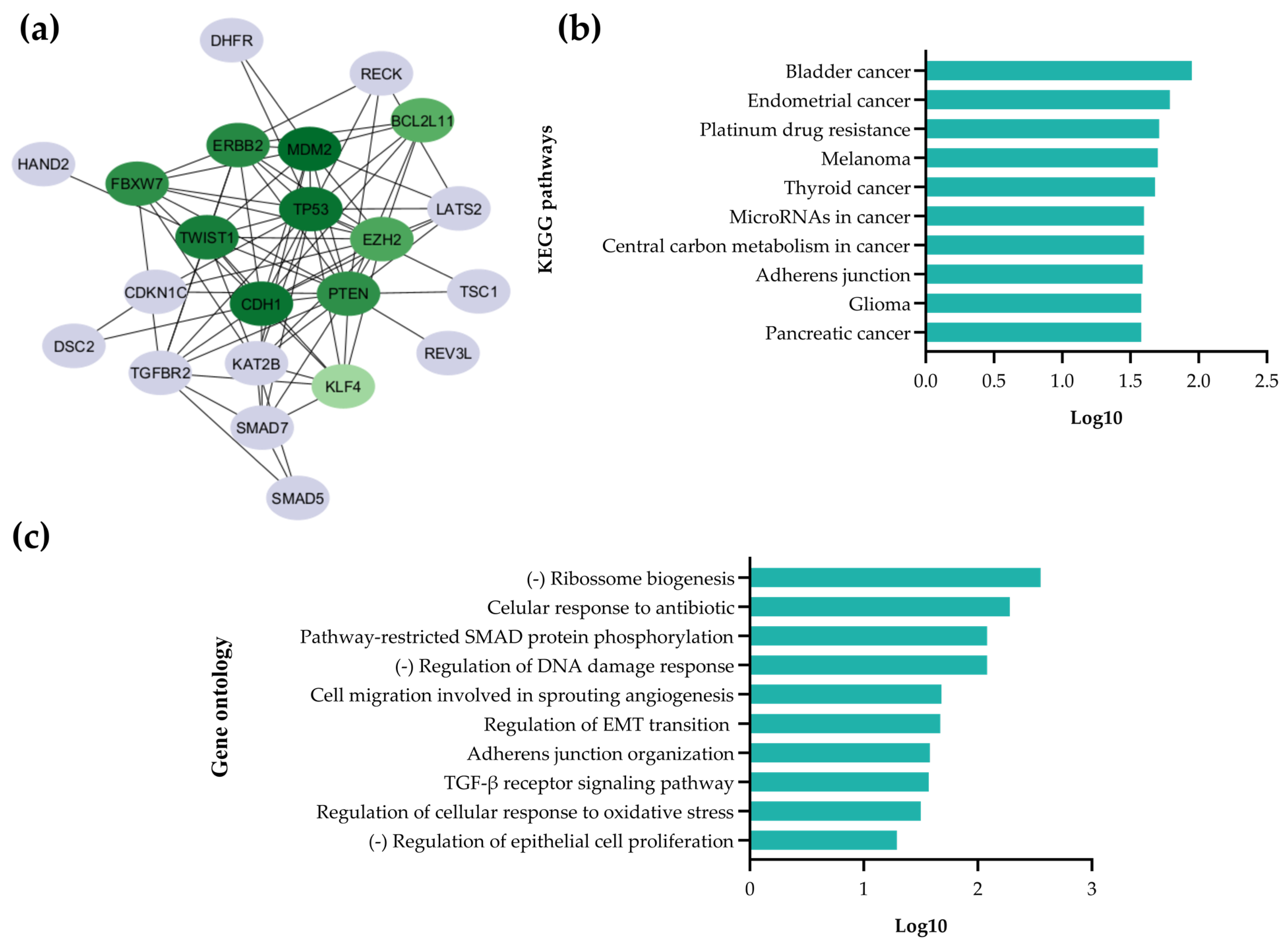
2.1.1. Identification of miR-25-3p Target Genes
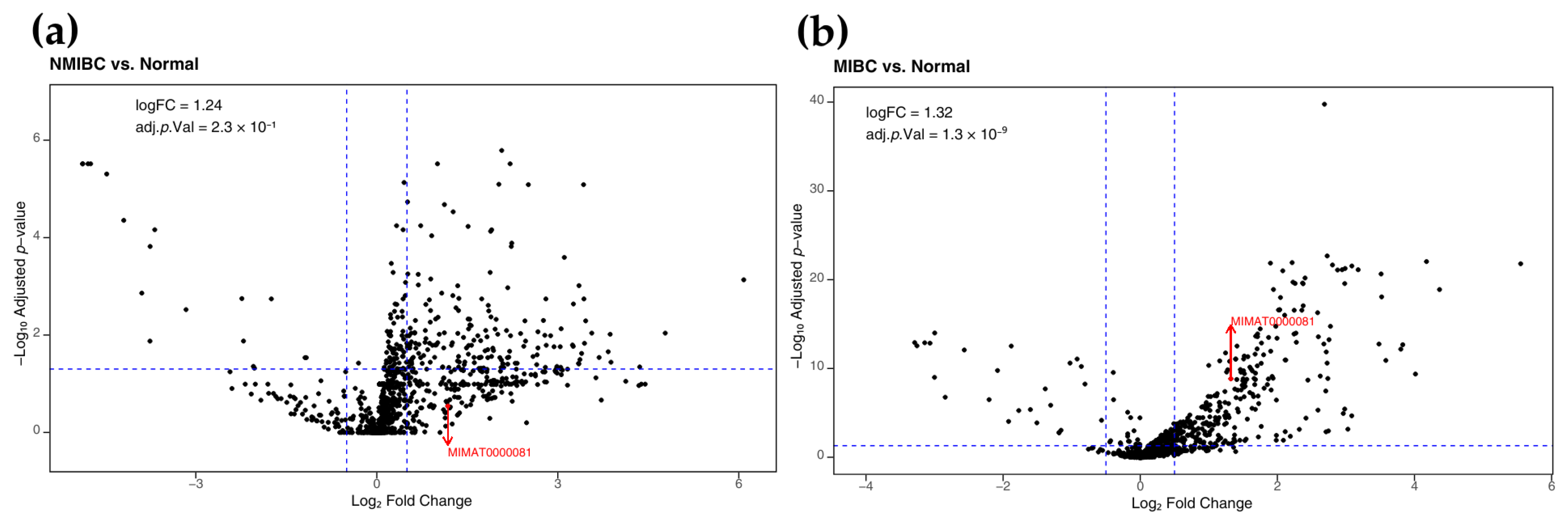
2.1.2. Increased Expression of miR-25-3p in Bladder Cancer
2.2. Functional Assays in Invasive UBC T24 Cells
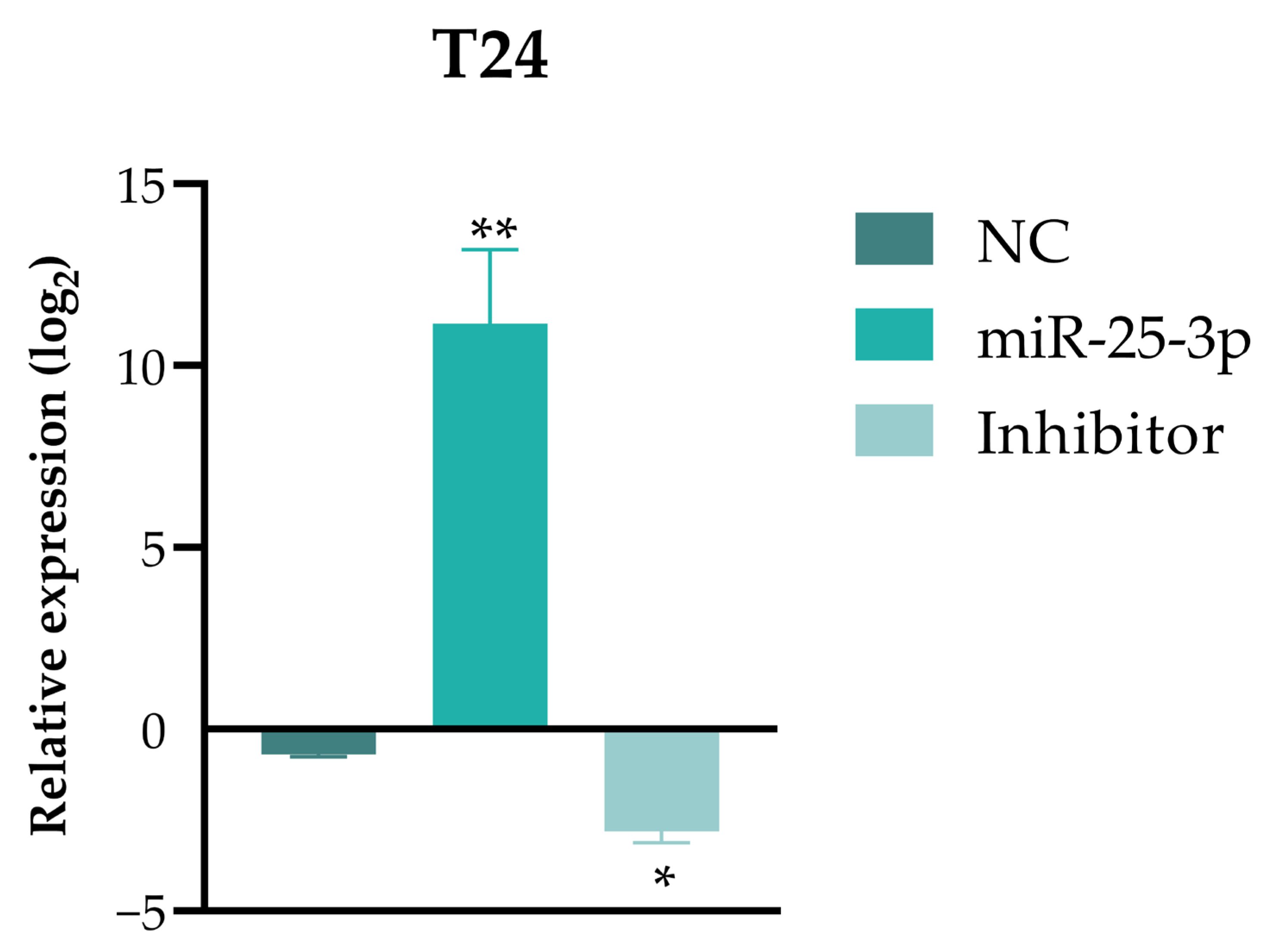
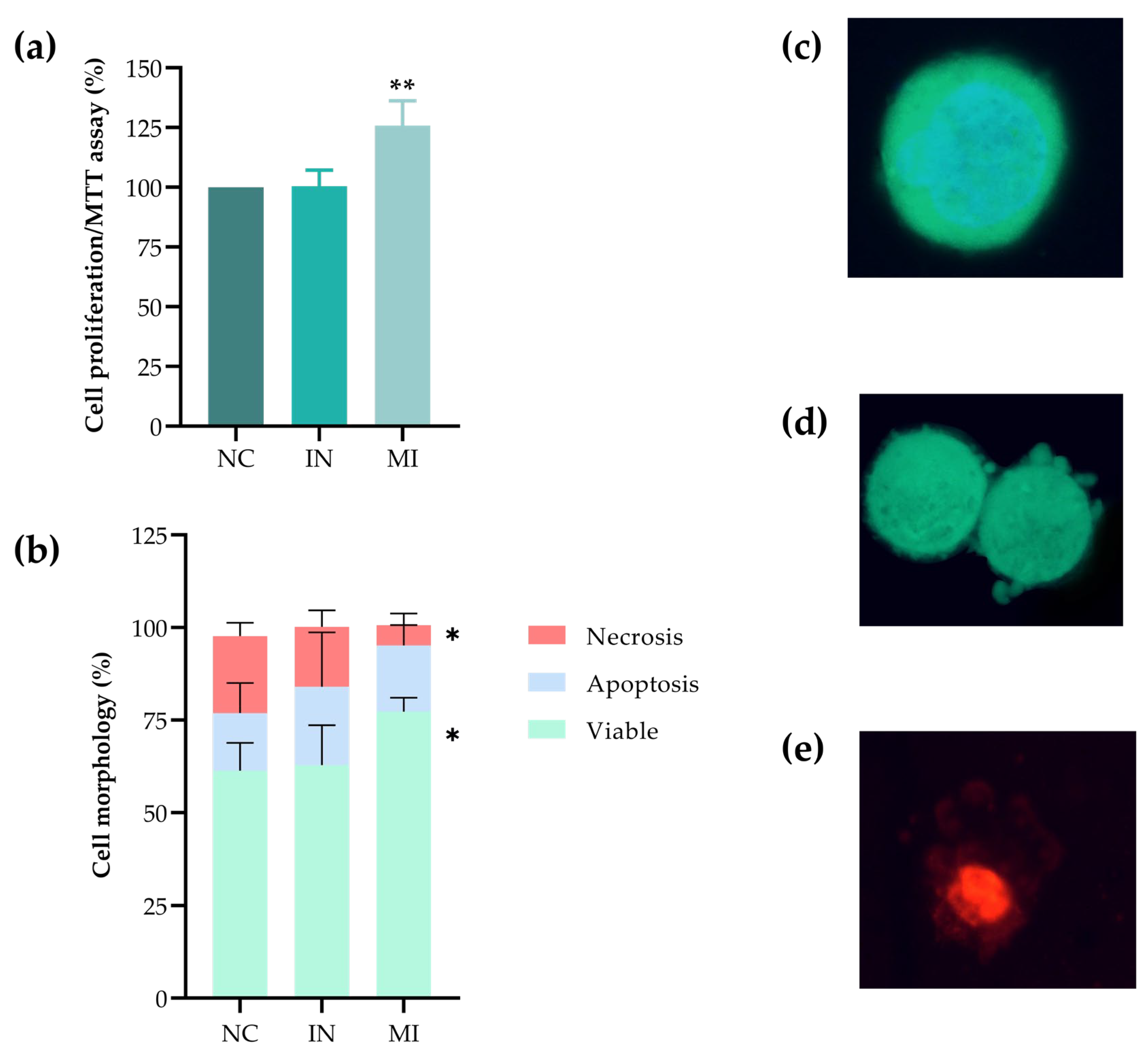
2.3. Overexpression of miR-25-3p Increases Cell Proliferation and Plays a Role in Cell Viability
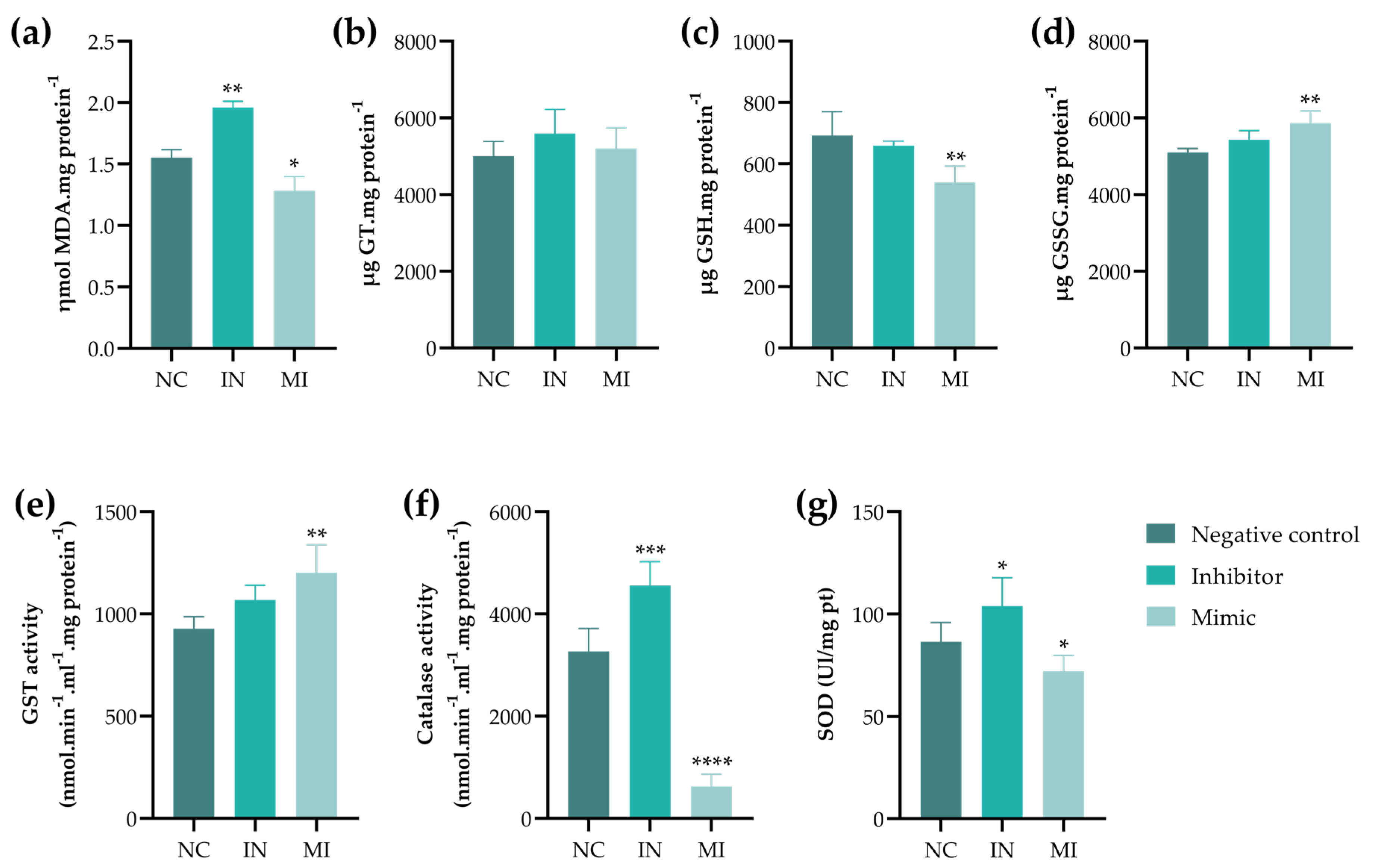
2.4. miR-25-3p Modulates the Redox Status
2.5. miR-25-3p Modulates T24 Cell Migration
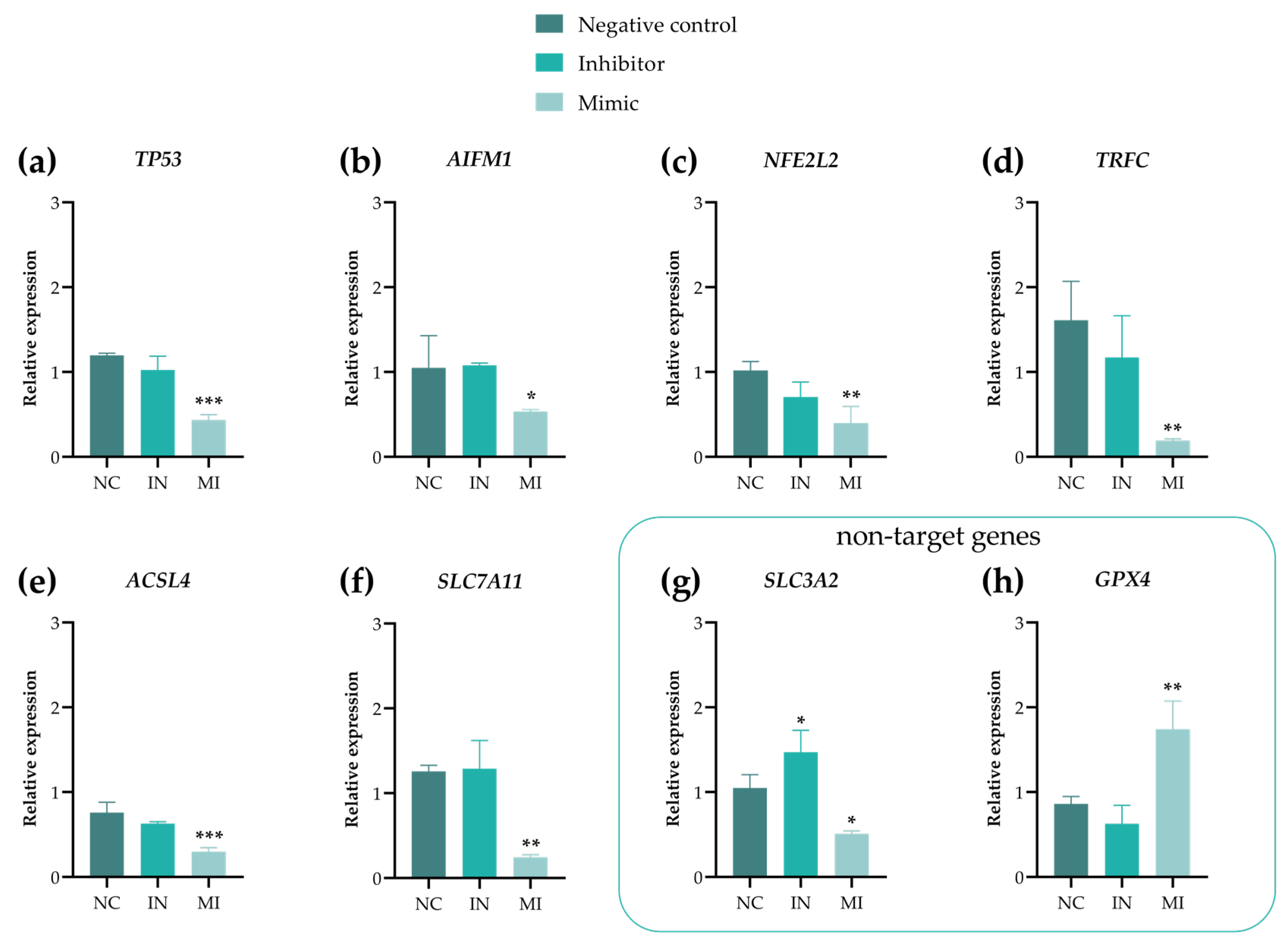
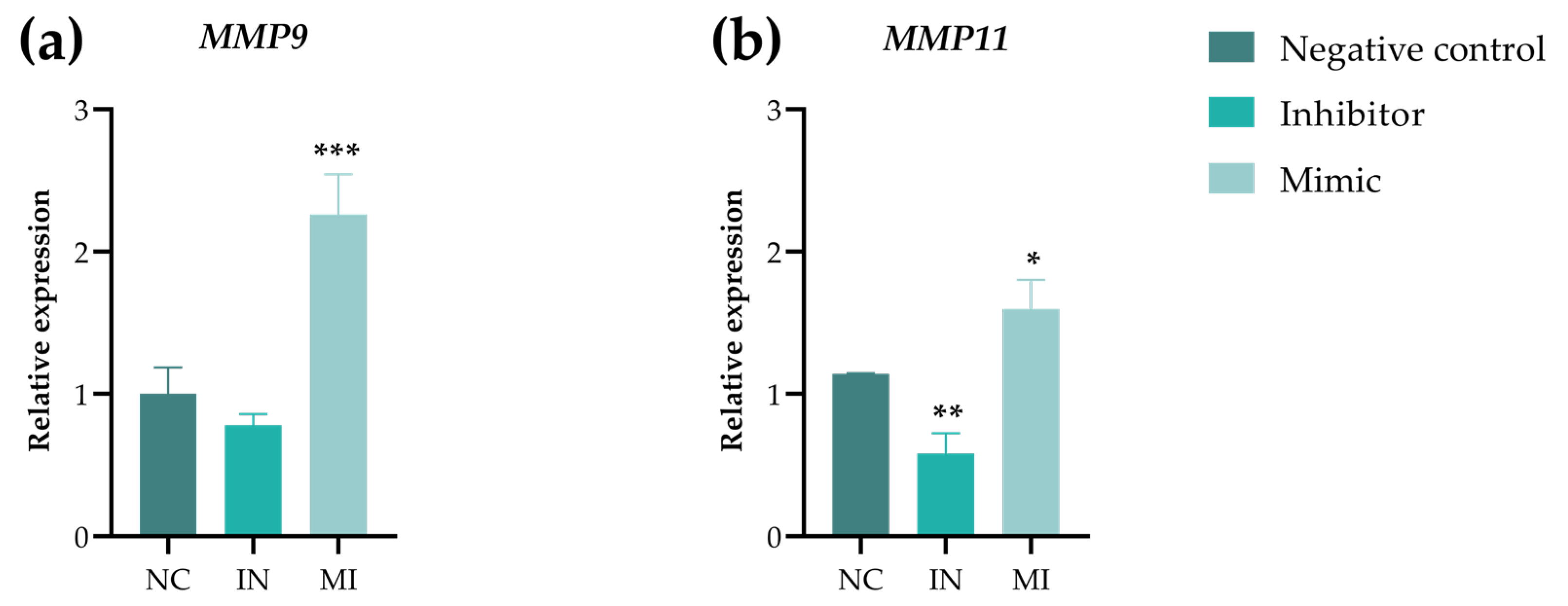
2.6. Gene Expression Showed That miR-25-3p Modulates Ferroptosis and MMPs Pathways
3. Discussion
4. Materials and Methods
4.1. Bioinformatic Analysis
4.1.1. Selection of Putative Targets Regulated by miR-25-3p
4.1.2. Survival Analysis
4.1.3. Analysis of miRNA–mRNA Association of Ferroptosis-Related Genes
4.2. Cell Line and Cultivation Conditions
4.3. Transfection of T24 Cells with miR-25-3p, miRNA Extraction, and RT-qPCR
4.4. Preparation of Monolayer Cell Culture (2D)
4.5. MTT Assay
4.6. Morphological Assay (Triple Staining) for Cell Death Assessment
4.7. Wound Healing (Scratch Assay)
4.8. Oxidative Stress
4.8.1. Quantification of TBARS
4.8.2. Assessment of GT, GSH, and GSSG Concentrations
4.8.3. GST Activity
4.8.4. CAT Activity
4.8.5. SOD Activity
4.9. Gene Expression Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2−ΔΔCt | 2-Delta-delta Ct |
| μg | Microgram |
| μL | Microliter |
| μM | Micromole |
| ABC | ATP-binding cassette |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 |
| AIFM1 | Apoptosis-inducing factor mitochondria-associated 1 |
| BCL2L11 | BCL2-like 11 |
| BLCA | Bladder cancer |
| BP | Biological process |
| CAT | Catalase |
| CC | Cellular component |
| cDDP | Cisplatin |
| CDH1 | Cadherin 1 |
| cDNA | Complementary DNA |
| CDNB | 1-Chloro-2,4-dinitrobenzene |
| DMSO | Dimethylsulfoxide |
| DTNB | Ellman’s reagent |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| ERBB2 | Erb-b2 receptor tyrosine kinase 2 |
| EZH2 | Enhancer of zeste 2 polycomb repressive complex 2 subunit |
| FBS | Fetal bovine serum |
| FBXW7 | F-Box and WD40 domain-containing protein 7 gene |
| FC | Fold change |
| FDA | Fluorescein diacetate |
| FDR | False discovery rate |
| FTH1 | Ferritin heavy chain 1 |
| GO | Gene Ontology |
| GPX4 | Glutathione peroxidase 4 |
| GR | Glutathione reductase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| GST | Glutathione S-transferase |
| GT | Glutathione |
| h | Hour |
| HO | Hoescht 33342 |
| IN | Inhibitor |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| KLF4 | Kruppel-like factor 4 |
| KM | Kaplan–Meier |
| MCM7 | Minichromosome maintenance complex component 7 |
| MDM2 | MDM2 proto-oncogene |
| MF | Molecular function |
| MI | Mimic |
| MIBC | Muscle-invasive bladder cancer |
| min | Minute |
| miRNA | Micro-RNA |
| MMP | Matrix metalloproteinase |
| MMP9 | Matrix metallopeptidase 9 |
| MMP11 | Matrix metallopeptidase 11 |
| mRNA | Messenger RNA |
| MTT | 3-(4,5-Dimethylthiazol-2yl)-2,5-di-diphenyltetrazoline bromide |
| NADP | Nicotinamide-adenine dinucleotide phosphate |
| NBT | Nitroblue tetrazolium |
| NC | Negative control |
| NFE2L2 | Nuclear factor erythroid 2-related factor 2 |
| NH2OH·HCl | Hydroxylamine hydrochloride |
| NMIBC | Non-muscle-invasive bladder cancer |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| pmol | Picomole |
| PPI | Protein–protein interaction |
| PTEN | Phosphatase and tensin homologue |
| qPCR | Real-time quantitative PCR |
| RC | Radical cystectomy |
| RPM | Revolutions per minute |
| RPMI 1640 | Roswell Park Memorial Institute 1640 |
| RT-PCR | Reverse-transcription polymerase chain reaction |
| RT-qPCR | Quantitative reverse-transcription PCR |
| sec | Second |
| SLC3A2 | Solute carrier family 3 member 2 |
| SLC7A10 | Solute carrier family 7 member 10 |
| SLC7A11 | Solute carrier family 7 member 11 |
| SMAD | Suppressor of mothers against decapentaplegic |
| SMAD2 | Suppressor of mothers against decapentaplegic 2 |
| SMAD3 | Suppressor of mothers against decapentaplegic 3 |
| SMAD7 | Suppressor of mothers against decapentaplegic 7 |
| SOD | Superoxide dismutase |
| STR | Short tandem repeat |
| TBARS | Thiobarbituric acid-reactive substance |
| TBA | Thiobarbituric acid |
| TCA | Trichloroacetic acid |
| TCGA | The Cancer Genome Atlas |
| TGF-β1 | Transforming growth factor β1 |
| TP53 | Tumor protein P53 |
| TRFC | Transferrin receptor |
| TWIST1 | Twist family BHLH transcription factor 1 |
| UBC | Urothelial carcinoma of the bladder |
| UTR | Untranslated region |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Van Hoogstraten, L.M.C.; Vrieling, A.; van der Heijden, A.G.; Kogevinas, M.; Richters, A.; Kiemeney, L.A. Global Trends in the Epidemiology of Bladder Cancer: Challenges for Public Health and Clinical Practice. Nat. Rev. Clin. Oncol. 2023, 20, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Understanding the Biology of Urothelial Cancer Metastasis. Asian J. Urol. 2016, 3, 211–222. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; MSHS, M. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in Bladder Cancer Biology and Therapy. Nat. Rev. Cancer 2020, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Compérat, E.; Amin, M.B.; Cathomas, R.; Choudhury, A.; De Santis, M.; Kamat, A.; Stenzl, A.; Thoeny, H.C.; Witjes, J.A. Current Best Practice for Bladder Cancer: A Narrative Review of Diagnostics and Treatments. Lancet 2022, 400, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Max Bruins, H.; Carrión, A.; Cathomas, R.; Compérat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2024, 85, 17–31. [Google Scholar] [CrossRef] [PubMed]
- The microRNA Lifecycle in Health and Cancer. Available online: https://www.mdpi.com/2072-6694/14/23/5748?utm_source=chatgpt.com (accessed on 2 August 2025).
- Martino, M.T.D.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Rouas, R.; Lewalle, P. OncomiRs as Noncoding RNAs Having Functions in Cancer: Their Role in Immune Suppression and Clinical Implications. Front. Immunol. 2022, 13, 913951. [Google Scholar] [CrossRef] [PubMed]
- Mehlich, D.; Garbicz, F.; Włodarski, P.K. The Emerging Roles of the Polycistronic miR-106b∼25 Cluster in Cancer—A Comprehensive Review. Biomed. Pharmacother. 2018, 107, 1183–1195. [Google Scholar] [CrossRef]
- Sárközy, M.; Kahán, Z.; Csont, T. A Myriad of Roles of miR-25 in Health and Disease. Oncotarget 2018, 9, 21580–21612. [Google Scholar] [CrossRef]
- Ning, J.; Chu, C.; Du, Y.; Zuo, L. MiR-25 Regulates Cell Proliferation and Metastasis in Bladder Urothelial Carcinoma. J. Cancer 2021, 12, 6706–6714. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Dong, S.; Wang, H.; Pan, C.; Yang, S. Functional Mechanism of MicroRNA-25-3p in Hilar Cholangiocarcinoma Cell Proliferation and Migration Through Regulation of Dual Specificity Phosphatase 5. J. Investig. Surg. 2023, 36, 2202768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bai, R.; Li, M.; Ye, H.; Wu, C.; Wang, C.; Li, S.; Tan, L.; Mai, D.; Li, G.; et al. Excessive miR-25-3p Maturation via N6-Methyladenosine Stimulated by Cigarette Smoke Promotes Pancreatic Cancer Progression. Nat. Commun. 2019, 10, 1858. [Google Scholar] [CrossRef]
- Ning, L.; Zhang, M.; Zhu, Q.; Hao, F.; Shen, W.; Chen, D. miR-25-3p Inhibition Impairs Tumorigenesis and Invasion in Gastric Cancer Cells in Vitro and in Vivo. Bioengineered 2020, 11, 81–90. [Google Scholar] [CrossRef]
- Li, H.; Lin, W.; Zhang, G.; Liu, R.; Qu, M.; Zhang, J.; Xing, X. BMSC-Exosomes miR-25-3p Regulates the P53 Signaling Pathway Through PTEN to Inhibit Cell Apoptosis and Ameliorate Liver Ischemia–reperfusion Injury. Stem Cell Rev. Rep. 2023, 19, 2820–2836. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Jafari-Gharabaghlou, D.; Mohammadi, M.; Hashemzadeh, M.S. Zinc Oxide Nanoparticles and Cancer Chemotherapy: Helpful Tools for Enhancing Chemo-Sensitivity and Reducing Side Effects? Biol. Trace Elem. Res. 2024, 202, 1878–1900. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dai, J.; Hou, G.; Liu, H.; Zheng, S.; Wang, X.; Lin, Q.; Zhang, Y.; Lu, M.; Gong, Y.; et al. SMURF2 Predisposes Cancer Cell toward Ferroptosis in GPX4-Independent Manners by Promoting GSTP1 Degradation. Mol. Cell 2023, 83, 4352–4369.e8. [Google Scholar] [CrossRef]
- JS-K Induces Ferroptosis in Renal Carcinoma Cells by Regulating the c-Myc-GSTP1 Axis|Scientific Reports. Available online: https://www.nature.com/articles/s41598-025-97887-3?utm_source=chatgpt.com (accessed on 2 August 2025).
- Nor, W.F.S.B.W.; Chung, I.; Said, N.A.B. MicroRNA-548m Suppresses Cell Migration and Invasion by Targeting Aryl Hydrocarbon Receptor in Breast Cancer Cells. Oncol. Res. 2021, 28, 615–629. [Google Scholar] [CrossRef]
- Hwang, T.I.-S.; Cuiu, Y.-C.; Chen, Y.-C.; Chen, P.-C.; Tsai, T.-F.; Chou, K.-Y.; Ho, C.-Y.; Chen, H.-E.; Chang, P.-H.; Chang, A.-C. Tumor Suppressive Functions of hsa-miR-34a on Cell Cycle, Migration and Protective Autophagy in Bladder Cancer. Int. J. Oncol. 2023, 62, 66. [Google Scholar] [CrossRef]
- Xu, C.; Zhou, G.; Sun, Z.; Zhang, Z.; Zhao, H.; Jiang, X. miR-148a-3p Inhibits the Proliferation and Migration of Bladder Cancer via Regulating the Expression of ROCK-1. PeerJ 2022, 10, e12724. [Google Scholar] [CrossRef]
- Peng, H.; Li, H. The Encouraging Role of Long Noncoding RNA Small Nuclear RNA Host Gene 16 in Epithelial-Mesenchymal Transition of Bladder Cancer via Directly Acting on miR-17-5p/Metalloproteinases 3 Axis. Mol. Carcinog. 2019, 58, 1465–1480. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Xu, W.; Xia, X.; Ding, J. Methyltransferase-like 3 (METTL3) Mediated N6-Methyladenosine (m6A) Modifications Facilitate Mir-25-3p Maturation to Promote Gastrointestinal Stromal Tumors (GISTs) Progression. Genes Genom. 2022, 44, 1519–1530. [Google Scholar] [CrossRef]
- Wu, T.; Hu, H.; Zhang, T.; Jiang, L.; Li, X.; Liu, S.; Zheng, C.; Yan, G.; Chen, W.; Ning, Y.; et al. miR-25 Promotes Cell Proliferation, Migration, and Invasion of Non-Small-Cell Lung Cancer by Targeting the LATS2/YAP Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9719723. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lv, W.; Ding, W.; Li, J. Sevoflurane Inhibits the Proliferation and Invasion of Hepatocellular Carcinoma Cells through Regulating the PTEN/Akt/GSK-3β/β-Catenin Signaling Pathway by Downregulating miR-25-3p. Int. J. Mol. Med. 2020, 46, 97–106. [Google Scholar] [CrossRef]
- Xu, D.; Wang, Y.; Wu, J.; Zhang, Z.; Chen, J.; Xie, M.; Tang, R.; Cheng, C.; Chen, L.; Lin, S.; et al. MTIF2 Impairs 5 Fluorouracil-Mediated Immunogenic Cell Death in Hepatocellular Carcinoma in Vivo: Molecular Mechanisms and Therapeutic Significance. Pharmacol. Res. 2021, 163, 105265. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, T.; Lai, J.; Zhang, J.; Wang, T.; Ling, Y.; He, S.; Hu, Z. MicroRNA-25/ATXN3 Interaction Regulates Human Colon Cancer Cell Growth and Migration. Mol. Med. Rep. 2019, 19, 4213–4221. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Xu, R.; Wang, W.; Zhang, W. Ferroptosis and the Bidirectional Regulatory Factor P53. Cell Death Discov. 2023, 9, 197. [Google Scholar] [CrossRef]
- Shan, P.; Yang, F.; Yu, J.; Wang, L.; Qu, Y.; Qiu, H.; Zhang, H.; Zhu, S. A Novel Histone Deacetylase Inhibitor Exerts Promising Anti-breast Cancer Activity via Triggering AIFM1-dependent Programmed Necrosis. Cancer Commun. 2022, 42, 1207. [Google Scholar] [CrossRef]
- Shan, Z.; Tang, W.; Shi, Z.; Shan, T. Ferroptosis: An Emerging Target for Bladder Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 8201–8214. [Google Scholar] [CrossRef]
- Zeng, F.; Lan, Y.; Wang, N.; Huang, X.; Zhou, Q.; Wang, Y. Ferroptosis: A New Therapeutic Target for Bladder Cancer. Front. Pharmacol. 2022, 13, 1043283. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R. NFE2L2 and Ferroptosis Resistance in Cancer Therapy. Cancer Drug Resist. 2024, 7, 41. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Kang, R.; Tang, D. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Jia, B.; Li, J.; Song, Y.; Luo, C. ACSL4-Mediated Ferroptosis and Its Potential Role in Central Nervous System Diseases and Injuries. Int. J. Mol. Sci. 2023, 24, 10021. [Google Scholar] [CrossRef] [PubMed]
- Mbah, N.E.; Lyssiotis, C.A. Metabolic Regulation of Ferroptosis in the Tumor Microenvironment. J. Biol. Chem. 2022, 298, 101617. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Tang, D.; Kroemer, G.; Ren, J. Ferroptosis in Hepatocellular Carcinoma: Mechanisms and Targeted Therapy. Br. J. Cancer 2023, 128, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhong, T.; Jiang, L.; Huang, J.; Xia, Y.; Hu, R. miR-25 Enhances Cell Migration and Invasion in Non-Small-Cell Lung Cancer Cells via ERK Signaling Pathway by Inhibiting KLF4. Mol. Med. Rep. 2018, 17, 7005–7016. [Google Scholar] [CrossRef]
- Augoff, K.; Hryniewicz-Jankowska, A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Jiang, J.; Li, S.; Wang, G.; Ju, L.; Wang, F.; Liu, T.; Li, S. Matrix Metalloproteinase 11 Is a Potential Biomarker in Bladder Cancer Diagnosis and Prognosis. OncoTargets Ther. 2020, 13, 9059–9069. [Google Scholar] [CrossRef]
- Ma, B.; Ran, R.; Liao, H.-Y.; Zhang, H.-H. The Paradoxical Role of Matrix Metalloproteinase-11 in Cancer. Biomed. Pharmacother. 2021, 141, 111899. [Google Scholar] [CrossRef]
- Raju, S.C.; Hauff, S.J.; Lemieux, A.J.; Orosco, R.K.; Gross, A.M.; Nguyen, L.T.; Savariar, E.; Moss, W.; Whitney, M.; Cohen, E.E.; et al. Combined TP53 Mutation/3p Loss Correlates with Decreased Radiosensitivity and Increased Matrix-Metalloproteinase Activity in Head and Neck Carcinoma. Oral Oncol. 2015, 51, 470–475. [Google Scholar] [CrossRef]
- Xu, G.; Ou, L.; Liu, Y.; Wang, X.; Liu, K.; Li, J.; Li, J.; Wang, S.; Huang, D.; Zheng, K.; et al. Upregulated Expression of MMP Family Genes Is Associated with Poor Survival in Patients with Esophageal Squamous Cell Carcinoma via Regulation of Proliferation and Epithelial-mesenchymal Transition. Oncol. Rep. 2020, 44, 29–42. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Li, X.; Wang, J.; Zhang, N.; Amin, B.; Xu, G.; Zhu, B. Cancer-Associated Fibroblast-Derived MMP11 Promotes Tumor Progression in Pancreatic Cancer. Cancer Sci. 2025, 116, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Kochumon, S.; Al-Sayyar, A.; Jacob, T.; Bahman, F.; Akhter, N.; Wilson, A.; Sindhu, S.; Hannun, Y.A.; Ahmad, R.; Al-Mulla, F. TGF-β and TNF-α Interaction Promotes the Expression of MMP-9 through H3K36 Dimethylation: Implications in Breast Cancer Metastasis. Front. Immunol. 2024, 15, 1430187. [Google Scholar] [CrossRef]
- Lian, G.-Y.; Wang, Q.-M.; Mak, T.S.-K.; Huang, X.-R.; Yu, X.-Q.; Lan, H.-Y. Inhibition of Tumor Invasion and Metastasis by Targeting TGF-β-Smad-MMP2 Pathway with Asiatic Acid and Naringenin. Mol. Ther.-Oncolytics 2021, 20, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Jacobsen, A.; Silber, J.; Harinath, G.; Huse, J.T.; Schultz, N.; Sander, C. Analysis of microRNA-Target Interactions across Diverse Cancer Types. Nat. Struct. Mol. Biol. 2013, 20, 1325–1332. [Google Scholar] [CrossRef]
- Souza, M.F.; Cólus, I.M.S.; Fonseca, A.S.; Antunes, V.C.; Kumar, D.; Cavalli, L.R. MiR-182-5p Modulates Prostate Cancer Aggressive Phenotypes by Targeting EMT Associated Pathways. Biomolecules 2022, 12, 187. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Proietti De Santis, L.; Balajee, A.S.; Lorenti Garcia, C.; Pepe, G.; Worboys, A.M.; Palitti, F. Inhibition of P53, P21 and Bax by Pifithrin-Alpha Does Not Affect UV Induced Apoptotic Response in CS-B Cells. DNA Repair 2003, 2, 891–900. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbeláez, P.; Cruz, J.C.; Muñoz-Camargo, C. An Image J Plugin for the High Throughput Image Analysis of in Vitro Scratch Wound Healing Assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Federici, G.; Shaw, B.J.; Handy, R.D. Toxicity of Titanium Dioxide Nanoparticles to Rainbow Trout (Oncorhynchus mykiss): Gill Injury, Oxidative Stress, and Other Physiological Effects. Aquat. Toxicol. 2007, 84, 415–430. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for Quantitative Determination of Glutathione and Glutathione Disulfide Levels Using Enzymatic Recycling Method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Carrara, I.M.; Melo, G.P.; Bernardes, S.S.; Neto, F.S.; Ramalho, L.N.Z.; Marinello, P.C.; Luiz, R.C.; Cecchini, R.; Cecchini, A.L. Looking beyond the Skin: Cutaneous and Systemic Oxidative Stress in UVB-Induced Squamous Cell Carcinoma in Hairless Mice. J. Photochem. Photobiol. B 2019, 195, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.H.; Habig, W.H.; Jakoby, W.B. Mechanism for the Several Activities of the Glutathione S-Transferases. J. Biol. Chem. 1976, 251, 6183–6188. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Oxygen Radicals in Biological Systems; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, A.H.; Pereira, É.R.; Santos, A.G.P.d.; Quadreli, D.H.; Lima, L.V.A.d.; Ribeiro, D.L.; Rahimirad, S.; Mathias, C.; Nóbrega, M.d.; Mantovani, M.S.; et al. miR-25-3p Modulates Tumor Aggressiveness and Ferroptosis Escape in T24 Bladder Cancer Cells In Vitro. Pharmaceuticals 2025, 18, 1382. https://doi.org/10.3390/ph18091382
Sakai AH, Pereira ÉR, Santos AGPd, Quadreli DH, Lima LVAd, Ribeiro DL, Rahimirad S, Mathias C, Nóbrega Md, Mantovani MS, et al. miR-25-3p Modulates Tumor Aggressiveness and Ferroptosis Escape in T24 Bladder Cancer Cells In Vitro. Pharmaceuticals. 2025; 18(9):1382. https://doi.org/10.3390/ph18091382
Chicago/Turabian StyleSakai, Andresa Hiromi, Érica Romão Pereira, Anna Gabriele Prado dos Santos, Débora Hipólito Quadreli, Luan Vitor Alves de Lima, Diego Luis Ribeiro, Samira Rahimirad, Carolina Mathias, Monyse de Nóbrega, Mário Sérgio Mantovani, and et al. 2025. "miR-25-3p Modulates Tumor Aggressiveness and Ferroptosis Escape in T24 Bladder Cancer Cells In Vitro" Pharmaceuticals 18, no. 9: 1382. https://doi.org/10.3390/ph18091382
APA StyleSakai, A. H., Pereira, É. R., Santos, A. G. P. d., Quadreli, D. H., Lima, L. V. A. d., Ribeiro, D. L., Rahimirad, S., Mathias, C., Nóbrega, M. d., Mantovani, M. S., Scantamburlo Alves Fernandes, G., Cólus, I. M. d. S., & Serpeloni, J. M. (2025). miR-25-3p Modulates Tumor Aggressiveness and Ferroptosis Escape in T24 Bladder Cancer Cells In Vitro. Pharmaceuticals, 18(9), 1382. https://doi.org/10.3390/ph18091382









